Future Prospects for the Development of Cost-Effective Adenovirus Vaccines
Abstract
:1. Introduction
2. Vectored Vaccines
2.1. Gold Standard, the Example of Rabies Vaccines
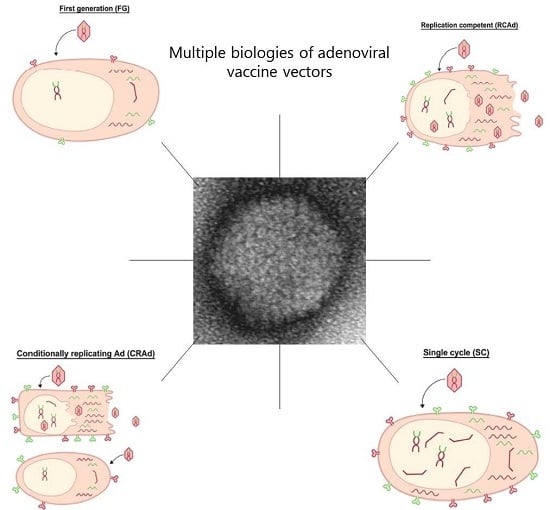
2.2. Lessons Learned from Non-Replicating Adenovirus-Vectored Vaccines
3. Advantages of Replication-Competent Adenovirus Compared to Other Adenovirus Vectors
4. Induction of Transgene Immunodominance by Modulation of the Adenovirus Vector Replication
4.1. Cell- or Tissue-Specific Adenovirus Attenuation
4.2. Increase of Transgene Immunodominance
5. Conclusions
Acknowledgments
Conflicts of Interest
Abbreviations
| Ad: | Adenovirus |
| CRAd: | Conditionally replicating adenovirus |
| DNA: | Deoxyribonucleic-acid |
| FG: | First generation |
| HC: | High capacity |
| HIV: | Human immunodeficiency virus |
| MHC: | Major histocompatibility complex |
| RC: | Replication competent |
| RCAd: | Replication competent adenovirus |
| RNA: | Ribonucleic acid |
| VLP: | Virus like particle |
| WT: | Wild-type |
References
- Andre, F.E.; Booy, R.; Bock, H.L.; Clemens, J.; Datta, S.K.; John, T.J.; Lee, B.W.; Lolekha, S.; Peltola, H.; Ruff, T.A.; et al. Vaccination greatly reduces disease, disability, death and inequity worldwide. Bull. World Health Organ. 2008, 86, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Folb, P.I.; Bernatowska, E.; Chen, R.; Clemens, J.; Dodoo, A.N.; Ellenberg, S.S.; Farrington, C.P.; John, T.J.; Lambert, P.H.; Macdonald, N.E.; et al. A global perspective on vaccine safety and public health: The Global Advisory Committee on Vaccine Safety. Am. J. Public Health 2004, 94, 1926–1931. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Pool, V.; Iskander, J.K.; English-Bullard, R.; Ball, R.; Wise, R.P.; Haber, P.; Pless, R.P.; Mootrey, G.; Ellenberg, S.S.; et al. Surveillance for safety after immunization: Vaccine Adverse Event Reporting System (VAERS)—United States, 1991–2001. MMWR Surveill. Summ. 2003, 52, 1–24. [Google Scholar] [PubMed]
- Shimizu, H.; Thorley, B.; Paladin, F.J.; Brussen, K.A.; Stambos, V.; Yuen, L.; Utama, A.; Tano, Y.; Arita, M.; Yoshida, H.; et al. Circulation of type 1 vaccine-derived poliovirus in the Philippines in 2001. J. Virol. 2004, 78, 13512–13521. [Google Scholar] [CrossRef] [PubMed]
- Artenstein, A.W.; Opal, J.M.; Opal, S.M.; Tramont, E.C.; Peter, G.; Russell, P.K. History of U.S. military contributions to the study of vaccines against infectious diseases. Mil. Med. 2005, 170 (Suppl. S4), 3–11. [Google Scholar] [CrossRef] [PubMed]
- Choudhry, A.; Mathena, J.; Albano, J.D.; Yacovone, M.; Collins, L. Safety evaluation of adenovirus type 4 and type 7 vaccine live, oral in military recruits. Vaccine 2016, 34, 4558–4564. [Google Scholar] [CrossRef] [PubMed]
- Pastoret, P.P.; Brochier, B. The development and use of a vaccinia-rabies recombinant oral vaccine for the control of wildlife rabies; a link between Jenner and Pasteur. Epidemiol. Infect. 1996, 116, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Brochier, B.; Aubert, M.F.; Pastoret, P.P.; Masson, E.; Schon, J.; Lombard, M.; Chappuis, G.; Languet, B.; Desmettre, P. Field use of a vaccinia-rabies recombinant vaccine for the control of sylvatic rabies in Europe and North America. Rev. Sci. Tech. 1996, 15, 947–970. [Google Scholar] [CrossRef] [PubMed]
