The Current State of Nanoparticle-Induced Macrophage Polarization and Reprogramming Research
Abstract
:1. Introduction
2. Macrophage Polarization
2.1. Characterization Polarized Macrophage Phenotype
2.2. Mechanisms of Macrophage Polarization
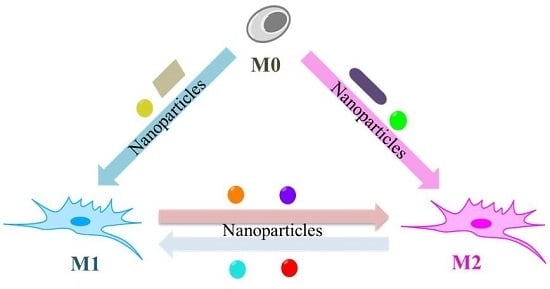
3. NPs Modulate Macrophage Polarization and Reprogramming
3.1. NPs Induce M0 Macrophage toward M1 Polarization
3.1.1. Chemical Composition
3.1.2. Size
3.1.3. Surface Chemical Modification
3.2. NPs Drive Primary Macrophage Polarization toward the M2 Phenotype
3.3. NPs Stimulated Macrophage Reprogramming
3.3.1. The Switch from the M2 Phenotype to M1 Phenotype
3.3.2. The Switch from the M1 Phenotype to M2 Phenotype
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Taki, A.; Smooker, P. Small wonders—The use of nanoparticles for delivering antigen. Vaccines 2015, 3, 638–661. [Google Scholar] [CrossRef] [PubMed]
- Kaittanis, C.; Shaffer, T.M.; Thorek, D.L.J.; Grimm, J. Dawn of advanced molecular medicine: Nanotechnological advancements in cancer imaging and therapy. Crit. Rev. Oncog. 2014, 19, 143–176. [Google Scholar] [CrossRef] [PubMed]
- Mahapatro, A.; Singh, D.K. Biodegradable nanoparticles are excellent vehicle for site directed in vivo delivery of drugs and vaccines. J. Nanobiotechnol. 2011, 9, 55. [Google Scholar] [CrossRef] [PubMed]
- Newman, M.D.; Stotland, M.; Ellis, J.I. The safety of nanosized particles in titanium dioxide- and zinc oxide-based sunscreens. J. Am. Acad. Dermatol. 2009, 61, 685–692. [Google Scholar] [CrossRef] [PubMed]
- Getts, D.R.; Shea, L.D.; Miller, S.D.; King, N.J.C. Harnessing nanoparticles for immune modulation. Trends Immunol. 2015, 36, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Yakoot, S.M.; Salem, N.A. A sonochemical-assisted simple and green synthesis of silver nanoparticles and its use in cosmetics. Int. J. Pharmacol. 2016, 12, 572–575. [Google Scholar] [CrossRef]
- Martirosyan, A.; Schneider, Y. Engineered nanomaterials in food: Implications for food safety and consumer health. Int. J. Environ. Res. Public Health 2014, 11, 5720–5750. [Google Scholar] [CrossRef] [PubMed]
- Contado, C. Nanomaterials in consumer products: A challenging analytical problem. Front. Chem. 2015, 3, 48. [Google Scholar] [PubMed]
- Cho, W.S.; Duffin, R.; Thielbeer, F.; Bradley, M.; Megson, I.L.; MacNee, W.; Poland, C.A.; Tran, C.L.; Donaldson, K. Zeta potential and solubility to toxic ions as mechanisms of lung inflammation caused by metal/metal oxide nanoparticles. Toxicol. Sci. 2012, 126, 469–477. [Google Scholar] [CrossRef] [PubMed]
- Rster, G.O.; Ferin, J.; Lehnert, B.E. Correlation between particle size, in vivo particle persistence, and lung injury. Environ. Health Perspect. 1994, 102, 173–179. [Google Scholar]
- Brandenberger, C.; Rowley, N.L.; Jackson-Humbles, D.N.; Zhang, Q.; Bramble, L.A.; Lewandowski, R.P.; Wagner, J.G.; Chen, W.; Kaplan, B.L.; Kaminski, N.E.; et al. Engineered silica nanoparticles act as adjuvants to enhance allergic airway disease in mice. Part. Fibre Toxicol. 2013, 10, 26. [Google Scholar] [CrossRef] [PubMed]
