A Histopathological Scheme for the Quantitative Scoring of Intervertebral Disc Degeneration and the Therapeutic Utility of Adult Mesenchymal Stem Cells for Intervertebral Disc Regeneration
Abstract
:1. Introduction
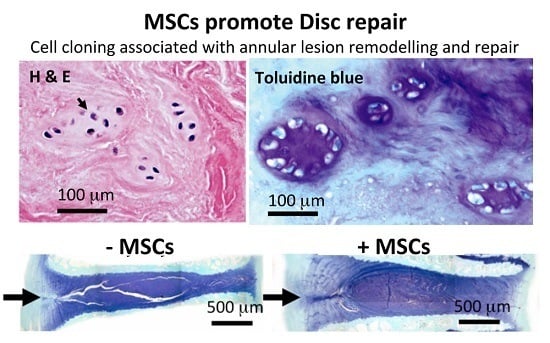
2. Results
3. Discussion
4. Materials and Methods
4.1. Ovine Models of DDD
4.2. Induction of Disc Degeneration and Administration of Mesenchymal Stem Cells
4.3. Isolation and Characterization of Mesenchymal Stem Cells
4.4. Histological Processing of IVDs
4.5. Histochemistry
4.5.1. Toluidine Blue Staining
4.5.2. Hematoxylin and Eosin Staining
4.6. Development of a Histopathological Scoring System for IVD Specimens
4.7. Statistics
5. Conclusions
Author Contributions
Conflicts of Interest
References
- Humzah, M.D.; Soames, R.W. Human intervertebral disc: Structure and function. Anat. Rec. 1988, 220, 337–356. [Google Scholar] [CrossRef] [PubMed]
- Iatridis, J.C.; MacLean, J.J.; Roughley, P.J.; Alini, M. Effects of mechanical loading on intervertebral disc metabolism in vivo. J. Bone Jt. Surg. Am. 2006, 88 (Suppl. S2), 41–46. [Google Scholar] [CrossRef]
- Roughley, P.J. Biology of intervertebral disc aging and degeneration: Involvement of the extracellular matrix. Spine 2004, 29, 2691–2699. [Google Scholar] [CrossRef] [PubMed]
- Little, J.P.; Pearcy, M.J.; Tevelen, G.; Evans, J.H.; Pettet, G.; Adam, C.J. The mechanical response of the ovine lumbar anulus fibrosus to uniaxial, biaxial and shear loads. J. Mech. Behav. Biomed. Mater. 2010, 3, 146–157. [Google Scholar] [CrossRef] [PubMed]
- Roughley, P.J.; Alini, M.; Antoniou, J. The role of proteoglycans in aging, degeneration and repair of the intervertebral disc. Biochem. Soc. Trans. 2002, 30, 869–874. [Google Scholar] [CrossRef] [PubMed]
- Melrose, J.; Whitelock, J.M. Chapter 467 Extracellular matrix. In Wiley Encyclopedia of Biomedical Engineering; Akay, M., Ed.; John Wiley and Son Inc.: Hoboken, NJ, USA, 1999. [Google Scholar]
- Iozzo, R.V.; Murdoch, A.D. Proteoglycans of the extracellular environment: Clues from the gene and protein side offer novel perspectives in molecular diversity and function. FASEB J. 1996, 10, 598–614. [Google Scholar] [PubMed]
- Roughley, P.J.; Melching, L.I.; Heathfield, T.F.; Pearce, R.H.; Mort, J.S. The structure and degradation of aggrecan in human intervertebral disc. Eur. Spine J. 2006, 15 (Suppl. S3), S326–S332. [Google Scholar] [CrossRef] [PubMed]
- Hardingham, T. Cartilage: Aggrecan-link protein-hyaluronan aggregates. In Glycoforum: Hyaluronan Today; Mizutani Foundation for Glycoscience: Tokyo, Japan, 1998. [Google Scholar]
- Eyre, D.R. Biochemistry of the intervertebral disc. Int. Rev. Connect. Tissue Res. 1979, 8, 227–291. [Google Scholar] [PubMed]
- Eyre, D.R.; Muir, H. Types I and II collagens in intervertebral disc. Interchanging radial distributions in annulus fibrosus. Biochem. J. 1976, 157, 267–270. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.; Melrose, J.; Caterson, B.; Roughley, P.; Eisenstein, S.M.; Roberts, S. A comparative evaluation of the small leucine-rich proteoglycans of pathological human intervertebral discs. Eur. Spine J. 2012, 21 (Suppl. S2), S154–S159. [Google Scholar] [CrossRef] [PubMed]
- Melrose, J.; Fuller, E.S.; Roughley, P.J.; Smith, M.M.; Kerr, B.; Hughes, C.E.; Caterson, B.; Little, C.B. Fragmentation of decorin, biglycan, lumican and keratocan is elevated in degenerate human meniscus, knee and hip articular cartilages compared with age-matched macroscopically normal and control tissues. Arthritis Res. Ther. 2008, 10, R79. [Google Scholar] [CrossRef] [PubMed]
- Hayes, A.J.; Shu, C.C.; Lord, M.S.; Little, C.B.; Whitelock, J.M.; Melrose, J. Pericellular colocalisation and interactive properties of type VI collagen and perlecan in the intervertebral disc. Eur. Cell Mater. 2016, 32, 40–57. [Google Scholar] [CrossRef] [PubMed]
- Melrose, J.; Roughley, P.J. Proteoglycans of the intervertebral disc. In The Intervertebral Disc Molecular and Structural Studies of the Disc in Health and Disease; Shapiro, I., Risbud, M.V., Eds.; Springer: Vienna, Austria, 2014; pp. 53–78. [Google Scholar]
- Stanton, H.; Melrose, J.; Little, C.B.; Fosang, A.J. Proteoglycan degradation by the ADAMTS family of proteinases. Biochim. Biophys. Acta 2011, 1812, 1616–1629. [Google Scholar] [CrossRef] [PubMed]
- Richardson, S.; Freemont, A.J.; Hoyland, J.A. Pathogenesis of Intervertebral disc degeneration. In The Intervertebral Disc Molecular and Structural Studies of the Disc in Health and Disease; Shapiro, I., Risbud, M.V., Eds.; Springer: Vienna, Austria, 2014; pp. 177–200. [Google Scholar]
- Richardson, S.M.; Doyle, P.; Minogue, B.M.; Gnanalingham, K.; Hoyland, J.A. Increased expression of matrix metalloproteinase-10, nerve growth factor and substance P in the painful degenerate intervertebral disc. Arthritis Res. Ther. 2009, 11, R126. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.J.; Yu, X.H.; Wang, C.; Yang, W.; He, W.S.; Zhang, S.J.; Yan, Y.G.; Zhang, J. MMPs and ADAMTSs in intervertebral disc degeneration. Clin. Chim. Acta 2015, 448, 238–246. [Google Scholar] [CrossRef] [PubMed]
- Blain, E.J. Mechanical regulation of matrix metalloproteinases. Front. Biosci. 2007, 12, 507–527. [Google Scholar] [CrossRef] [PubMed]
- Melrose, J.; Smith, S.M.; Little, C.B.; Moore, R.J.; Vernon-Roberts, B.; Fraser, R.D. Recent advances in annular pathobiology provide insights into rim-lesion mediated intervertebral disc degeneration and potential new approaches to annular repair strategies. Eur. Spine J. 2008, 17, 1131–1148. [Google Scholar] [CrossRef] [PubMed]
- Adams, M.A.; Roughley, P.J. What is intervertebral disc degeneration, and what causes it? Spine 2006, 31, 2151–2161. [Google Scholar] [CrossRef] [PubMed]
- Johnson, W.E.; Caterson, B.; Eisenstein, S.M.; Hynds, D.L.; Snow, D.M.; Roberts, S. Human intervertebral disc aggrecan inhibits nerve growth in vitro. Arthritis Rheum. 2002, 46, 2658–2664. [Google Scholar] [CrossRef] [PubMed]
- Johnson, W.E.; Caterson, B.; Eisenstein, S.M.; Roberts, S. Human intervertebral disc aggrecan inhibits endothelial cell adhesion and cell migration in vitro. Spine 2005, 30, 1139–1147. [Google Scholar] [CrossRef] [PubMed]
- Andersson, G. The Epidemiology of Spinal Disorders. In The Adult Spine: Principles and Practice; Lippincott-Raven: Philadelphia, PA, USA, 1997; pp. 93–141. [Google Scholar]
- Melrose, J. Strategies in regenerative medicine for intervertebral disc repair using mesenchymal stem cells and bioscaffolds. Regen. Med. 2016, 11, 705–724. [Google Scholar] [CrossRef] [PubMed]
- Maidhof, R.; Alipui, D.O.; Rafiuddin, A.; Levine, M.; Grande, D.A.; Chahine, N.O. Emerging trends in biological therapy for intervertebral disc degeneration. Discov. Med. 2012, 14, 401–411. [Google Scholar] [PubMed]
- Than, K.D.; Rahman, S.U.; Wang, L.; Khan, A.; Kyere, K.A.; Than, T.T.; Miyata, Y.; Park, Y.S.; La Marca, F.; Kim, H.M.; et al. Intradiscal injection of simvastatin results in radiologic, histologic, and genetic evidence of disc regeneration in a rat model of degenerative disc disease. Spine J. 2014, 14, 1017–1028. [Google Scholar] [CrossRef] [PubMed]
