A Dual-Modality System for Both Multi-Color Ultrasound-Switchable Fluorescence and Ultrasound Imaging
Abstract
:1. Introduction
2. Dual-Modality Imaging System
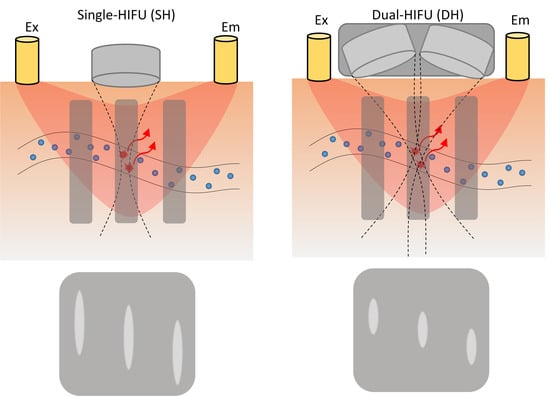
2.1. Basic Principles of USF and US Imaging
2.2. Hardware of the System
2.2.1. USF Sub-System
2.2.2. US Sub-System
2.2.3. Synchronization of USF and US Sub-Systems
2.3. Materials and Synthesis of USF Contrast Agents
2.4. Processing of USF and US Data
3. Results and Discussion
3.1. Single Target
3.2. Simultaneous Imaging of Multiple Targets Using Dual Modality Imaging
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Dorrell, M.I.; Aguilar, E.; Scheppke, L.; Barnett, F.H.; Friedlander, M. Combination angiostatic therapy completely inhibits ocular and tumor angiogenesis. Proc. Natl. Acad. Sci. USA 2007, 104, 967–972. [Google Scholar] [CrossRef] [PubMed]
- Abdollahi, A.; Folkman, J. Evading tumor evasion: Current concepts and perspectives of anti-angiogenic cancer therapy. Drug Resist. Updates 2010, 13, 16–28. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Chen, X. Multimodality molecular imaging of tumor angiogenesis. J. Nucl. Med. 2008, 49 (Suppl. S2), 113S–128S. [Google Scholar] [CrossRef] [PubMed]
- Niu, G.; Chen, X.Y. Has Molecular and Cellular Imaging Enhanced Drug Discovery and Drug Development? Drugs R D 2008, 9, 351–368. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.W.; Hao, Y.W.; Nyagilo, J.; Dave, D.P.; Xu, L.F.; Sun, X.K. Porous Hollow Gold Nanoparticles for Cancer SERS Imaging. J. Nano Res. 2010, 10, 137–148. [Google Scholar] [CrossRef]
- Faivre, S.; Djelloul, S.; Raymond, E. New paradigms in anticancer therapy: Targeting multiple signaling pathways with kinase inhibitors. Semin. Oncol. 2006, 33, 407–420. [Google Scholar] [CrossRef] [PubMed]
- Hecht, J.R.; Mitchell, E.; Chidiac, T.; Scroggin, C.; Hagenstad, C.; Spigel, D.; Marshall, J.; Cohn, A.; McCollum, D.; Stellaet, P.; et al. A Randomized Phase IIIB Trial of Chemotherapy, Bevacizumab, and Panitumumab Compared with Chemotherapy and Bevacizumab Alone for Metastatic Colorectal Cancer. J. Clin. Oncol. 2009, 27, 672–680. [Google Scholar] [CrossRef] [PubMed]
- Quesada, A.R.; Medina, M.A.; Alba, E. Playing only one instrument may be not enough: Limitations and future of the antianglogenic treatment of cancer. Bioessays 2007, 29, 1159–1168. [Google Scholar] [CrossRef] [PubMed]
- Shariat, S.F.; Karakiewicz, P.I.; Ashfaq, R.; Lerner, S.P.; Palapattu, G.S.; Cote, R.J.; Sagalowsky, A.I.; Lotan, Y. Multiple biomarkers improve prediction of bladder cancer recurrence and mortality in patients undergoing cystectomy. Cancer 2008, 112, 315–325. [Google Scholar] [CrossRef] [PubMed]
- Zavaleta, C.L.; Smith, B.R.; Walton, I.; Doering, W.; Davis, G.; Shojaei, B.; Natan, M.J.; Gambhir, S.S. Multiplexed imaging of surface enhanced Raman scattering nanotags in living mice using noninvasive Raman spectroscopy. Proc. Natl. Acad. Sci. USA 2009, 106, 13511–13516. [Google Scholar] [CrossRef] [PubMed]
- Gambhir, S.S. Molecular imaging of cancer with positron emission tomography. Nat. Rev. Cancer 2002, 2, 683–693. [Google Scholar] [CrossRef] [PubMed]