- Zanganeh, S.; Hutter, G.; Spitler, R.; Lenkov, O.; Mahmoudi, M.; Shaw, A.; Pajarinen, J.S.; Nejadnik, H.; Goodman, S.; Moseley, M.; et al. Iron oxide nanoparticles inhibit tumour growth by inducing pro-inflammatory macrophage polarization in tumour tissues. Nat. Nanotechnol. 2016, 11, 986–994. [Google Scholar] [CrossRef] [PubMed]
- Laskin, D.L.; Sunil, V.R.; Gardner, C.R.; Laskin, J.D. Macrophages and tissue injury: Agents of defense or destruction? Annu. Rev. Pharmacol. 2011, 51, 267–288. [Google Scholar] [CrossRef] [PubMed]
- Wynn, T.A.; Chawla, A.; Pollard, J.W. Origins and hallmarks of macrophages: Development, homeostasis, and disease. Nature 2013, 496, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Martinez, F.O. Regulators of macrophage activation. Eur. J. Immunol. 2011, 41, 1531–1534. [Google Scholar] [CrossRef] [PubMed]
- Hill, A.A.; Bolus, W.R.; Hasty, A.H. A decade of progress in adipose tissue macrophage biology. Immunol. Rev. 2014, 262, 134–152. [Google Scholar] [CrossRef] [PubMed]
- Rőszer, T. Understanding the mysterious M2 macrophage through activation markers and effector mechanisms. Mediat. Inflamm. 2015, 2015, 816460. [Google Scholar] [CrossRef] [PubMed]
- Mills, C.D.; Kincaid, K.; Alt, J.M.; Heilman, M.J.; Hill, A.M. M-1/M-2 Macrophages and the Th1/Th2 paradigm. J. Immunol. 2000, 164, 6166–6173. [Google Scholar] [CrossRef] [PubMed]
- Dobrovolskaia, M.A.; McNeil, S.E. Immunological properties of engineered nanomaterials. Nat. Nanotechnol. 2007, 2, 469–478. [Google Scholar] [CrossRef] [PubMed]
- MacParland, S.A.; Tsoi, K.M.; Ouyang, B.; Ma, X.; Manuel, J.; Fawaz, A.; Ostrowski, M.A.; Alman, B.A.; Zilman, A.; Chan, W.C.W.; et al. Gold Nanoparticle Uptake by Human Derived Macrophages and Primary Liver Phagocytic Cells is Dependent on Phenotype. ACS Nano 2016. [Google Scholar] [CrossRef]
- Lucarelli, M.; Gatti, A.M.; Savarino, G.; Quattroni, P.; Martinelli, L.; Monari, E.; Boraschi, D. Innate defence functions of macrophages can be biased by nano-sized ceramic and metallic particles. Eur. Cytokine Netw. 2004, 15, 339–346. [Google Scholar] [PubMed]
- Laskar, A.; Eilertsen, J.; Li, W.; Yuan, X. SPION primes THP1 derived M2 macrophages towards M1-like macrophages. Biochem. Biophys. Res. Commun. 2013, 441, 737–742. [Google Scholar] [CrossRef] [PubMed]
- Bartneck, M.; Ritz, T.; Keul, H.A.; Wambach, M.; Bornemann, J.; Gbureck, U.; Ehling, J.; Lammers, T.; Heymann, F.; Gassler, N.; et al. Peptide-Functionalized gold nanorods increase liver injury in hepatitis. ACS Nano 2012, 6, 8767–8777. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, A.; Syrovets, T.; Haas, K.A.; Loos, C.; Musyanovych, A.; Mailänder, V.; Landfester, K.; Simmet, T. Carboxyl- and amino-functionalized polystyrene nanoparticles differentially affect the polarization profile of M1 and M2 macrophage subsets. Biomaterials 2016, 85, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Talekar, M.; Tran, T.; Amiji, M. Translational nano-medicines: Targeted therapeutic delivery for cancer and inflammatory diseases. AAPS J. 2015, 17, 813–827. