Hydroxytyrosol and Cytoprotection: A Projection for Clinical Interventions
Abstract
:1. Introduction
2. Material and Methods
3. General Characteristics of Hydroxytyrosol
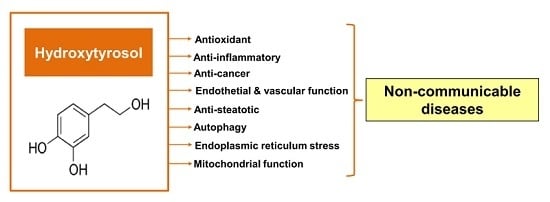
4. Protective Properties of Hydroxytyrosol
4.1. Antioxidant Effect
4.2. Anti-Inflammatory Effect
4.3. Anti-Cancerogenic Effect
4.4. Effects on Endothelial and Vascular Function
4.5. Anti-Steatotic Effect
4.6. Effects on Endoplasmic Reticulum (ER) Stress and Autophagy
4.7. Effects on Mitochondrial Function
4.8. Other Effects
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Global Health Observatory 2011. Available online: http://www.who.int/gho/ncd/en/index.html (accessed on 9 March 2017).
- Camps, J.; García-Heredia, A.; Hernández-Aguilera, A.; Joven, J. Paraoxonases, mitochondrial dysfunction and non-communicable diseases. Chem. Biol. Interact. 2016, 259, 382–387. [Google Scholar] [CrossRef] [PubMed]
- Adams, R. Prevention is better than cure: Tackling non-communicable diseases in developing countries by focusing on prevention. Perspect. Public Health 2015, 135, 121–122. [Google Scholar] [CrossRef] [PubMed]
- Granados-Principal, S.; Quiles, J.L.; Ramirez-Tortosa, C.L.; Sanchez-Rovira, P.; Ramirez-Tortosa, M.C. Hydroxytyrosol: From laboratory investigations to future clinical trials. Nutr. Rev. 2010, 68, 191–206. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.; Zhang, B.; Yao, J.; Duan, D.; Fang, J. Dual protection of hydroxytyrosol, an olive oil polyphenol, against oxidative damage in PC12 cells. Food Funct. 2015, 6, 2091–2100. [Google Scholar] [CrossRef] [PubMed]
- Gordon, M.H.; Paiva-Martins, F.; Almeida, M. Antioxidant activity of hydroxytyrosol acetate compared with that of other olive oil polyphenols. J. Agric. Food Chem. 2001, 49, 2480–2485. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela, R.; Echeverría, F.; Ortiz, M.; Rincón-Cervera, M.Á.; Espinosa, A.; Hernández-Rodas, M.C.; Illesca, P.; Valenzuela, A.; Videla, L.A. Hydroxytyrosol prevents reduction in liver activity of Δ-5 and Δ-6 desaturases, oxidative stress, and depletion in long chain polyunsaturated fatty acid content in different tissues of high-fat diet fed mice. Lipids Health Dis. 2017, 16, 64. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; He, X.W.; Jiang, J.G.; Xu, X.L. Hydroxytyrosol and its potential therapeutic effects. J. Agric. Food Chem. 2014, 62, 1449–1455. [Google Scholar] [CrossRef] [PubMed]
- Richard, N.; Arnold, S.; Kilpert, C.; Wertz, K. Hydroxytyrosol is the major anti-inflammatory compound in aqueous olive extracts and impairs cytokine and chemokine production in macrophages.pdf. Planta Med. 2011, 77, 1890–1897. [Google Scholar] [CrossRef] [PubMed]
- Bernini, R.; Merendino, N.; Romani, A.; Velotti, F. 7: Naturally Occurring Hydroxytyrosol: Synthesis and Anticancer Potential. Curr. Med. Chem. 2013, 20, 655–670. [Google Scholar] [CrossRef] [PubMed]
- Catalán, Ú.; López de las Hazas, M.C.; Rubió, L.; Fernández-Castillejo, S.; Pedret, A.; de la Torre, R.; Motilva, M.J.; Solà, R. Protective effect of hydroxytyrosol and its predominant plasmatic human metabolites against endothelial dysfunction in human aortic endothelial cells. Mol. Nutr. Food Res. 2015, 59, 2523–2536. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela, R.; Illesca, P.; Echeverría, F.; Espinosa, A.; Rincón, M.Á.; Ortiz, M.; Hernández-Rodas, M.C.; Valenzuela, A.; Videla, L. Molecular adaptations underlying the beneficial effects of hydroxytyrosol in the pathogenic alterations induced by a high-fat diet in mouse liver: PPAR-α and Nrf2 activation, and NF-κB down-regulation. Food Funct. 2017, 8, 1526–1537. [Google Scholar] [CrossRef] [PubMed]