- Wuertz, K.; Quero, L.; Sekiguchi, M.; Klawitter, M.; Nerlich, A.; Konno, S.; Kikuchi, S.; Boos, N. The red wine polyphenol resveratrol shows promising potential for the treatment of nucleus pulposus-mediated pain in vitro and in vivo. Spine 2011, 36, E1373–E1384. [Google Scholar] [CrossRef] [PubMed]
- Anderson, D.G.; Tannoury, C. Molecular pathogenic factors in symptomatic disc degeneration. Spine J. 2005, 5, 260S–266S. [Google Scholar] [CrossRef] [PubMed]
- Fuller, E.S.; Shu, C.; Smith, M.M.; Little, C.B.; Melrose, J. Hyaluronan oligosaccharides stimulate MMP and anabolic gene expression in vitro by intervertebral disc cells and annular repair in vivo. J. Tissue Eng. Regen. Med. 2016. [Google Scholar] [CrossRef]
- Gawri, R.; Antoniou, J.; Ouellet, J.; Awwad, W.; Steffen, T.; Roughley, P.; Haglund, L.; Mwale, F. Best paper NASS 2013: Link-N can stimulate proteoglycan synthesis in the degenerated human intervertebral discs. Eur. Cell Mater. 2013, 26, 107–119. [Google Scholar] [CrossRef] [PubMed]
- Mwale, F.; Masuda, K.; Pichika, R.; Epure, L.M.; Yoshikawa, T.; Hemmad, A.; Roughley, P.J.; Antoniou, J. The efficacy of Link N as a mediator of repair in a rabbit model of intervertebral disc degeneration. Arthritis Res. Ther. 2011, 13, R120. [Google Scholar] [CrossRef] [PubMed]
- Petit, A.; Yao, G.; Rowas, S.A.; Gawri, R.; Epure, L.; Antoniou, J.; Mwale, F. Effect of synthetic link N peptide on the expression of type I and type II collagens in human intervertebral disc cells. Tissue Eng. Part A 2011, 17, 899–904. [Google Scholar] [CrossRef] [PubMed]
- Mwale, F.; Demers, C.N.; Petit, A.; Roughley, P.; Poole, A.R.; Steffen, T.; Aebi, M.; Antoniou, J. A synthetic peptide of link protein stimulates the biosynthesis of collagens II, IX and proteoglycan by cells of the intervertebral disc. J. Cell. Biochem. 2003, 88, 1202–1213. [Google Scholar] [CrossRef] [PubMed]
- Antoniou, J.; Wang, H.T.; Alaseem, A.M.; Haglund, L.; Roughley, P.J.; Mwale, F. The effect of Link N on differentiation of human bone marrow-derived mesenchymal stem cells. Arthritis Res. Ther. 2012, 14, R267. [Google Scholar] [CrossRef] [PubMed]
- Mwale, F.; Wang, H.T.; Roughley, P.; Antoniou, J.; Haglund, L. Link N and mesenchymal stem cells can induce regeneration of the early degenerate Intervertebral Disc. Tissue Eng. Part A 2014. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.J.; Lee, J.W.; Moon, E.J.; Chung, Y.G.; Kim, O.S.; Kim, H.J. Anabolic effects of Peniel 2000, a peptide that regulates TGF-β1 signaling on intervertebral disc degeneration. Spine 2013, 38, E49–E58. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.J. Resveratrol has anabolic effects on disc degeneration in a rabbit model. J. Korean Med. Sci. 2013, 28, 939–945. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Phillips, F.M.; An, H.S.; Ellman, M.; Thonar, E.J.; Wu, W.; Park, D.; Im, H.J. The action of resveratrol, a phytoestrogen found in grapes, on the intervertebral disc. Spine 2008, 33, 2586–2595. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Lin, C.Y. Simvastatin stimulates chondrogenic phenotype of intervertebral disc cells partially through BMP-2 pathway. Spine 2008, 33, E525–E531. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, L.; Park, J.B.; Park, P.; Yang, V.C.; Hollister, S.J.; La Marca, F.; Lin, C.Y. Intradiscal injection of simvastatin retards progression of intervertebral disc degeneration induced by stab injury. Arthritis Res. Ther. 2009, 11, R172. [Google Scholar] [CrossRef] [PubMed]
- Gologorsky, Y.; Chi, J. Statins for disc degeneration. Neurosurgery 2014, 74, N18–N19. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.H.; Yang, K.C.; Chen, Y.J.; Sun, Y.H.; Yang, S.H. Lovastatin prevents discography-associated degeneration and maintains the functional morphology of intervertebral discs. Spine J. 2014, 14, 2459–2466. [Google Scholar] [CrossRef] [PubMed]
- Karamouzian, S.; Eskandary, H.; Saeed, A.; Reihani-Kermani, H.; Aboosaeedi, H.R.; Malekpoor-Afshar, R.; Safizade, H.; Eskandari, M. Effect of atorvastatin on angiogenesis in degenerated intervertebral disc in rat. Spine 2011, 36, 1824–1828. [Google Scholar] [CrossRef] [PubMed]
- Wong, R.W.; Rabie, B.; Bendeus, M.; Hagg, U. The effects of Rhizoma Curculiginis and Rhizoma Drynariae extracts on bones. Chin. Med. 2007, 2, 13. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.A.; Subhan, N.; Rahman, M.M.; Uddin, S.J.; Reza, H.M.; Sarker, S.D. Effect of citrus flavonoids, naringin and naringenin, on metabolic syndrome and their mechanisms of action. Adv. Nutr. 2014, 5, 404–417. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Whitaker, C.; Xu, Z.; Heggeness, M.; Yang, S.Y. Therapeutic effects of naringin on degenerative human nucleus pulposus cells for discogenic low back pain. Spine J. 2016, 16, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Freeman, B.J.; Kuliwaba, J.S.; Jones, C.F.; Shu, C.C.; Colloca, C.J.; Zarrinkalam, M.R.; Mulaibrahimovic, A.; Gronthos, S.; Zannettino, A.C.; Howell, S. Allogeneic mesenchymal precursor cells promote healing in postero-lateral annular lesions and improve indices of lumbar intervertebral disc degeneration in an ovine model. Spine 2016, 41, 1331–1339. [Google Scholar] [CrossRef] [PubMed]
- Pettine, K.; Suzuki, R.; Sand, T.; Murphy, M. Treatment of discogenic back pain with autologous bone marrow concentrate injection with minimum two year follow-up. Int. Orthop. 2016, 40, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Sakai, D.; Andersson, G.B. Stem cell therapy for intervertebral disc regeneration: Obstacles and solutions. Nat. Rev. Rheumatol. 2015, 11, 243–256. [Google Scholar] [CrossRef] [PubMed]
- Zeckser, J.; Wolff, M.; Tucker, J.; Goodwin, J. Multipotent mesenchymal stem cell treatment for discogenic low back pain and disc degeneration. Stem Cells Int. 2016, 2016, 3908389. [Google Scholar] [CrossRef] [PubMed]
- Crevensten, G.; Walsh, A.J.; Ananthakrishnan, D.; Page, P.; Wahba, G.M.; Lotz, J.C.; Berven, S. Intervertebral disc cell therapy for regeneration: Mesenchymal stem cell implantation in rat intervertebral discs. Ann. Biomed. Eng. 2004, 32, 430–434. [Google Scholar] [CrossRef] [PubMed]
- Vadala, G.; Sowa, G.; Hubert, M.; Gilbertson, L.G.; Denaro, V.; Kang, J.D. Mesenchymal stem cells injection in degenerated intervertebral disc: Cell leakage may induce osteophyte formation. J. Tissue Eng. Regen. Med. 2012, 6, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Longo, U.G.; Papapietro, N.; Petrillo, S.; Franceschetti, E.; Maffulli, N.; Denaro, V. Mesenchymal stem cell for prevention and management of intervertebral disc degeneration. Stem Cells Int. 2012, 2012, 921053. [Google Scholar] [CrossRef] [PubMed]
- Oehme, D.; Goldschlager, T.; Ghosh, P.; Rosenfeld, J.V.; Jenkin, G. Cell-based therapies used to treat lumbar degenerative disc disease: A systematic review of animal studies and human clinical trials. Stem Cells Int. 2015, 2015, 946031. [Google Scholar] [CrossRef] [PubMed]