- Yarosh, O.K.; Wandeler, A.I.; Graham, F.L.; Campbell, J.B.; Prevec, L. Human adenovirus type 5 vectors expressing rabies glycoprotein. Vaccine 1996, 14, 1257–1264. [Google Scholar] [CrossRef]
- Rosatte, R.C.; Donovan, D.; Davies, J.C.; Allan, M.; Bachmann, P.; Stevenson, B.; Sobey, K.; Brown, L.; Silver, A.; Bennett, K.; et al. Aerial distribution of ONRAB baits as a tactic to control rabies in raccoons and striped skunks in Ontario, Canada. J. Wildl. Dis. 2009, 45, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Ontario Resuming Rabies Vaccination Bait Drops. Available online: https://news.ontario.ca/mnr/en/2016/03/ontario-resuming-rabies-vaccination-bait-drops.html (accessed on 31 March 2016).
- Knowles, M.K.; Nadin-Davis, S.A.; Sheen, M.; Rosatte, R.; Mueller, R.; Beresford, A. Safety studies on an adenovirus recombinant vaccine for rabies (AdRG1.3-ONRAB) in target and non-target species. Vaccine 2009, 27, 6619–6626. [Google Scholar] [CrossRef] [PubMed]
- Rosatte, R.C.; Donovan, D.; Davies, J.C.; Brown, L.; Allan, M.; von Zuben, V.; Bachmann, P.; Sobey, K.; Silver, A.; Bennett, K.; et al. High-density baiting with ONRAB(R) rabies vaccine baits to control Arctic-variant rabies in striped skunks in Ontario, Canada. J. Wildl. Dis. 2011, 47, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Alkhatib, G.; Briedis, D.J. High-level eucaryotic in vivo expression of biologically active measles virus hemagglutinin by using an adenovirus type 5 helper-free vector system. J. Virol. 1988, 62, 2718–2727. [Google Scholar] [PubMed]
- Lasaro, M.O.; Ertl, H.C. New insights on adenovirus as vaccine vectors. Mol. Ther. 2009, 17, 1333–1339. [Google Scholar] [CrossRef] [PubMed]
- Kamen, A.; Henry, O. Development and optimization of an adenovirus production process. J. Gene Med. 2004, 6 (Suppl. 1), S184–S192. [Google Scholar] [CrossRef] [PubMed]
- Danthinne, X.; Imperiale, M.J. Production of first generation adenovirus vectors: A review. Gene Ther. 2000, 7, 1707–1714. [Google Scholar] [CrossRef] [PubMed]
- Randrianarison-Jewtoukoff, V.; Perricaudet, M. Recombinant adenoviruses as vaccines. Biologicals 1995, 23, 145–157. [Google Scholar] [CrossRef] [PubMed]
- Imler, J.L. Adenovirus vectors as recombinant viral vaccines. Vaccine 1995, 13, 1143–1151. [Google Scholar] [CrossRef]
- Vorburger, S.A.; Hunt, K.K. Adenoviral gene therapy. Oncologist 2002, 7, 46–59. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.C.; Dayball, K.; Wan, Y.H.; Bramson, J. Detailed analysis of the CD8+ T-cell response following adenovirus vaccination. J. Virol. 2003, 77, 13407–13411. [Google Scholar] [CrossRef] [PubMed]
- Vanniasinkam, T.; Ertl, H.C. Adenoviral gene delivery for HIV-1 vaccination. Curr. Gene Ther. 2005, 5, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Bett, A.J.; Prevec, L.; Graham, F.L. Packaging capacity and stability of human adenovirus type 5 vectors. J. Virol. 1993, 67, 5911–5921. [Google Scholar] [PubMed]
- Ferreira, T.B.; Alves, P.M.; Aunins, J.G.; Carrondo, M.J. Use of adenoviral vectors as veterinary vaccines. Gene Ther. 2005, 12 (Suppl. 1), S73–S83. [Google Scholar] [CrossRef] [PubMed]
- Haj-Ahmad, Y.; Graham, F.L. Development of a helper-independent human adenovirus vector and its use in the transfer of the herpes simplex virus thymidine kinase gene. J. Virol. 1986, 57, 267–274. [Google Scholar] [PubMed]
- Russell, W.C. Update on adenovirus and its vectors. J. Gen. Virol. 2000, 81 Pt 11, 2573–2604. [Google Scholar] [CrossRef] [PubMed]