- Giordano, E.; Davalos, A.; Nicod, N.; Visioli, F. Hydroxytyrosol attenuates tunicamycin-induced endoplasmic reticulum stress in human hepatocarcinoma cells. Mol. Nutr. Food Res. 2014, 58, 954–962. [Google Scholar] [CrossRef] [PubMed]
- Cetrullo, S.; D’Adamo, S.; Guidotti, S.; Borzì, R.M.; Flamigni, F. Hydroxytyrosol prevents chondrocyte death under oxidative stress by inducing autophagy through sirtuin 1-dependent and -independent mechanisms. Biochim. Biophys. Acta 2016, 1860, 1181–1191. [Google Scholar] [CrossRef] [PubMed]
- Zheng, A.; Li, H.; Xu, J.; Cao, K.; Li, H.; Pu, W.; Yang, Z.; Peng, Y.; Long, J.; Liu, J.; et al. Hydroxytyrosol improves mitochondrial function and reduces oxidative stress in the brain of db/db mice: Role of AMP-activated protein kinase activation. Br. J. Nutr. 2015, 113, 1667–1676. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Dietetic Products and Nutrition and Allergies (NDA). Scientific Opinion on the substantiation of health claims related to polyphenols in olive and protection of LDL particles from oxidative damage (ID 1333, 1638, 1639, 1696, 2865), maintenance of normal blood HDL-cholesterol concentrations (ID 1639), maintenance of normal blood pressure (ID 3781), “anti-inflammatory properties” (ID 1882), “contributes to the upper respiratory tract health” (ID 3468), “can help to maintain a normal function of gastrointestinal tract” (3779), and “contributes to body defences against external agents” (ID 3467) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA J. 2011, 9, 2033. [Google Scholar]
- Cheng, Z.; Schmelz, E.M.; Liu, D.; Hulver, M.W. Targeting mitochondrial alterations to prevent type 2 diabetes-evidence from studies of dietary redox-active compounds. Mol. Nutr. Food Res. 2014, 58, 1739–1749. [Google Scholar] [CrossRef] [PubMed]
- Achmon, Y.; Fishman, A. The antioxidant hydroxytyrosol: Biotechnological production challenges and opportunities. Appl. Microbiol. Biotechnol. 2015, 99, 1119–1130. [Google Scholar] [CrossRef] [PubMed]
- Cao, K.; Xu, J.; Zou, X.; Li, Y.; Chen, C.; Zheng, A.; Li, H.; Szeto, I.M.Y.; Shi, Y.; Long, J.; et al. Hydroxytyrosol prevents diet-induced metabolic syndrome and attenuates mitochondrial abnormalities in obese mice. Free Radic. Biol. Med. 2014, 67, 396–407. [Google Scholar] [CrossRef] [PubMed]
- Waterman, E.; Lockwood, B. Active components and clinical applications of olive oil. Altern. Med. Rev. 2007, 12, 331–342. [Google Scholar] [PubMed]
- De La Cruz, J.P.; Ruiz-Moreno, M.I.; Guerrero, A.; López-Villodres, J.A.; Reyes, J.J.; Espartero, J.L.; Labajos, M.T.; González-Correa, J.A. Role of the catechol group in the antioxidant and neuroprotective effects of virgin olive oil components in rat brain. J. Nutr. Biochem. 2015, 26, 549–555. [Google Scholar] [CrossRef] [PubMed]
- Visioli, F.; Poli, A.; Gall, C. Antioxidant and other biological activities of phenols from olives and olive oil. Med. Res. Rev. 2002, 22, 65–75. [Google Scholar] [CrossRef] [PubMed]