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Oh, N. Endocytosis and exocytosis of nanoparticles in mammalian cells. Int. J. Nanomed. 2014, 9, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Gao, T.; Hong, H.; Sun, J. Applications of gold nanoparticles in cancer nanotechnology. Nanotechnol. Sci. Appl. 2011, 2008, 17–32. [Google Scholar] [CrossRef] [PubMed]
- Taylor, P.R.; Martinez-Pomares, L.; Stacey, M.; Lin, H.; Brown, G.D.; Gordon, S. Macrophage receptors and immune recognition. Annu. Rev. Immunol. 2005, 23, 901–944. [Google Scholar] [CrossRef] [PubMed]
- Giovannia, M.; Yueb, J.; Zhangb, L.; Xiea, J.; Ong, C.N.; Leonga, D.T. Pro-Inflammatory responses of RAW264.7 macrophages when treated with ultralow concentrations of silver, titanium dioxide, and zinc oxide nanoparticles. J. Hazard. Mater. 2015, 297, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Laskin, D.L. Macrophages and inflammatory mediators in chemical toxicity: A battle of forces. Chem. Res. Toxicol. 2009, 22, 1376–1385. [Google Scholar] [CrossRef] [PubMed]
- Mills, C.D. M1 and M2 macrophages: Oracles of health and disease. Crit. Rev. Immunol. 2012, 32, 463–488. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Sica, A.; Sozzani, S.; Allavena, P.; Vecchi, A.; Locat, M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004, 25, 677–686. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Kim, Y.J.; Jang, J.H.; Park, J.W. Modulating macrophage polarization with divalent cations in nanostructured titanium implant surfaces. Nanotechnology 2016, 27, 85101. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Tran, T.H.; Amiji, M. Macrophage repolarization with targeted alginate nanoparticles containing IL-10 plasmid DNA for the treatment of experimental arthritis. Biomaterials 2015, 61, 162–177. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.K.; Chittezhath, M.; Shalova, I.N.; Lim, J. Macrophage polarization and plasticity in health and disease. Immunl. Res. 2012, 53, 11–24. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.C.; Zou, X.B.; Chai, Y.F.; Yao, Y.M. Macrophage polarization in inflammatory diseases. Int. J. Biol. Sci. 2014, 10, 520–529. [Google Scholar] [CrossRef] [PubMed]
- Su, L.; Zhang, W.; Wu, X.; Zhang, Y.; Chen, X.; Liu, G.; Chen, G.; Jiang, M. Glycocalyx-mimicking nanoparticles for stimulation and polarization of macrophages via specific interactions. Small 2015, 11, 4191–4200. [Google Scholar] [CrossRef] [PubMed]
- Mosser, D.M. The many faces of macrophage activation. J. Leukoc. Biol. 2003, 73, 209–212. [Google Scholar] [CrossRef] [PubMed]
- Jablonski, K.A.; Amici, S.A.; Webb, L.M.; Ruiz-Rosado, J.D.D.; Popovich, P.G.; Partida-Sanchez, S.; Guerau-de-Arellano, M. Novel markers to delineate murine M1 and M2 macrophages. PLoS ONE 2015, 10, e145342. [Google Scholar] [CrossRef] [PubMed]