- Liniger, M.; Zuniga, A.; Naim, H.Y. Use of viral vectors for the development of vaccines. Expert Rev. Vaccines 2007, 6, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Schagen, F.H.; Ossevoort, M.; Toes, R.E.; Hoeben, R.C. Immune responses against adenoviral vectors and their transgene products: A review of strategies for evasion. Crit. Rev. Oncol. Hematol. 2004, 50, 51–70. [Google Scholar] [CrossRef]
- Nazerai, L.; Bassi, M.R.; Uddback, I.E.; Holst, P.J.; Christensen, J.P.; Thomsen, A.R. Early life vaccination: Generation of adult-quality memory CD8+ T cells in infant mice using non-replicating adenoviral vectors. Sci. Rep. 2016, 6, 38666. [Google Scholar] [CrossRef] [PubMed]
- Kochanek, S. High-capacity adenoviral vectors for gene transfer and somatic gene therapy. Hum. Gene Ther. 1999, 10, 2451–2459. [Google Scholar] [CrossRef] [PubMed]
- Weaver, E.A.; Nehete, P.N.; Buchl, S.S.; Senac, J.S.; Palmer, D.; Ng, P.; Sastry, K.J.; Barry, M.A. Comparison of replication-competent, first generation, and helper-dependent adenoviral vaccines. PLoS ONE 2009, 4, e5059. [Google Scholar] [CrossRef] [PubMed]
- Peng, B.; Wang, L.R.; Gomez-Roman, V.R.; Davis-Warren, A.; Montefiori, D.C.; Kalyanaraman, V.S.; Venzon, D.; Zhao, J.; Kan, E.; Rowell, T.J.; et al. Replicating rather than nonreplicating adenovirus-human immunodeficiency virus recombinant vaccines are better at eliciting potent cellular immunity and priming high-titer antibodies. J. Virol. 2005, 79, 10200–10209. [Google Scholar] [CrossRef] [PubMed]
- Kron, M.W.; Engler, T.; Schmidt, E.; Schirmbeck, R.; Kochanek, S.; Kreppel, F. High-capacity adenoviral vectors circumvent the limitations of ΔE1 and ΔE1/ΔE3 adenovirus vectors to induce multispecific transgene product-directed CD8 T-cell responses. J. Gene Med. 2011, 13, 648–657. [Google Scholar] [CrossRef] [PubMed]
- Demberg, T.; Florese, R.H.; Heath, M.J.; Larsen, K.; Kalisz, I.; Kalyanaraman, V.S.; Lee, E.M.; Pal, R.; Venzon, D.; Grant, R.; et al. A replication-competent adenovirus-human immunodeficiency virus (Ad-HIV) tat and Ad-HIV env priming/Tat and envelope protein boosting regimen elicits enhanced protective efficacy against simian/human immunodeficiency virus SHIV89.6P challenge in rhesus macaques. J. Virol. 2007, 81, 3414–3427. [Google Scholar] [PubMed]
- Wang, Y.; Xiang, Z.; Pasquini, S.; Ertl, H.C. The use of an E1-deleted, replication-defective adenovirus recombinant expressing the rabies virus glycoprotein for early vaccination of mice against rabies virus. J. Virol. 1997, 71, 3677–3683. [Google Scholar] [PubMed]
- Khurana, S.; Coyle, E.M.; Manischewitz, J.; King, L.R.; Ishioka, G.; Alexander, J.; Smith, J.; Gurwith, M.; Golding, H. Oral priming with replicating adenovirus serotype 4 followed by subunit H5N1 vaccine boost promotes antibody affinity maturation and expands H5N1 cross-clade neutralization. PLoS ONE 2015, 10, e0115476. [Google Scholar] [CrossRef] [PubMed]
- Pinschewer, D.D.; Perez, M.; Jeetendra, E.; Bachi, T.; Horvath, E.; Hengartner, H.; Whitt, M.A.; de la Torre, J.C.; Zinkernagel, R.M. Kinetics of protective antibodies are determined by the viral surface antigen. J. Clin. Invest. 2004, 114, 988–993. [Google Scholar] [CrossRef] [PubMed]
- Zygraich, N.; Lobmann, M.; Peetermans, J.; Vascoboinic, E.; Huygelen, C. Local and systemic response after simultaneous intranasal inoculation of temperature-sensitive mutants of parainfluenza 3, IBR and bovine adenovirus 3. Dev. Biol. Stand. 1975, 28, 482–488. [Google Scholar] [PubMed]