- Oehme, D.; Goldschlager, T.; Rosenfeld, J.V.; Ghosh, P.; Jenkin, G. The role of stem cell therapies in degenerative lumbar spine disease: A review. Neurosurg. Rev. 2015, 38, 429–445. [Google Scholar] [CrossRef] [PubMed]
- Richardson, S.M.; Hoyland, J.A.; Mobasheri, R.; Csaki, C.; Shakibaei, M.; Mobasheri, A. Mesenchymal stem cells in regenerative medicine: Opportunities and challenges for articular cartilage and intervertebral disc tissue engineering. J. Cell. Physiol. 2010, 222, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Krock, E.; Rosenzweig, D.H.; Haglund, L. The inflammatory milieu of the degenerate disc: Is mesenchymal stem cell-based therapy for intervertebral disc repair a feasible approach? Curr. Stem Cell Res. Ther. 2015, 10, 317–328. [Google Scholar] [CrossRef] [PubMed]
- Melrose, J. The promise of mesenchymal stem cells for intervertebral disc repair. J. Stem Cell Res. Ther. 2017, 2, 61–64. [Google Scholar]
- Shim, E.K.; Lee, J.S.; Kim, D.E.; Kim, S.K.; Jung, B.J.; Choi, E.Y.; Kim, C.S. Autogenous mesenchymal stem cells from the vertebral body enhance intervertebral disc regeneration by paracrine interaction: An in vitro pilot study. Cell Transplant. 2016. [Google Scholar] [CrossRef] [PubMed]
- An, H.S.; Thonar, E.J.; Masuda, K. Biological repair of intervertebral disc. Spine 2003, 28, S86–S92. [Google Scholar] [CrossRef] [PubMed]
- Masuda, K. Biological repair of the degenerated intervertebral disc by the injection of growth factors. Eur. Spine J. 2008, 17 (Suppl. S4), 441–451. [Google Scholar] [CrossRef] [PubMed]
- Meisel, H.J.; Siodla, V.; Ganey, T.; Minkus, Y.; Hutton, W.C.; Alasevic, O.J. Clinical experience in cell-based therapeutics: Disc chondrocyte transplantation A treatment for degenerated or damaged intervertebral disc. Biomol. Eng. 2007, 24, 5–21. [Google Scholar] [CrossRef] [PubMed]
- Orozco, L.; Soler, R.; Morera, C.; Alberca, M.; Sanchez, A.; Garcia-Sancho, J. Intervertebral disc repair by autologous mesenchymal bone marrow cells: A pilot study. Transplantation 2011, 92, 822–828. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, T.; Ueda, Y.; Miyazaki, K.; Koizumi, M.; Takakura, Y. Disc regeneration therapy using marrow mesenchymal cell transplantation: A report of two case studies. Spine 2010, 35, E475–E480. [Google Scholar] [CrossRef] [PubMed]
- Bae, H.W.; Amirdelfan, K.; Coric, D.; McJunkin, T.L.; Pettine, K.A.; Hong, H.J.; DePalma, M.J.; Kim, K.D.; Beckworth, W.J.; et al. A phase II study demonstrating efficacy and safety of mesenchymal precursor cells in low back pain due to disc degeneration. Spine J. 2014, 14, S31–S32. [Google Scholar] [CrossRef]
- Alini, M.; Eisenstein, S.M.; Ito, K.; Little, C.; Kettler, A.A.; Masuda, K.; Melrose, J.; Ralphs, J.; Stokes, I.; Wilke, H.J. Are animal models useful for studying human disc disorders/degeneration? Eur. Spine J. 2008, 17, 2–19. [Google Scholar] [CrossRef] [PubMed]
- Daly, C.; Ghosh, P.; Jenkin, G.; Oehme, D.; Goldschlager, T. A Review of Animal Models of Intervertebral Disc Degeneration: Pathophysiology, Regeneration, and Translation to the Clinic. BioMed Res. Int. 2016, 2016, 5952165. [Google Scholar] [CrossRef] [PubMed]
- Melrose, J.; Shu, C.; Young, C.; Ho, R.; Smith, M.M.; Young, A.A.; Smith, S.S.; Gooden, B.; Dart, A.; Podadera, J.; et al. Mechanical destabilization induced by controlled annular incision of the intervertebral disc dysregulates metalloproteinase expression and induces disc degeneration. Spine 2012, 37, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Osti, O.L.; Vernon-Roberts, B.; Fraser, R.D. 1990 Volvo Award in experimental studies. Anulus tears and intervertebral disc degeneration. An experimental study using an animal model. Spine 1990, 15, 762–767. [Google Scholar] [PubMed]
- Melrose, J.; Ghosh, P.; Taylor, T.K.; Hall, A.; Osti, O.L.; Vernon-Roberts, B.; Fraser, R.D. A longitudinal study of the matrix changes induced in the intervertebral disc by surgical damage to the annulus fibrosus. J. Orthop. Res. 1992, 10, 665–676. [Google Scholar] [CrossRef] [PubMed]
- Detiger, S.E.; Hoogendoorn, R.J.; van der Veen, A.J.; van Royen, B.J.; Helder, M.N.; Koenderink, G.H.; Smit, T.H. Biomechanical and rheological characterization of mild intervertebral disc degeneration in a large animal model. J. Orthop. Res. 2013, 31, 703–709. [Google Scholar] [CrossRef] [PubMed]
- Hoogendoorn, R.J.; Wuisman, P.I.; Smit, T.H.; Everts, V.E.; Helder, M.N. Experimental intervertebral disc degeneration induced by chondroitinase ABC in the goat. Spine 2007, 32, 1816–1825. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Drapeau, S.; An, H.S.; Markova, D.; Lenart, B.A.; Anderson, D.G. Histological features of the degenerating intervertebral disc in a goat disc-injury model. Spine 2011, 36, 1519–1527. [Google Scholar] [CrossRef] [PubMed]
- Bergknut, N.; Meij, B.P.; Hagman, R.; de Nies, K.S.; Rutges, J.P.; Smolders, L.A.; Creemers, L.B.; Lagerstedt, A.S.; Hazewinkel, H.A.; Grinwis, G.C. Intervertebral disc disease in dogs—Part 1: A new histological grading scheme for classification of intervertebral disc degeneration in dogs. Vet. J. 2013, 195, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Bergknut, N.; Rutges, J.P.; Kranenburg, H.J.; Smolders, L.A.; Hagman, R.; Smidt, H.J.; Lagerstedt, A.S.; Penning, L.C.; Voorhout, G.; Hazewinkel, H.A.; et al. The dog as an animal model for intervertebral disc degeneration? Spine 2012, 37, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Seiler, G.; Hani, H.; Scheidegger, J.; Busato, A.; Lang, J. Staging of lumbar intervertebral disc degeneration in nonchondrodystrophic dogs using low-field magnetic resonance imaging. Vet. Radiol. Ultrasound 2003, 44, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Omlor, G.W.; Nerlich, A.G.; Wilke, H.J.; Pfeiffer, M.; Lorenz, H.; Schaaf-Keim, M.; Bertram, H.; Richter, W.; Carstens, C.; Guehring, T. A new porcine in vivo animal model of disc degeneration: Response of anulus fibrosus cells, chondrocyte-like nucleus pulposus cells, and notochordal nucleus pulposus cells to partial nucleotomy. Spine 2009, 34, 2730–2739. [Google Scholar] [CrossRef] [PubMed]
- Chiang, C.J.; Cheng, C.K.; Sun, J.S.; Liao, C.J.; Wang, Y.H.; Tsuang, Y.H. The effect of a new anular repair after discectomy in intervertebral disc degeneration: An experimental study using a porcine spine model. Spine 2011, 36, 761–769. [Google Scholar] [CrossRef] [PubMed]
- Kaigle, A.M.; Holm, S.H.; Hansson, T.H. 1997 Volvo Award winner in biomechanical studies. Kinematic behavior of the porcine lumbar spine: A chronic lesion model. Spine 1997, 22, 2796–2806. [Google Scholar] [CrossRef] [PubMed]
- Moore, R.J.; Osti, O.L.; Vernon-Roberts, B.; Fraser, R.D. Changes in endplate vascularity after an outer anulus tear in the sheep. Spine 1992, 17, 874–878. [Google Scholar] [CrossRef] [PubMed]