- De Bock, M.; Derraik, J.G.B.; Brennan, C.M.; Biggs, J.B.; Morgan, P.E.; Hodgkinson, S.C.; Hofman, P.L.; Cutfield, W.S. Olive (Olea europaea L.) leaf polyphenols improve insulin sensitivity in middle-aged overweight men: A randomized, placebo-controlled, crossover trial. PLoS ONE 2013, 8, e57622. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Morató, J.; Boronat, A.; Kotronoulas, A.; Pujadas, M.; Pastor, A.; Olesti, E.; Pérez-Mañá, C.; Khymenets, O.; Fitó, M.; Farré, M.; et al. Metabolic disposition and biological significance of simple phenols of dietary origin: Hydroxytyrosol and tyrosol. Drug Metab. Rev. 2016, 48, 218–236. [Google Scholar] [CrossRef] [PubMed]
- Tripoli, E.; Giammanco, M.; Tabacchi, G.; Di Majo, D.; Giammanco, S.; La Guardia, M. The phenolic compounds of olive oil: Structure, biological activity and beneficial effects on human health. Nutr. Res. Rev. 2005, 18, 98–112. [Google Scholar] [CrossRef] [PubMed]
- Piñeiro, Z.; Cantos-Villar, E.; Palma, M.; Puertas, B. Direct liquid chromatography method for the simultaneous quantification of hydroxytyrosol and tyrosol in red wines. J. Agric. Food Chem. 2011, 59, 11683–11689. [Google Scholar] [CrossRef] [PubMed]
- De La Torre, R.; Covas, M.I.; Pujadas, M.A.; Fitó, M.; Farré, M. Is dopamine behind the health benefits of red wine? Eur. J. Nutr. 2006, 45, 307–310. [Google Scholar] [CrossRef] [PubMed]
- Mosele, J.I.; Martín-Peláes, S.; Maciá, A.; Farrás, M.; Valls, R.M.; Catalán, U.; Motilva, M.J. Faecal microbial metabolism of olive oil phenolic compounds: In Vitro and in vivo approacges. Mol. Nutr. Food Res. 2014, 58, 1809–1819. [Google Scholar] [CrossRef] [PubMed]
- Martín-Peláes, S.; Mosele, J.I.; Pizarro, N.; Farrás, M.; de la Torre, R.; Subirana, I.; Pérez-Cano, F.J.; Castañer, O.; Solá, R.; Fernández-Castillejo, S.; et al. Effect of virgin olive oil and thyme phenolic compounds on blood lipid profile: Implications of human gut microbiota. Eur. J. Nutr. 2017, 56, 119–131. [Google Scholar] [CrossRef] [PubMed]
- López de las Hazas, M.C.; Rubiió, L.; Kotronoulas, A.; de la Torre, R.; Solá, R.; Motilva, M.J. Dose effect on the uptake and accumulation of hydroxytyrosol and its metabolites in target tissues in rats. Mol. Nutr. Food Res. 2015, 59, 1395–1399. [Google Scholar] [CrossRef] [PubMed]
- Auñon-Calles, D.; Canut, L.; Visioli, F. Toxicological evaluation of pure hydroxytyrosol. Food Chem. Toxicol. 2013, 55, 498–504. [Google Scholar] [CrossRef] [PubMed]
- Auñon-Calles, D.; Giordano, E.; Bohnenberger, S.; Visioli, F. Hydroxytyrosol is not genotoxic in vitro. Pharmacol. Res. 2013, 74, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Miró-Casas, E.; Farré Albaladejo, M.; Covas, M.I.; Rodriguez, J.O.; Menoyo Colomer, E.; Lamuela Raventós, R.M.; de la Torre, R. Capillary gas chromatography-mass spectrometry quantitative determination of hydroxytyrosol and tyrosol in human urine after olive oil intake. Anal. Biochem. 2001, 294, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Miró-Casas, E.; Covas, M.I.; Fitó, M.; Farré-Albadalejo, M.; Marrugat, J.; de la Torre, R. Tyrosol and hydroxytyrosol are absorbed from moderate and sustained doses of virgin olive oil in humans. Eur. J. Clin. Nutr. 2003, 57, 186–190. [Google Scholar] [CrossRef] [PubMed]
- Bulotta, S.; Celano, M.; Lepore, S.M.; Montalcini, T.; Pujia, A.; Russo, D. Beneficial effects of the olive oil phenolic components oleuropein and hydroxytyrosol: Focus on protection against cardiovascular and metabolic diseases. J. Transl. Med. 2014, 12, 219. [Google Scholar] [CrossRef] [PubMed]