- Ntziachristos, V.; Ripoll, J.; Wang, L.H.V.; Weissleder, R. Looking and listening to light: The evolution of whole-body photonic imaging. Nat. Biotechnol. 2005, 23, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Corlu, A.; Choe, R.; Durduran, T.; Rosen, M.A.; Schweiger, M.; Arridge, S.R.; Schnall, M.D.; Yodh, A.G. Three-dimensional in vivo fluorescence diffuse optical tomography of breast cancer in humans. Opt. Express 2007, 15, 6696–6716. [Google Scholar] [CrossRef] [PubMed]
- Culver, J.; Akers, W.; Achilefu, S. Multimodality molecular imaging with combined optical and SPECT/PET modalities. J. Nucl. Med. 2008, 49, 169–172. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.H.V. Ultrasound-mediated biophotonic imaging: A review of acousto-optical tomography and photo-acoustic tomography. Dis. Markers 2003, 19, 123–138. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.V. Photoacoustic Imaging and Spectroscopy; CRC: Boca Raton, FL, USA, 2009; 499p. [Google Scholar]
- Wang, L.V. Multiscale photoacoustic microscopy and computed tomography. Nat. Photonics 2009, 3, 503–509. [Google Scholar] [CrossRef] [PubMed]
- Yuan, B.; Zhu, Q. Separately reconstructing the structural and functional parameters of a fluorescent inclusion embedded in a turbid medium. Opt. Express 2006, 14, 7172–7187. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Chang, J.H.; Wilson, B.C.; Veilleux, I.; Bai, Y.H.; DaCosta, R.; Kim, K.; Ha, S.; Lee, J.G.; Kim, J.S.; et al. A prototype hand-held tri-modal instrument for in vivo ultrasound, photoacoustic, and fluorescence imaging. Rev. Sci. Instrum. 2015, 86, 034901. [Google Scholar] [CrossRef] [PubMed]
- James, J.; Murukeshan, V.M.; Woh, L.S. Integrated photoacoustic, ultrasound and fluorescence platform for diagnostic medical imaging-proof of concept study with a tissue mimicking phantom. Biomed. Opt. Express 2014, 5, 2135–2144. [Google Scholar] [CrossRef] [PubMed]
- Yuan, B. Ultrasound-modulated fluorescence based on a fluorophore-quencher-labeled microbubble system. J. Biomed. Opt. 2009, 14, 024043. [Google Scholar] [CrossRef] [PubMed]
- Yuan, B.; Liu, Y. Ultrasound-modulated fluorescence from rhodamine B aqueous solution. J. Biomed. Opt. 2010, 15, 021321. [Google Scholar] [CrossRef] [PubMed]
- Yuan, B.; Liu, Y.; Mehl, P.; Vignola, J. Microbubble-enhanced ultrasound-modulated fluorescence in a turbid medium. Appl. Phys. Lett. 2009, 95, 181113. [Google Scholar] [CrossRef]
- Yuan, B.; Gamelin, J.; Zhu, Q. Mechanisms of the ultrasonic modulation of fluorescence in turbid media. J. Appl. Phys. 2008, 104, 103102. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yuan, B.; Vignola, J. Effect of Fluorescent Particle Size on the Modulation Efficiency of Ultrasound-Modulated Fluorescence. Int. J. Opt. 2012, 2012, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Yuan, B.; Uchiyama, S.; Liu, Y.; Nguyen, N.T.; Alexandrakis, G. High-resolution imaging in a deep turbid medium based on an ultrasound-switchable fluorescence technique. Appl. Phys. Lett. 2012, 101, 033703. [Google Scholar] [CrossRef] [PubMed]