- Moore, R.J.; Crotti, T.N.; Osti, O.L.; Fraser, R.D.; Vernon-Roberts, B. Osteoarthrosis of the facet joints resulting from anular rim lesions in sheep lumbar discs. Spine 1999, 24, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Moore, R.J.; Vernon-Roberts, B.; Osti, O.L.; Fraser, R.D. Remodeling of vertebral bone after outer anular injury in sheep. Spine 1996, 21, 936–940. [Google Scholar] [CrossRef] [PubMed]
- Melrose, J.; Roberts, S.; Smith, S.; Menage, J.; Ghosh, P. Increased nerve and blood vessel ingrowth associated with proteoglycan depletion in an ovine anular lesion model of experimental disc degeneration. Spine 2002, 27, 1278–1285. [Google Scholar] [CrossRef] [PubMed]
- Melrose, J.; Smith, S.; Little, C.B.; Kitson, J.; Hwa, S.Y.; Ghosh, P. Spatial and temporal localization of transforming growth factor-beta, fibroblast growth factor-2, and osteonectin, and identification of cells expressing alpha-smooth muscle actin in the injured anulus fibrosus: Implications for extracellular matrix repair. Spine 2002, 27, 1756–1764. [Google Scholar] [CrossRef] [PubMed]
- Osti, O.L.; Vernon-Roberts, B.; Moore, R.; Fraser, R.D. Annular tears and disc degeneration in the lumbar spine. A post-mortem study of 135 discs. J. Bone Jt. Surg. Br. 1992, 74, 678–682. [Google Scholar]
- De Visser, H.; Rowe, C.; Pearcy, M. A robotic testing facility for the measurement of the mechanics of spinal joints. Proc. Inst. Mech. Eng. H 2007, 221, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Thompson, R.E.; Pearcy, M.J.; Downing, K.J.; Manthey, B.A.; Parkinson, I.H.; Fazzalari, N.L. Disc lesions and the mechanics of the intervertebral joint complex. Spine 2000, 25, 3026–3035. [Google Scholar] [CrossRef] [PubMed]
- Hilton, R.C.; Ball, J. Vertebral rim lesions in the dorsolumbar spine. Ann. Rheum. Dis. 1984, 43, 302–307. [Google Scholar] [CrossRef] [PubMed]
- Melrose, J. A global pictorial assessment of the intervertebral disc cell in several species reveals a remarkable biodiversity in this cell type which should be taken into account in experimental studies on intervertebral disc repair. Spine Res. 2016, 2, 1. [Google Scholar]
- Fagan, A.; Moore, R.; Vernon Roberts, B.; Blumbergs, P.; Fraser, R. ISSLS prize winner: The innervation of the intervertebral disc: A quantitative analysis. Spine 2003, 28, 2570–2576. [Google Scholar] [CrossRef] [PubMed]
- Vos, T.; Flaxman, A.D.; Naghavi, M.; Lozano, R.; Michaud, C.; Ezzati, M.; Shibuya, K.; Salomon, J.A.; Abdalla, S.; Aboyans, V.; et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2163–2196. [Google Scholar] [CrossRef]
- Maniadakis, N.; Gray, A. The economic burden of back pain in the UK. Pain 2000, 84, 95–103. [Google Scholar] [CrossRef]
- Walker, B.F.; Muller, R.; Grant, W.D. Low back pain in Australian adults: The economic burden. Asia Pac. J. Public Health 2003, 15, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Briggs, A.M.; Buchbinder, R. Back pain: A national health priority area in australia? Med. J. Aust. 2009, 190, 499–502. [Google Scholar] [PubMed]
- Ehrlich, G.E.; Khaltaev, N.G.; Chronic Respiratory Diseases and Arthritis Team. Low Back Pain Initiative; World Health Organization: Geneva, Switzerland, 1999; p. 152. [Google Scholar]
- Walker, B.F.; Muller, R.; Grant, W.D. Low back pain in Australian adults: Prevalence and associated disability. J. Manip. Physiol. Ther. 2004, 27, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Hoy, D.; March, L.; Brooks, P.; Blyth, F.; Woolf, A.; Bain, C.; Williams, G.; Smith, E.; Vos, T.; Barendregt, J.; et al. The global burden of low back pain: Estimates from the Global Burden of Disease 2010 study. Ann. Rheum. Dis. 2014, 73, 968–974. [Google Scholar] [CrossRef] [PubMed]
- Hoy, D.; March, L.; Brooks, P.; Woolf, A.; Blyth, F.; Vos, T.; Buchbinder, R. Measuring the global burden of low back pain. Best Pract. Res. Clin. Rheumatol. 2010, 24, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Hoy, D.G.; Smith, E.; Cross, M.; Sanchez-Riera, L.; Buchbinder, R.; Blyth, F.M.; Brooks, P.; Woolf, A.D.; Osborne, R.H.; Fransen, M.; et al. The global burden of musculoskeletal conditions for 2010: An overview of methods. Ann. Rheum. Dis. 2014, 73, 982–989. [Google Scholar] [CrossRef] [PubMed]
- Ho, G.; Leung, V.Y.; Cheung, K.M.; Chan, D. Effect of severity of intervertebral disc injury on mesenchymal stem cell-based regeneration. Connect. Tissue Res. 2008, 49, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.H.; Jin, E.S.; Min, J.K.; Jeon, S.R.; Park, C.S.; Kim, H.S.; Choi, K.H. Human mesenchymal stem cells implantation into the degenerated coccygeal disc of the rat. Cytotechnology 2009, 59, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Leung, V.Y.; Aladin, D.M.; Lv, F.; Tam, V.; Sun, Y.; Lau, R.Y.; Hung, S.C.; Ngan, A.H.; Tang, B.; Lim, C.T.; et al. Mesenchymal stem cells reduce intervertebral disc fibrosis and facilitate repair. Stem Cells 2014, 32, 2164–2177. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Leung, V.Y.; Luk, K.D.; Chan, D.; Cheung, K.M. Mesenchymal stem cells arrest intervertebral disc degeneration through chondrocytic differentiation and stimulation of endogenous cells. Mol. Ther. 2009, 17, 1959–1966. [Google Scholar] [CrossRef] [PubMed]
- Cheung, K.M.; Karppinen, J.; Chan, D.; Ho, D.W.; Song, Y.Q.; Sham, P.; Cheah, K.S.; Leong, J.C.; Luk, K.D. Prevalence and pattern of lumbar magnetic resonance imaging changes in a population study of one thousand forty-three individuals. Spine 2009, 34, 934–940. [Google Scholar] [CrossRef] [PubMed]
- Livshits, G.; Popham, M.; Malkin, I.; Sambrook, P.N.; Macgregor, A.J.; Spector, T.; Williams, F.M. Lumbar disc degeneration and genetic factors are the main risk factors for low back pain in women: The UK Twin Spine Study. Ann. Rheum. Dis. 2011, 70, 1740–1745. [Google Scholar] [CrossRef] [PubMed]
- Bogduk, N. The lumbar disc and low back pain. Neurosurg. Clin. N. Am. 1991, 2, 791–806. [Google Scholar] [PubMed]
- Freemont, A.J. The cellular pathobiology of the degenerate intervertebral disc and discogenic back pain. Rheumatology 2009, 48, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Freemont, A.J.; Peacock, T.E.; Goupille, P.; Hoyland, J.A.; O’Brien, J.; Jayson, M.I. Nerve ingrowth into diseased intervertebral disc in chronic back pain. Lancet 1997, 350, 178–181. [Google Scholar] [CrossRef]
- Ahn, S.H.; Cho, Y.W.; Ahn, M.W.; Jang, S.H.; Sohn, Y.K.; Kim, H.S. mRNA expression of cytokines and chemokines in herniated lumbar intervertebral discs. Spine 2002, 27, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Friberg, S.; Hirsch, C. Anatomical and clinical studies on lumbar disc degeneration. Clin. Orthop. Relat. Res. 1992, 19, 222–242. [Google Scholar] [CrossRef]