- Sica, A.; Erreni, M.; Allavena, P.; Porta, C. Macrophage polarization in pathology. Cell. Mol. Life Sci. 2015, 72, 4111–4126. [Google Scholar] [CrossRef] [PubMed]
- McWhorter, F.Y.; Davis, C.T.; Liu, W.F. Physical and mechanical regulation of macrophage phenotype and function. Cell. Mol. Life Sci. 2015, 72, 1303–1316. [Google Scholar] [CrossRef] [PubMed]
- Sica, A.; Mantovani, A. Macrophage plasticity and polarization: In vivo veritas. J. Clin. Investig. 2012, 122, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Mills, C.D.; Ley, K. M1 and M2 macrophages: The chicken and the egg of immunity. J. Innate Immun. 2014, 6, 716–726. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Huang, C.; Lin, Z.; Zhan, S.; Kong, L.; Fang, C.; Li, J. Macrophage polarization and function with emphasis on the evolving roles of coordinated regulation of cellular signaling pathways. Cell Signal. 2014, 26, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Liu, R.; Wang, X.; Liu, Q.; Chen, Y.; Valle, R.P.; Zuo, Y.Y.; Xia, T.; Liu, S. Crucial role of lateral size for graphene oxide in activating macrophages and stimulating pro-inflammatory responses in cells and animals. ACS Nano 2015, 9, 10498–10515. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.; Rastogi, R.; Shelke, J.; Amiji, M.M. Modulation of macrophage functional polarity towards anti-inflammatory phenotype with plasmid DNA delivery in CD44 targeting hyaluronic acid nanoparticles. Sci. Rep. 2015, 5, 16632. [Google Scholar] [CrossRef] [PubMed]
- Yen, H.; Hsu, S.; Tsai, C. Cytotoxicity and immunological response of gold and silver nanoparticles of different sizes. Small 2009, 5, 1553–1561. [Google Scholar] [CrossRef] [PubMed]
- Saxena, A.; Tripathi, R.M.; Zafar, F.; Singh, P. Green synthesis of silver nanoparticles using aqueous solution of Ficus benghalensis leaf extract and characterization of their antibacterial activity. Mater. Lett. 2012, 67, 91–94. [Google Scholar] [CrossRef]
- Nishanth, R.P.; Jyotsna, R.G.; Schlager, J.J.; Hussain, S.M.; Reddanna, P. Inflammatory responses of RAW 264.7 macrophages upon exposure to nanoparticles: Role of ROS-NFκB signaling pathway. Nanotoxicology 2011, 5, 502–516. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, M.; Toshima, H.; Yonezawa, T.; Kawai, K.; Narushima, T.; Kaga, M.; Endo, K. Responses of RAW264.7 macrophages to water-dispersible gold and silver nanoparticles stabilized by metal-carbon sigma-bonds. J. Biomed. Mater. Res. A 2014, 102, 1838–1849. [Google Scholar] [CrossRef] [PubMed]
- Tyner, K.; Bancos, S.; Stevens, D. Effect of silica and gold nanoparticles on macrophage proliferation, activation markers, cytokine production, and phagocytosis in vitro. Int. J. Nanomed. 2015, 10, 183–206. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Hitchins, V.M.; Schrand, A.M.; Hussain, S.M.; Goering, P.L. Uptake of gold nanoparticles in murine macrophage cells without cytotoxicity or production of pro-inflammatory mediators. Nanotoxicity 2011, 5, 284–295. [Google Scholar] [CrossRef] [PubMed]