- Crosby, C.M.; Nehete, P.; Sastry, K.J.; Barry, M.A. Amplified and persistent immune responses generated by single-cycle replicating adenovirus vaccines. J. Virol. 2015, 89, 669–75. [Google Scholar] [CrossRef] [PubMed]
- Crosby, C.M.; Matchett, W.E.; Anguiano-Zarate, S.S.; Parks, C.A.; Weaver, E.A.; Pease, L.R.; Webby, R.J.; Barry, M.A. Replicating Single-Cycle Adenovirus Vectors Generate Amplified Influenza Vaccine Responses. J. Virol. 2017, 91. [Google Scholar] [CrossRef] [PubMed]
- Curiel, D.T. The development of conditionally replicative adenoviruses for cancer therapy. Clin. Cancer Res. 2000, 6, 3395–3399. [Google Scholar] [PubMed]
- Langlois, R.A.; Albrecht, R.A.; Kimble, B.; Sutton, T.; Shapiro, J.S.; Finch, C.; Angel, M.; Chua, M.A.; Gonzalez-Reiche, A.S.; Xu, K.; et al. MicroRNA-based strategy to mitigate the risk of gain-of-function influenza studies. Nat. Biotechnol. 2013, 31, 844–847. [Google Scholar] [CrossRef] [PubMed]
- Mizuguchi, Y.; Takizawa, T.; Uchida, E. Host cellular microRNA involvement in the control of hepatitis B virus gene expression and replication. World J. Hepatol. 2015, 7, 696–702. [Google Scholar] [CrossRef] [PubMed]
- Ylosmaki, E.; Hakkarainen, T.; Hemminki, A.; Visakorpi, T.; Andino, R.; Saksela, K. Generation of a conditionally replicating adenovirus based on targeted destruction of E1A mRNA by a cell type-specific MicroRNA. J. Virol. 2008, 82, 11009–11015. [Google Scholar] [CrossRef] [PubMed]
- Mei, Y.F.; Wu, H.; Hultenby, K.; Silver, J. Complete replication-competent adenovirus 11p vectors with E1 or E3 insertions show improved heat stability. Virology 2016, 497, 198–210. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.J.; Wang, L.; Dong, F.; Zhang, L.; Guo, W.; Teraishi, F.; Xu, K.; Ji, L.; Fang, B. Oncolysis and suppression of tumor growth by a GFP-expressing oncolytic adenovirus controlled by an hTERT and CMV hybrid promoter. Cancer Gene Ther. 2006, 13, 720–723. [Google Scholar] [CrossRef] [PubMed]
- Richardson, J.S.; Yao, M.K.; Tran, K.N.; Croyle, M.A.; Strong, J.E.; Feldmann, H.; Kobinger, G.P. Enhanced protection against Ebola virus mediated by an improved adenovirus-based vaccine. PLoS ONE 2009, 4, e5308. [Google Scholar] [CrossRef] [PubMed]
- Sridhar, S.; Reyes-Sandoval, A.; Draper, S.J.; Moore, A.C.; Gilbert, S.C.; Gao, G.P.; Wilson, J.M.; Hill, A.V. Single-dose protection against Plasmodium berghei by a simian adenovirus vector using a human cytomegalovirus promoter containing intron A. J. Virol. 2008, 82, 3822–3833. [Google Scholar] [CrossRef] [PubMed]
- Alharbi, N.K.; Spencer, A.J.; Salman, A.M.; Tully, C.M.; Chinnakannan, S.K.; Lambe, T.; Yamaguchi, Y.; Morris, S.J.; Orubu, T.; Draper, S.J.; et al. Enhancing cellular immunogenicity of MVA-vectored vaccines by utilizing the F11L endogenous promoter. Vaccine 2016, 34, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Spencer, A.J.; Cottingham, M.G.; Jenks, J.A.; Longley, R.J.; Capone, S.; Colloca, S.; Folgori, A.; Cortese, R.; Nicosia, A.; Bregu, M.; et al. Enhanced vaccine-induced CD8+ T cell responses to malaria antigen ME-TRAP by fusion to MHC class ii invariant chain. PLoS ONE 2014, 9, e100538. [Google Scholar] [CrossRef] [PubMed]