- Battie, M.C.; Videman, T.; Levalahti, E.; Gill, K.; Kaprio, J. Heritability of low back pain and the role of disc degeneration. Pain 2007, 131, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Leung, V.Y.; Chan, D.; Cheung, K.M. Regeneration of intervertebral disc by mesenchymal stem cells: Potentials, limitations, and future direction. Eur. Spine J. 2006, 15 (Suppl. S3), S406–S413. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chee, A.; Thonar, E.J.; An, H.S. Intervertebral disk repair by protein, gene, or cell injection: A framework for rehabilitation-focused biologics in the spine. PM R 2011, 3, S88–S94. [Google Scholar] [CrossRef] [PubMed]
- Guterl, C.C.; See, E.Y.; Blanquer, S.B.; Pandit, A.; Ferguson, S.J.; Benneker, L.M.; Grijpma, D.W.; Sakai, D.; Eglin, D.; Alini, M.; et al. Challenges and strategies in the repair of ruptured annulus fibrosus. Eur. Cell Mater. 2013, 25, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Hegewald, A.A.; Zouhair, S.; Endres, M.; Cabraja, M.; Woiciechowsky, C.; Thome, C.; Kaps, C. Towards biological anulus repair: TGF-β3, FGF-2 and human serum support matrix formation by human anulus fibrosus cells. Tissue Cell 2013, 45, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Vernengo, J.; Fussell, G.W.; Smith, N.G.; Lowman, A.M. Synthesis and characterization of injectable bioadhesive hydrogels for nucleus pulposus replacement and repair of the damaged intervertebral disc. J. Biomed. Mater. Res. B Appl. Biomater. 2010, 93, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Bergknut, N.; Grinwis, G.; Pickee, E.; Auriemma, E.; Lagerstedt, A.S.; Hagman, R.; Hazewinkel, H.A.; Meij, B.P. Reliability of macroscopic grading of intervertebral disk degeneration in dogs by use of the Thompson system and comparison with low-field magnetic resonance imaging findings. Am. J. Vet. Res. 2011, 72, 899–904. [Google Scholar] [CrossRef] [PubMed]
- Hodges, P.W.; Galea, M.P.; Holm, S.; Holm, A.K. Corticomotor excitability of back muscles is affected by intervertebral disc lesion in pigs. Eur. J. Neurosci. 2009, 29, 1490–1500. [Google Scholar] [CrossRef] [PubMed]
- Colloca, C.J.; Gunzburg, R.; Freeman, B.J.; Szpalski, M.; Afifi, M.; Moore, R.J. Biomechancial quantification of pathologic manipulable spinal lesions: An in vivo ovine model of spondylolysis and intervertebral disc degeneration. J. Manip. Physiol. Ther. 2012, 35, 354–366. [Google Scholar] [CrossRef] [PubMed]
- Colloca, C.J.; Keller, T.S.; Harrison, D.E.; Moore, R.J.; Gunzburg, R.; Harrison, D.D. Spinal manipulation force and duration affect vertebral movement and neuromuscular responses. Clin. Biomech. 2006, 21, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Colloca, C.J.; Keller, T.S.; Moore, R.J.; Gunzburg, R.; Harrison, D.E. Intervertebral disc degeneration reduces vertebral motion responses. Spine 2007, 32, E544–E550. [Google Scholar] [CrossRef] [PubMed]
- Colloca, C.J.; Keller, T.S.; Moore, R.J.; Gunzburg, R.; Harrison, D.E. Effects of disc degeneration on neurophysiological responses during dorsoventral mechanical excitation of the ovine lumbar spine. J. Electromyogr. Kinesiol. 2008, 18, 829–837. [Google Scholar] [CrossRef] [PubMed]
- Keller, T.S.; Colloca, C.J.; Moore, R.J.; Gunzburg, R.; Harrison, D.E.; Harrison, D.D. Three-dimensional vertebral motions produced by mechanical force spinal manipulation. J. Manip. Physiol. Ther. 2006, 29, 425–436. [Google Scholar] [CrossRef] [PubMed]
- Hodges, P.; James, G.; Blomster, L.; Hall, L.; Schmid, A.; Shu, C.; Little, C.B.; Melrose, J. Multifidus muscle changes after back injury are characterized by structural remodeling of muscle, adipose and connective tissue, but not muscle atrophy: Molecular and morphological evidence. Spine 2015, 40, 1057–1071. [Google Scholar] [CrossRef] [PubMed]
- Hodges, P.; Holm, A.K.; Hansson, T.; Holm, S. Rapid atrophy of the lumbar multifidus follows experimental disc or nerve root injury. Spine 2006, 31, 2926–2933. [Google Scholar] [CrossRef] [PubMed]
- Hodges, P.W.; James, G.; Blomster, L.; Hall, L.; Schmid, A.B.; Shu, C.; Little, C.; Melrose, J. Can proinflammatory cytokine gene expression explain multifidus muscle fiber changes after an intervertebral disc lesion? Spine 2014, 39, 1010–1017. [Google Scholar] [CrossRef] [PubMed]
- Freeman, M.D.; Woodham, M.A.; Woodham, A.W. The role of the lumbar multifidus in chronic low back pain: A review. PM R 2010, 2, 142–146. [Google Scholar] [CrossRef] [PubMed]
- Holm, S.; Maroudas, A.; Urban, J.P.; Selstam, G.; Nachemson, A. Nutrition of the intervertebral disc: Solute transport and metabolism. Connect. Tissue Res. 1981, 8, 101–119. [Google Scholar] [CrossRef] [PubMed]
- Bibby, S.R.; Urban, J.P. Effect of nutrient deprivation on the viability of intervertebral disc cells. Eur. Spine J. 2004, 13, 695–701. [Google Scholar] [CrossRef] [PubMed]
- Holm, S.; Selstam, G. Oxygen tension alterations in the intervertebral disc as a response to changes in the arterial blood. Ups. J. Med. Sci. 1982, 87, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Horner, H.A.; Urban, J.P. 2001 Volvo Award Winner in Basic Science Studies: Effect of nutrient supply on the viability of cells from the nucleus pulposus of the intervertebral disc. Spine 2001, 26, 2543–2549. [Google Scholar] [CrossRef] [PubMed]
- Urban, J.P.; Smith, S.; Fairbank, J.C. Nutrition of the intervertebral disc. Spine 2004, 29, 2700–2709. [Google Scholar] [CrossRef] [PubMed]
- Diamant, B.; Karlsson, J.; Nachemson, A. Correlation between lactate levels and pH in discs of patients with lumbar rhizopathies. Experientia 1968, 24, 1195–1196. [Google Scholar] [CrossRef] [PubMed]
- Razaq, S.; Wilkins, R.J.; Urban, J.P. The effect of extracellular pH on matrix turnover by cells of the bovine nucleus pulposus. Eur. Spine J. 2003, 12, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Ellman, M.B.; An, H.S.; Muddasani, P.; Im, H.J. Biological impact of the fibroblast growth factor family on articular cartilage and intervertebral disc homeostasis. Gene 2008, 420, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Kurakawa, T.; Kakutani, K.; Morita, Y.; Kato, Y.; Yurube, T.; Hirata, H.; Miyazaki, S.; Terashima, Y.; Maeno, K.; Takada, T.; et al. Functional impact of integrin α5β1 on the homeostasis of intervertebral discs: A study of mechanotransduction pathways using a novel dynamic loading organ culture system. Spine J. 2015, 15, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Pratsinis, H.; Kletsas, D. Growth factors in intervertebral disc homeostasis. Connect. Tissue Res. 2008, 49, 273–276. [Google Scholar] [CrossRef] [PubMed]
- Nachemson, A. Intradiscal measurements of pH in patients with lumbar rhizopathies. Acta Orthop. Scand. 1969, 40, 23–42. [Google Scholar] [CrossRef] [PubMed]
- Naqvi, S.M.; Buckley, C.T. Extracellular matrix production by nucleus pulposus and bone marrow stem cells in response to altered oxygen and glucose microenvironments. J. Anat. 2015, 227, 757–766. [Google Scholar] [CrossRef] [PubMed]
- Naqvi, S.M.; Buckley, C.T. Bone marrow stem cells in response to intervertebral disc-like matrix acidity and oxygen concentration: Implications for cell-based regenerative therapy. Spine 2016, 41, 743–750. [Google Scholar] [CrossRef] [PubMed]