- Roy, R.; Parashar, V.; Chauhan, L.K.S.; Shanker, R.; Das, M.; Tripathi, A.; Dwivedi, P.D. Mechanism of uptake of ZnO nanoparticles and inflammatory responses in macrophages require PI3K mediated MAPKs signaling. Toxicol. In Vitro 2014, 28, 457–467. [Google Scholar] [CrossRef] [PubMed]
- Ansari, C.; Tikhomirov, G.A.; Hong, S.H.; Falconer, R.A.; Loadman, P.M.; Gill, J.H.; Castaneda, R.; Hazard, F.K.; Tong, L.; Lenkov, O.D.; et al. Development of Novel Tumor-Targeted Theranostic Nanoparticles Activated by Membrane-Type Matrix Metalloproteinases for Combined Cancer Magnetic Resonance Imaging and Therapy. Small 2016, 10, 566–575. [Google Scholar] [CrossRef] [PubMed]
- Neuwelt, E.A.; Várallyay, C.G.; Manninger, S.; Solymosi, D.; Haluska, M.; Hunt, M.A.; Nesbit, G.; Stevens, A.; Jerosch-Herold, M.; Jacobs, P.M.; et al. The potential of ferumoxytol nanoparticle magnetic resonance imaging, perfusion, and angiography in central nervous system. Neurosurgery 2007, 60, 601–612. [Google Scholar] [CrossRef] [PubMed]
- Go, D.P.; Palmer, J.A.; Gras, S.L.; O’Connor, A.J. Coating and release of an anti-inflammatory hormone from PLGA microspheres for tissue engineering. J. Biomed. Mater. Res. A 2012, 100, 507–517. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.; Krishnan, S.; Amiji, M.M. MicroRNA-223 induced repolarization of peritoneal macrophages using CD44 targeting hyaluronic acid nanoparticles for anti-inflammatory effects. PLoS ONE 2016, 11, e152024. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, G.; Meng, C.; Guo, X.; Cheruku, P.S.; Shi, L.; Xu, H.; Li, H.; Wang, G.; Evans, A.R.; Safe, S.; et al. Molecular Cardiology A Novel Regulator of Macrophage Activation. Circulation 2012, 125, 2892–2903. [Google Scholar] [CrossRef] [PubMed]

| NPs | Shape | Zeta Potential (mV) | Hydrodynamic Diameter (nm) | Core Size (nm) | Surface Modification | Initial PhenoType | Polarized Phenotype | Model | Cell Line | Marker | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ag | - | −11.30 ± 1.01 in medium | 168.57 ± 2.47 in medium | 35.53 ± 14.92 | - | M0 | M1 | in vitro | RAW264.7 | IL-6 , IL-1β↑ | [27] |
| sphere | 13.69 ± 0.25 in H2O | - | 3.08 ± 1.16 | J774.A1 | IL-1, IL-6, TNF-α↑ | [44] | |||||
| 15.43 ± 2.72 in H2O | 5.75 ± 1.12 | ||||||||||
| 5.35 ± 1.26 in H2O | 24.85 ± 6.06 | ||||||||||
| −39.5 ± 3.5 in H2O | 15 ± 3 | RAW 264.7 | TNF-α, IL-6↑ | [47] | |||||||
| −34.5 ± 3.7 in H2O | 40 ± 4 | ||||||||||
| Au | sphere | −56.64 ± 1.84 in H2O | - | 2.81 ± 0.84 | - | M0 | M1 | in vitro | J774.A1 | IL-1, IL-6, TNF-α↑ | [44] |
| −60.85 ± 2.88 in H2O | 5.52 ± 0.95 | ||||||||||
| −78.81 ± 1.97 in H2O | 38.05 ± 11.88 | ||||||||||
| Co | - | - | - | 50–200 | - | M0 | M1 | in vitro | U-937 | TNF-α↑ | [19] |
| ZnO | - | −8.62 ± 0.26 in medium | 89.92 ± 1.58 in medium | 31.89 ± 12.63 | - | M0 | M1 | in vitro | RAW264.7 | IL-6 , IL-1β↑ | [27] |