- Pei, Y.B.; Wei, M.Y.; Cheng, B.B.; Liu, Y.; Xie, Z.W.; Nguyen, K.; Yuan, B. High resolution imaging beyond the acoustic diffraction limit in deep tissue via ultrasound-switchable NIR fluorescence. Sci. Rep. 2014, 4, 4690. [Google Scholar] [CrossRef] [PubMed]
- Cheng, B.B.; Wei, M.Y.; Liu, Y.; Pitta, H.; Xie, Z.W.; Hong, Y.; Nguyen, K.T.; Yuan, B. Development of Ultrasound-Switchable Fluorescence Imaging Contrast Agents Based on Thermosensitive Polymers and Nanoparticles. IEEE J. Sel. Top. Quantum Electron. 2014, 20, 67–80. [Google Scholar] [CrossRef] [PubMed]
- Cheng, B.B.; Bandi, V.; Wei, M.Y.; Pei, Y.B.; D’Souza, F.; Nguyen, K.T.; Hong, Y.; Yuan, B. High-Resolution Ultrasound-Switchable Fluorescence Imaging in Centimeter-Deep Tissue Phantoms with High Signal-to-Noise Ratio and High Sensitivity via Novel Contrast Agents. PLoS ONE 2016, 11, e0165963. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Cheng, B.; Yao, T.; Xu, C.; Nguyen, K.T.; Hong, Y.; Yuan, B. New generation ICG-based contrast agents for ultrasound-switchable fluorescence imaging. Sci. Rep. 2016, 6, 35942. [Google Scholar] [CrossRef] [PubMed]
- Bandi, V.; El-Khouly, M.E.; Ohkubo, K.; Nesterov, V.N.; Zandler, M.E.; Fukuzumi, S.; D’Souza, F. Excitation-wavelength-dependent, ultrafast photoinduced electron transfer in bisferrocene/BF2-chelated-azadipyrromethene/fullerene tetrads. Chemistry 2013, 19, 7221–7230. [Google Scholar] [CrossRef] [PubMed]
- Yuan, B.H.; Pei, Y.B.; Kandukuri, J. Breaking the acoustic diffraction limit via nonlinear effect and thermal confinement for potential deep-tissue high-resolution imaging. Appl. Phys. Lett. 2013, 102, 063703. [Google Scholar] [CrossRef] [PubMed]
- Kandukuri, J.; Liu, Y.; Yuan, B. Cost-efficient and multi-functional systems for ultrasound measurement and imaging. Austin J. Biomed. Eng. 2014, 1, 1001. [Google Scholar] [PubMed]




© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kandukuri, J.; Yu, S.; Cheng, B.; Bandi, V.; D’Souza, F.; Nguyen, K.T.; Hong, Y.; Yuan, B. A Dual-Modality System for Both Multi-Color Ultrasound-Switchable Fluorescence and Ultrasound Imaging. Int. J. Mol. Sci. 2017, 18, 323. https://doi.org/10.3390/ijms18020323
Kandukuri J, Yu S, Cheng B, Bandi V, D’Souza F, Nguyen KT, Hong Y, Yuan B. A Dual-Modality System for Both Multi-Color Ultrasound-Switchable Fluorescence and Ultrasound Imaging. International Journal of Molecular Sciences. 2017; 18(2):323. https://doi.org/10.3390/ijms18020323
Chicago/Turabian StyleKandukuri, Jayanth, Shuai Yu, Bingbing Cheng, Venugopal Bandi, Francis D’Souza, Kytai T. Nguyen, Yi Hong, and Baohong Yuan. 2017. "A Dual-Modality System for Both Multi-Color Ultrasound-Switchable Fluorescence and Ultrasound Imaging" International Journal of Molecular Sciences 18, no. 2: 323. https://doi.org/10.3390/ijms18020323
APA StyleKandukuri, J., Yu, S., Cheng, B., Bandi, V., D’Souza, F., Nguyen, K. T., Hong, Y., & Yuan, B. (2017). A Dual-Modality System for Both Multi-Color Ultrasound-Switchable Fluorescence and Ultrasound Imaging. International Journal of Molecular Sciences, 18(2), 323. https://doi.org/10.3390/ijms18020323