- Wuertz, K.; Godburn, K.; Iatridis, J.C. MSC response to pH levels found in degenerating intervertebral discs. Biochem. Biophys. Res. Commun. 2009, 379, 824–829. [Google Scholar] [CrossRef] [PubMed]
- Wuertz, K.; Godburn, K.; Neidlinger-Wilke, C.; Urban, J.; Iatridis, J.C. Behavior of mesenchymal stem cells in the chemical microenvironment of the intervertebral disc. Spine 2008, 33, 1843–1849. [Google Scholar] [CrossRef] [PubMed]
- Schleich, C.; Muller-Lutz, A.; Eichner, M.; Schmitt, B.; Matuschke, F.; Bittersohl, B.; Zilkens, C.; Wittsack, H.J.; Antoch, G.; Miese, F. Glycosaminoglycan chemical exchange saturation transfer of lumbar intervertebral discs in healthy volunteers. Spine 2016, 41, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Schleich, C.; Muller-Lutz, A.; Matuschke, F.; Sewerin, P.; Sengewein, R.; Schmitt, B.; Ostendorf, B.; Wittsack, H.J.; Stanke, K.; Antoch, G.; et al. Glycosaminoglycan chemical exchange saturation transfer of lumbar intervertebral discs in patients with spondyloarthritis. J. Magn. Reson. Imaging 2015, 42, 1057–1063. [Google Scholar] [CrossRef] [PubMed]
- Melrose, J.; Ghosh, P.; Taylor, T.K.; Latham, J.; Moore, R. Topographical variation in the catabolism of aggrecan in an ovine annular lesion model of experimental disc degeneration. J. Spinal Disord. 1997, 10, 55–67. [Google Scholar] [CrossRef] [PubMed]
- Melrose, J.; Ghosh, P.; Taylor, T.K.; Vernon-Roberts, B.; Latham, J.; Moore, R. Elevated synthesis of biglycan and decorin in an ovine annular lesion model of experimental disc degeneration. Eur. Spine J. 1997, 6, 376–384. [Google Scholar] [CrossRef] [PubMed]
- Melrose, J.; Smith, S.M.; Fuller, E.S.; Young, A.A.; Roughley, P.J.; Dart, A.; Little, C.B. Biglycan and fibromodulin fragmentation correlates with temporal and spatial annular remodelling in experimentally injured ovine intervertebral discs. Eur. Spine J. 2007, 16, 2193–2205. [Google Scholar] [CrossRef] [PubMed]
- Schollum, M.L.; Appleyard, R.C.; Little, C.B.; Melrose, J. A detailed microscopic examination of alterations in normal anular structure induced by mechanical destabilization in an ovine model of disc degeneration. Spine 2010, 35, 1965–1973. [Google Scholar] [CrossRef] [PubMed]
- Kettler, A.; Wilke, H.J. Review of existing grading systems for cervical or lumbar disc and facet joint degeneration. Eur. Spine J. 2006, 15, 705–718. [Google Scholar] [CrossRef] [PubMed]
- Gunzburg, R.; Parkinson, R.; Moore, R.; Cantraine, F.; Hutton, W.; Vernon-Roberts, B.; Fraser, R. A cadaveric study comparing discography, magnetic resonance imaging, histology, and mechanical behavior of the human lumbar disc. Spine 1992, 17, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Tertti, M.; Paajanen, H.; Laato, M.; Aho, H.; Komu, M.; Kormano, M. Disc degeneration in magnetic resonance imaging. A comparative biochemical, histologic, and radiologic study in cadaver spines. Spine 1991, 16, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Gibson, M.J.; Buckley, J.; Mawhinney, R.; Mulholland, R.C.; Worthington, B.S. Magnetic resonance imaging and discography in the diagnosis of disc degeneration. A comparative study of 50 discs. J. Bone Jt. Surg. Br. 1986, 68, 369–373. [Google Scholar]
- Schneiderman, G.; Flannigan, B.; Kingston, S.; Thomas, J.; Dillin, W.H.; Watkins, R.G. Magnetic resonance imaging in the diagnosis of disc degeneration: Correlation with discography. Spine 1987, 12, 276–281. [Google Scholar] [CrossRef] [PubMed]
- Melhem, E.R.; Caruthers, S.D.; Jara, H. Cervical spine: Three-dimensional MR imaging with magnetization transfer prepulsed turbo field echo techniques. Radiology 1998, 207, 815–821. [Google Scholar] [CrossRef] [PubMed]
- Rumboldt, Z.; Marotti, M. Magnetization transfer, HASTE, and FLAIR imaging. Magn. Reson. Imaging Clin. N. Am. 2003, 11, 471–492. [Google Scholar] [CrossRef]
- Taso, M.; Girard, O.M.; Duhamel, G.; Le Troter, A.; Feiweier, T.; Guye, M.; Ranjeva, J.P.; Callot, V. Tract-specific and age-related variations of the spinal cord microstructure: A multi-parametric MRI study using diffusion tensor imaging (DTI) and inhomogeneous magnetization transfer (ihMT). NMR Biomed. 2016, 29, 817–832. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, H.; Onaya, H.; Itai, Y.; Nishimura, H.; Matsumura, A.; Tsunoda, T.; Kandatsu, S.; Koga, M.; Yoshikawa, K.; Kato, H.; et al. Comparison between magnetization transfer contrast and fast spin-echo MR imaging of degenerative disease of the cervical spine at 0.3 T. Magn. Reson. Imaging 1997, 15, 37–45. [Google Scholar] [CrossRef]
- Haneder, S.; Ong, M.M.; Budjan, J.M.; Schmidt, R.; Konstandin, S.; Morelli, J.N.; Schad, L.R.; Schoenberg, S.O.; Kerl, U.H. 23Na-magnetic resonance imaging of the human lumbar vertebral discs: In vivo measurements at 3.0 T in healthy volunteers and patients with low back pain. Spine J. 2014, 14, 1343–1350. [Google Scholar] [CrossRef] [PubMed]
- Insko, E.K.; Clayton, D.B.; Elliott, M.A. In vivo sodium MR imaging of the intervertebral disk at 4 T. Acad. Radiol. 2002, 9, 800–804. [Google Scholar] [CrossRef]
- Malzacher, M.; Kalayciyan, R.; Konstandin, S.; Haneder, S.; Schad, L.R. Sodium-23 MRI of whole spine at 3 Tesla using a 5-channel receive-only phased-array and a whole-body transmit resonator. Z. Med. Phys. 2016, 26, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Noebauer-Huhmann, I.M.; Juras, V.; Pfirrmann, C.W.; Szomolanyi, P.; Zbyn, S.; Messner, A.; Wimmer, J.; Weber, M.; Friedrich, K.M.; Stelzeneder, D.; et al. Sodium MR imaging of the lumbar intervertebral disk at 7 T: Correlation with T2 mapping and modified Pfirrmann score at 3 T–preliminary results. Radiology 2012, 265, 555–564. [Google Scholar] [CrossRef] [PubMed]
- Solanky, B.S.; Riemer, F.; Golay, X.; Wheeler-Kingshott, C.A. Sodium quantification in the spinal cord at 3 T. Magn. Reson. Med. 2013, 69, 1201–1208. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; McArdle, E.; Fenty, M.; Witschey, W.; Elliott, M.; Sochor, M.; Reddy, R.; Borthakur, A. Validation of sodium magnetic resonance imaging of intervertebral disc. Spine 2010, 35, 505–510. [Google Scholar] [CrossRef] [PubMed]
- Antoniou, J.; Epure, L.M.; Michalek, A.J.; Grant, M.P.; Iatridis, J.C.; Mwale, F. Analysis of quantitative magnetic resonance imaging and biomechanical parameters on human discs with different grades of degeneration. J. Magn. Reson. Imaging 2013, 38, 1402–1414. [Google Scholar] [CrossRef] [PubMed]
- Antoniou, J.; Pike, G.B.; Steffen, T.; Baramki, H.; Poole, A.R.; Aebi, M.; Alini, M. Quantitative magnetic resonance imaging in the assessment of degenerative disc disease. Magn. Reson. Med. 1998, 40, 900–907. [Google Scholar] [CrossRef] [PubMed]
- Kellgren, J.H.; Lawrence, J.S. Radiological assessment of osteo-arthrosis. Ann. Rheum. Dis. 1957, 16, 494–502. [Google Scholar] [CrossRef] [PubMed]
- Kirkaldy-Willis, W.H.; Wedge, J.H.; Yong-Hing, K.; Reilly, J. Pathology and pathogenesis of lumbar spondylosis and stenosis. Spine 1978, 3, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Adams, M.A.; Dolan, P.; Hutton, W.C. The stages of disc degeneration as revealed by discograms. J. Bone Jt. Surg. Br. 1986, 68, 36–41. [Google Scholar]