- Holst, P.J.; Sorensen, M.R.; Mandrup Jensen, C.M.; Orskov, C.; Thomsen, A.R.; Christensen, J.P. MHC class II-associated invariant chain linkage of antigen dramatically improves cell-mediated immunity induced by adenovirus vaccines. J. Immunol. 2008, 180, 3339–3346. [Google Scholar] [CrossRef] [PubMed]
- Ragonnaud, E.; Andersson, A.M.; Pedersen, A.E.; Laursen, H.; Holst, P.J. An adenoviral cancer vaccine co-encoding a tumor associated antigen together with secreted 4-1BBL leads to delayed tumor progression. Vaccine 2016, 34, 2147–2156. [Google Scholar] [CrossRef] [PubMed]
- Forbes, E.K.; de Cassan, S.C.; Llewellyn, D.; Biswas, S.; Goodman, A.L.; Cottingham, M.G.; Long, C.A.; Pleass, R.J.; Hill, A.V.; Hill, F.; et al. T cell responses induced by adenoviral vectored vaccines can be adjuvanted by fusion of antigen to the oligomerization domain of C4b-binding protein. PLoS ONE 2012, 7, e44943. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Leneghan, D.B.; Miura, K.; Nikolaeva, D.; Brian, I.J.; Dicks, M.D.; Fyfe, A.J.; Zakutansky, S.E.; de Cassan, S.; Long, C.A.; et al. Enhancing immunogenicity and transmission-blocking activity of malaria vaccines by fusing Pfs25 to IMX313 multimerization technology. Sci. Rep. 2016, 6, 18848. [Google Scholar] [CrossRef] [PubMed]
- Matthews, Q.L. Capsid-incorporation of antigens into adenovirus capsid proteins for a vaccine approach. Mol. Pharm. 2011, 8, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Krause, A.; Joh, J.H.; Hackett, N.R.; Roelvink, P.W.; Bruder, J.T.; Wickham, T.J.; Kovesdi, I.; Crystal, R.G.; Worgall, S. Epitopes expressed in different adenovirus capsid proteins induce different levels of epitope-specific immunity. J. Virol. 2006, 80, 5523–5530. [Google Scholar] [CrossRef] [PubMed]
- Meulenbroek, R.A.; Sargent, K.L.; Lunde, J.; Jasmin, B.J.; Parks, R.J. Use of adenovirus protein IX (pIX) to display large polypeptides on the virion—Generation of fluorescent virus through the incorporation of pIX-GFP. Mol. Ther. 2004, 9, 617–624. [Google Scholar] [CrossRef] [PubMed]
- Karen, K.A.; Deal, C.; Adams, R.J.; Nielsen, C.; Ward, C.; Espinosa, D.A.; Xie, J.; Zavala, F.; Ketner, G. A replicating adenovirus capsid display recombinant elicits antibodies against Plasmodium falciparum sporozoites in Aotus nancymaae monkeys. Infect. Immun. 2015, 83, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Andersson, A.C.; Resende, M.; Salanti, A.; Nielsen, M.A.; Holst, P.J. Novel adenovirus encoded virus-like particles displaying the placental malaria associated VAR2CSA antigen. Vaccine 2017, 35, 1140–1147. [Google Scholar] [CrossRef] [PubMed]



© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fougeroux, C.; Holst, P.J. Future Prospects for the Development of Cost-Effective Adenovirus Vaccines. Int. J. Mol. Sci. 2017, 18, 686. https://doi.org/10.3390/ijms18040686
Fougeroux C, Holst PJ. Future Prospects for the Development of Cost-Effective Adenovirus Vaccines. International Journal of Molecular Sciences. 2017; 18(4):686. https://doi.org/10.3390/ijms18040686
Chicago/Turabian StyleFougeroux, Cyrielle, and Peter J. Holst. 2017. "Future Prospects for the Development of Cost-Effective Adenovirus Vaccines" International Journal of Molecular Sciences 18, no. 4: 686. https://doi.org/10.3390/ijms18040686
APA StyleFougeroux, C., & Holst, P. J. (2017). Future Prospects for the Development of Cost-Effective Adenovirus Vaccines. International Journal of Molecular Sciences, 18(4), 686. https://doi.org/10.3390/ijms18040686