| TiO2 | - | −11.20 ± 1.11 in medium | 191.57 ± 1.52 in medium | 30.70 ± 9.18 | - | M0 | M1 | in vitro | RAW264.7 | IL-6 , IL-1β↑ | [27] |
| SiO2 | - | - | - | 15 | - | M0 | M1 | in vitro | U-937 | IL-1β, TNF-α↑ | [21] |
| Ferumoxytol | - | - | - | 15 nm | Carboxymet-hyldextran | M0 | M1 | in vitro | RAW264.7 | CD86, TNF-α↑ | [12] |
| in vivo | Liver and lung macrophages | CD80↑ CD206↓ (liver) CD206↓ (lung) | |||||||||
| GO | sheet | −31.88 ± 2.42 in H2O | - | 50–350 | - | M0 | M1 | in vitro | J774.A1 and THP-1 | TNF-α, IL-6, iNOS↑ (J774.A1) & IL-1β, TNF-α↑ (THP-1) | [45] |
| −30.42 ± 2.17 in H2O | 350–750 | ||||||||||
| −28.72 ± 2.36 in H2O | 750–1300 | ||||||||||
| Au | rod | - | - | 15 × 50 | GLF | M0 | M1 | isolated from mouse liver | mouse hepatic macrophage | Arg1, Retnla, IL-4↓, TNF-α↑ | [23] |
| RGD | M0 | M2 | Arg1, Retnla, IL-4↑, TNF-α↓ | ||||||||
| Ti | - | - | - | 30–50 | Ca and Sr | M0 | M2 | in vitro | J774.A1 | Arg1, MR, CD163↑ | [33] |
| Glyco-NP | - | - | 36 in PBS | - | Gal | M2 | M1 | isolated from mouse peritoneal cavity | mouse peritoneal macrophage | CD86, IL-12↑, CD206, CD23, IL-10↓, TNF-α↑ | [37] |
| 34 in PBS | Man | ||||||||||
| 34 in PBS | Fuc | ||||||||||
| SPIONs | - | −8.02 in 0.9% NaCl | - | 60.32 | - | M2 | M1 | in vitro | THP1 derived M2 macrophage | CD86, TNF-a↑ | [22] |
| HA-PEI NPs | - | - | 185.9 in PBS | 80–120 | plasmid DNA IL-10 | M1 | M2 | in vitro & isolated from mouse peritoneal cavity | J774.A1 & mouse peritoneal macrophage | Arg, CD163, IL-10↑, iNOS, CD80↓ (J774.A1) CD163, IL-10↑ TNF-α, IL-1β, iNOS↓ (peritoneal macrophage) | [47] |
| plasmid DNA IL-4 | Arg, CD206, CD163, IL-10↑, iNOS, CD80↓ (J774.A1) CD163, IL-10↑ iNOS↓ (peritoneal macrophage) | ||||||||||
| −14.7 in PBS | ~200 in PBS | ~200 | miR-223 plasmid DNA encapsulated | IL-1β, TNF-α, IL-6, iNOS↓, Arg↑in two cell lines | [57] | ||||||
| Alginate NPs | sphere | 15.8 ± 3.7 | 299.7 ± 2.2 | 180–250 | mIL-10 plasmid DNA | M1 | M2 | in vitro | synovial macrophage | IL-6, IL-1β, TNF-α↓ | [34] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miao, X.; Leng, X.; Zhang, Q. The Current State of Nanoparticle-Induced Macrophage Polarization and Reprogramming Research. Int. J. Mol. Sci. 2017, 18, 336. https://doi.org/10.3390/ijms18020336
Miao X, Leng X, Zhang Q. The Current State of Nanoparticle-Induced Macrophage Polarization and Reprogramming Research. International Journal of Molecular Sciences. 2017; 18(2):336. https://doi.org/10.3390/ijms18020336
Chicago/Turabian StyleMiao, Xiaoyuan, Xiangfeng Leng, and Qiu Zhang. 2017. "The Current State of Nanoparticle-Induced Macrophage Polarization and Reprogramming Research" International Journal of Molecular Sciences 18, no. 2: 336. https://doi.org/10.3390/ijms18020336
APA StyleMiao, X., Leng, X., & Zhang, Q. (2017). The Current State of Nanoparticle-Induced Macrophage Polarization and Reprogramming Research. International Journal of Molecular Sciences, 18(2), 336. https://doi.org/10.3390/ijms18020336