- Pathria, M.; Sartoris, D.J.; Resnick, D. Osteoarthritis of the facet joints: Accuracy of oblique radiographic assessment. Radiology 1987, 164, 227–230. [Google Scholar] [CrossRef] [PubMed]
- Modic, M.T.; Masaryk, T.J.; Ross, J.S.; Carter, J.R. Imaging of degenerative disk disease. Radiology 1988, 168, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Modic, M.T.; Steinberg, P.M.; Ross, J.S.; Masaryk, T.J.; Carter, J.R. Degenerative disk disease: Assessment of changes in vertebral body marrow with MR imaging. Radiology 1988, 166, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Mok, F.P.; Samartzis, D.; Karppinen, J.; Fong, D.Y.; Luk, K.D.; Cheung, K.M. Modic changes of the lumbar spine: Prevalence, risk factors, and association with disc degeneration and low back pain in a large-scale population-based cohort. Spine J. 2016, 16, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.P.; Pearce, R.H.; Schechter, M.T.; Adams, M.E.; Tsang, I.K.; Bishop, P.B. Preliminary evaluation of a scheme for grading the gross morphology of the human intervertebral disc. Spine 1990, 15, 411–415. [Google Scholar] [CrossRef] [PubMed]
- Weiner, D.K.; Distell, B.; Studenski, S.; Martinez, S.; Lomasney, L.; Bongiorni, D. Does radiographic osteoarthritis correlate with flexibility of the lumbar spine? J. Am. Geriatr. Soc. 1994, 42, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Pfirrmann, C.W.; Metzdorf, A.; Zanetti, M.; Hodler, J.; Boos, N. Magnetic resonance classification of lumbar intervertebral disc degeneration. Spine 2001, 26, 1873–1878. [Google Scholar] [CrossRef] [PubMed]
- Carragee, E.J.; Han, M.Y.; Suen, P.W.; Kim, D. Clinical outcomes after lumbar discectomy for sciatica: The effects of fragment type and anular competence. J. Bone Jt. Surg. Am. 2003, 85-A, 102–108. [Google Scholar] [CrossRef]
- Thalgott, J.S.; Albert, T.J.; Vaccaro, A.R.; Aprill, C.N.; Giuffre, J.M.; Drake, J.S.; Henke, J.P. A new classification system for degenerative disc disease of the lumbar spine based on magnetic resonance imaging, provocative discography, plain radiographs and anatomic considerations. Spine J. 2004, 4, 167S–172S. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Videman, T.; Battie, M.C. Lumbar vertebral endplate lesions: Prevalence, classification, and association with age. Spine 2012, 37, 1432–1439. [Google Scholar] [CrossRef] [PubMed]
- Rutges, J.P.; Duit, R.A.; Kummer, J.A.; Bekkers, J.E.; Oner, F.C.; Castelein, R.M.; Dhert, W.J.; Creemers, L.B. A validated new histological classification for intervertebral disc degeneration. Osteoarthr. Cartil. 2013, 21, 2039–2047. [Google Scholar] [CrossRef] [PubMed]
- Cunha, C.; Almeida, C.R.; Almeida, M.I.; Silva, A.M.; Molinos, M.; Lamas, S.; Pereira, C.L.; Teixeira, G.Q.; Monteiro, A.T.; Santos, S.G.; et al. Systemic delivery of bone marrow mesenchymal stem cells for in situ intervertebral disc regeneration. Stem Cells Transl. Med. 2017, 6, 1029–1039. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Li, D.P.; Zhang, Z.J.; Shu, H.M.; Hu, L.; Li, Z.N.; Huang, Y.H. Effect of basic fibroblast growth factor and transforming growth factor-β1 combined with bone marrow mesenchymal stem cells on the repair of degenerated intervertebral discs in rat models. Zhongguo Yi Xue Ke Xue Yuan Xue Bao 2015, 37, 456–465. [Google Scholar] [PubMed]
- Li, X.; Zhang, Y.; Song, B.; En, H.; Gao, S.; Zhang, S.; Cai, Y.; Li, Z.J.; Li, C.; Wang, W.; et al. Experimental application of bone marrow mesenchymal stem cells for the repair of intervertebral disc annulus fibrosus. Med. Sci. Monit. 2016, 22, 4426–4430. [Google Scholar] [CrossRef] [PubMed]
- Noriega, D.C.; Ardura, F.; Hernandez-Ramajo, R.; Martin-Ferrero, M.A.; Sanchez-Lite, I.; Toribio, B.; Alberca, M.; Garcia, V.; Moraleda, J.M.; Sanchez, A.; et al. Intervertebral disc repair by allogeneic mesenchymal bone marrow cells: A randomized controlled trial. Transplantation 2016. [Google Scholar] [CrossRef] [PubMed]
- Pereira, C.L.; Teixeira, G.Q.; Ribeiro-Machado, C.; Caldeira, J.; Costa, M.; Figueiredo, F.; Fernandes, R.; Aguiar, P.; Grad, S.; Barbosa, M.A.; et al. Mesenchymal stem/stromal cells seeded on cartilaginous endplates promote intervertebral disc regeneration through extracellular matrix remodeling. Sci. Rep. 2016, 6, 33836. [Google Scholar] [CrossRef] [PubMed]
- Thorpe, A.A.; Dougill, G.; Vickers, L.; Reeves, N.D.; Sammon, C.; Cooper, G.; Le Maitre, C.L. Thermally triggered hydrogel injection into bovine intervertebral disc tissue explants induces differentiation of mesenchymal stem cells and restores mechanical function. Acta Biomater. 2017. [Google Scholar] [CrossRef] [PubMed]
- Acosta, F.L., Jr.; Lotz, J.; Ames, C.P. The potential role of mesenchymal stem cell therapy for intervertebral disc degeneration: A critical overview. Neurosurg. Focus 2005, 19, E4. [Google Scholar] [CrossRef] [PubMed]
- Clarke, L.E.; Richardson, S.M.; Hoyland, J.A. Harnessing the potential of mesenchymal stem cells for IVD regeneration. Curr. Stem Cell Res. Ther. 2015, 10, 296–306. [Google Scholar] [CrossRef] [PubMed]
- Gou, S.; Oxentenko, S.C.; Eldrige, J.S.; Xiao, L.; Pingree, M.J.; Wang, Z.; Perez-Terzic, C.; Qu, W. Stem cell therapy for intervertebral disk regeneration. Am. J. Phys. Med. Rehabil. 2014, 93, S122–S131. [Google Scholar] [CrossRef] [PubMed]
- Hiyama, A.; Mochida, J.; Sakai, D. Stem cell applications in intervertebral disc repair. Cell. Mol. Biol. 2008, 54, 24–32. [Google Scholar] [PubMed]
- Bostelmann, R.; Bostelmann, T.; Nasaca, A.; Steiger, H.J.; Zaucke, F.; Schleich, C. Biochemical validity of imaging techniques (X-ray, MRI, and dGEMRIC) in degenerative disc disease of the human cervical spine-an in vivo study. Spine J. 2017, 17, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Fazzalari, N.L.; Costi, J.J.; Hearn, T.C.; Fraser, R.D.; Vernon-Roberts, B.; Hutchinson, J.; Manthey, B.A.; Parkinson, I.H.; Sinclair, C. Mechanical and pathologic consequences of induced concentric anular tears in an ovine model. Spine 2001, 26, 2575–2581. [Google Scholar] [CrossRef] [PubMed]
- Melrose, J.; Shu, C.C.; Dart, A.; Martin, J.; Bell, R.; Little, C.B. Prevention and treatment of intervertebral disc degeneration with bone marrpw derived stem (stromal) cells-an in vivo study in sheep. Glob. Spine J. 2014, 4, s-0034. [Google Scholar]
- Shu, C.; Dart, A.; Clarke, J.; Martin, J.; Little, C.B.; Melrose, J. Prevention and treatment of intervertebral disc degeneration with bone marrow derived stem (stromal) cells-an in vivo study in sheep. Osteoarthr. Cartil. 2014, 22, S28–S29. [Google Scholar] [CrossRef]
- Castaneda, S.; Roman-Blas, J.A.; Largo, R.; Herrero-Beaumont, G. Osteoarthritis: A progressive disease with changing phenotypes. Rheumatology 2014, 53, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Driban, J.B.; Sitler, M.R.; Barbe, M.F.; Balasubramanian, E. Is osteoarthritis a heterogeneous disease that can be stratified into subsets? Clin. Rheumatol. 2010, 29, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Knoop, J.; van der Leeden, M.; Thorstensson, C.A.; Roorda, L.D.; Lems, W.F.; Knol, D.L.; Steultjens, M.P.; Dekker, J. Identification of phenotypes with different clinical outcomes in knee osteoarthritis: Data from the Osteoarthritis Initiative. Arthritis Care Res. 2011, 63, 1535–1542. [Google Scholar] [CrossRef] [PubMed]
- Kraus, V.B.; Blanco, F.J.; Englund, M.; Karsdal, M.A.; Lohmander, L.S. Call for standardized definitions of osteoarthritis and risk stratification for clinical trials and clinical use. Osteoarthr. Cartil. 2015, 23, 1233–1241. [Google Scholar] [CrossRef] [PubMed]
- Loughlin, J. Osteoarthritis: All types of trouble–defining OA in the genomic era. Nat. Rev. Rheumatol. 2011, 7, 200–201. [Google Scholar] [CrossRef] [PubMed]
- Riddle, D.L.; Dumenci, L. Modeling longitudinal osteoarthritis data to identify homogeneous subgroups: Opportunities and challenges in a burgeoning literature. Osteoarthr. Cartil. 2015, 23, 1035–1037. [Google Scholar] [CrossRef] [PubMed]
- Talic-Tanovic, A.; Hadziahmetovic, Z.; Madjar-Simic, I.; Papovic, A. Comparison of clinical and radiological parameters at knee osteoarthritis. Med. Arch. 2017, 71, 48–51. [Google Scholar] [CrossRef] [PubMed]
- Waarsing, J.H.; Bierma-Zeinstra, S.M.; Weinans, H. Distinct subtypes of knee osteoarthritis: Data from the Osteoarthritis Initiative. Rheumatology 2015, 54, 1650–1658. [Google Scholar] [CrossRef] [PubMed]
- Shu, C.; Smith, S.M.; Little, C.B.; Melrose, J. Use of FGF-2 and FGF-18 to direct bone marrow stromal stem cells to chondrogenic and osteogenic lineages. Future Sci. OA 2016, 2, FSO142. [Google Scholar] [CrossRef] [PubMed]
- Melrose, J.; Smith, S.M.; Smith, M.M.; Little, C.B. The use of Histochoice for histological examination of articular and growth plate cartilages, intervertebral disc and meniscus. Biotech. Histochem. 2008, 83, 47–53. [Google Scholar] [CrossRef] [PubMed]













| Grade | Histopathological Features |
|---|---|
| A. Toluidine Blue/Fast Green Proteoglycan Staining | |
| 0 | Fast green staining only of OAF, metachromatic Toluidine blue staining of IAF, intense metachromatic staining in NP, well defined CEP staining. Alternate AF lamellae discernable due to differing metachromatic staining intensities of adjacent lamellae |
| 1 | Moderately reduced metachromatic staining of MAF/IAF in vicinity of lesion, fast green staining of OAF only, normal metachromatic staining of NP and CEP |
| 2 | Reduced patchy metachromatic staining around lesion, fast green staining in OAF (no metachromasia) |
| 3 | Reduced metachromatic staining in NP compared to sham or NOC IVD, very faint or no metachromatic staining in OAF/MAF, fast green staining only in OAF |
| B. IVD Structure/Lesion Characteristics | |
| 0 | Normal IVD structure with well defined annular lamellae, central NP and CEP |
| 1 | Lesion evident in MAF, normal NP morphology |
| 2 | Lesion evident in MAF/IAF, lesion but may not be apparent in OAF due to spontaneous repair, IAF lamellae may be inverted and have anomalous distortions in normal lamellar architecture |
| 3 | Bifurcation/propagation of lesion from MAF/IAF into NP margins, mild delamination, when more extensive may lead to concentric tears between lamellae in MAF/IAF |
| 4 | Propagation of lesion into NP, with disruption in normal NP structure, distortion of annular lamellae into atypical arrangements-severe delamination, separation of translamellar cross bridges |
| 5 | Lesion reaching through NP into contralateral posterior AF with disruption in normal NP structure |
| C. Cellular morphology | |
| 0 | Normal, sparse distribution of typical single AF and NP fibrochondrocytes |
| 1 | Small groups of rounded chondrocytic cells (two to four cells/group) in vicinity of annular lesion in IAF, occasional cell division in resident inner AF and NP cells |
| 2 | Moderate increase in well defined groups of rounded dividing cells (four to eight per group) in vicinity of lesion and with penetrating blood vessels associated with the lesion site, well defined chondroid cell colonies in NP contained within a dense basophilic matrix with little fibrillar material evident around the cells contrasting with NP cells |
| 3 | Numerous cell clones around IAF/MAF lesion, chondroid cell nests in NP containing >50 cells |
| D. Blood Vessel Ingrowth | |
| 0 | Very occasional vessels in outermost annular lamellae, occasional capillaries in CEP |
| 1 | Occasional blood vessels in OAF and MAF |
| 2 | Moderate number of blood vessels in IAF |
| 3 | Extensive ingrowth of vessels in IAF/MAF and along lesion margins |
| E. Influx of cells into the lesion site | |
| 0 | Normal cell distribution in OAF, MAF, IAF and NP |
| 1 | Slight influx of cells mainly in outer AF |
| 2 | Moderate influx of cells throughout AF |
| 3 | Large influx of cells throughout AF |
| 4 | Heavy influx of cells throughout AF particularly in inner AF and around lesion |
| F. Histolopathological features not covered elsewhere | |
| 2 | Chondroid metaplasia in AF |
| 2 | Cystic degeneration affecting ≥5% NP |
| 3 | Extensive cystic degeneration affecting ≥20% NP |
| 4 | “Bare” fibrillar elements in NP due to loss of ground substance, confirmed by a paucity of toluidine blue metachromasia affecting ≥20% of NP and also evident as a reduced disc height |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shu, C.C.; Smith, M.M.; Smith, S.M.; Dart, A.J.; Little, C.B.; Melrose, J. A Histopathological Scheme for the Quantitative Scoring of Intervertebral Disc Degeneration and the Therapeutic Utility of Adult Mesenchymal Stem Cells for Intervertebral Disc Regeneration. Int. J. Mol. Sci. 2017, 18, 1049. https://doi.org/10.3390/ijms18051049
Shu CC, Smith MM, Smith SM, Dart AJ, Little CB, Melrose J. A Histopathological Scheme for the Quantitative Scoring of Intervertebral Disc Degeneration and the Therapeutic Utility of Adult Mesenchymal Stem Cells for Intervertebral Disc Regeneration. International Journal of Molecular Sciences. 2017; 18(5):1049. https://doi.org/10.3390/ijms18051049
Chicago/Turabian StyleShu, Cindy C., Margaret M. Smith, Susan M. Smith, Andrew J. Dart, Christopher B. Little, and James Melrose. 2017. "A Histopathological Scheme for the Quantitative Scoring of Intervertebral Disc Degeneration and the Therapeutic Utility of Adult Mesenchymal Stem Cells for Intervertebral Disc Regeneration" International Journal of Molecular Sciences 18, no. 5: 1049. https://doi.org/10.3390/ijms18051049
APA StyleShu, C. C., Smith, M. M., Smith, S. M., Dart, A. J., Little, C. B., & Melrose, J. (2017). A Histopathological Scheme for the Quantitative Scoring of Intervertebral Disc Degeneration and the Therapeutic Utility of Adult Mesenchymal Stem Cells for Intervertebral Disc Regeneration. International Journal of Molecular Sciences, 18(5), 1049. https://doi.org/10.3390/ijms18051049