- Bulotta, S.; Oliverio, M.; Procopio, A. Biological activity of oleuropein and its derivatives. In Handbook of Natural Products; Ramawat, K., Merillon, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; Volume 156, pp. 1–33. [Google Scholar]
- Giordano, E.; Dávalos, A.; Visioli, F. Chronic hydroxytyrosol feeding modulates glutathione-mediated oxido-reduction pathways in adipose tissue: A nutrigenomic study. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 1144–1150. [Google Scholar] [CrossRef] [PubMed]
- Granados-Principal, S.; El-Azem, N.; Pamplona, R.; Ramirez-Tortosa, C.; Pulido-Moran, M.; Vera-Ramirez, L.; Quiles, J.L.; Sanchez-Rovira, P.; Naudí, A.; Portero-Otin, M.; et al. Hydroxytyrosol ameliorates oxidative stress and mitochondrial dysfunction in doxorubicin-induced cardiotoxicity in rats with breast cancer. Biochem. Pharmacol. 2014, 90, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Dinkova-Kostova, A.T.; Holtzclaw, W.D.; Cole, R.N.; Itoh, K.; Wakabayashi, N.; Katoh, Y.; Yamamoto, M.; Talalay, P. Direct evidence that sulfhydryl groups of Keap1 are the sensors regulating induction of phase 2 enzymes that protect against carcinogens and oxidants. Proc. Natl. Acad. Sci. USA 2002, 99, 11908–11913. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Liu, Z.; Feng, Z.; Hao, J.; Shen, W.; Li, X.; Sun, L.; Sharman, E.; Wang, Y.; Wertz, K.; et al. Hydroxytyrosol protects against oxidative damage by simultaneous activation of mitochondrial biogenesis and phase II detoxifying enzyme systems in retinal pigment epithelial cells. J. Nutr. Biochem. 2010, 21, 1089–1098. [Google Scholar] [CrossRef] [PubMed]
- Zrelli, H.; Matsuoka, M.; Kitazaki, S.; Zarrouk, M.; Miyazaki, H. Hydroxytyrosol reduces intracellular reactive oxygen species levels in vascular endothelial cells by upregulating catalase expression through the AMPK-FOXO3a pathway. Eur. J. Pharmacol. 2011, 660, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Pirozzi, C.; Lama, A.; Simeoli, R.; Paciello, O.; Pagano, T.B.; Mollica, M.P.; Di Guida, F.; Russo, R.; Magliocca, S.; Canani, R.B.; et al. Hydroxytyrosol prevents metabolic impairment reducing hepatic inflammation and restoring duodenal integrity in a rat model of NAFLD. J. Nutr. Biochem. 2016, 30, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Gloire, G.; Legrand-Poels, S.; Piette, J. NF-κB activation by reactive oxygen species: Fifteen years later. Biochem. Pharmacol. 2006, 72, 1493–1505. [Google Scholar] [CrossRef] [PubMed]
- Killeen, M.J.; Linder, M.; Pontoniere, P.; Crea, R. NF-κB signaling and chronic inflammatory diseases: Exploring the potential of natural products to drive new therapeutic opportunities. Drug Discov. Today 2014, 19, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Bieghs, V.; Trauwein, C. The innate immune response during liver inflammation and metabolic disease. Trends Immunol. 2013, 34, 446–452. [Google Scholar] [CrossRef] [PubMed]
- Lopez, S.; Montserrat-de la Paz, S.; Lucas, R.; Bermudez, B.; Abia, R.; Morales, J.C.; Muriana, F.J.G. Effect of metabolites of hydroxytyrosol on protection against oxidative stress and inflammation in human endothelial cells. J. Funct. Foods 2017, 29, 238–247. [Google Scholar] [CrossRef]
- Warleta, F.; Quesada, C.S.; Campos, M.; Allouche, Y.; Beltrán, G.; Gaforio, J.J. Hydroxytyrosol protects against oxidative DNA damage in human breast cells. Nutrients 2011, 3, 839–857. [Google Scholar] [CrossRef] [PubMed]
- Terzuoli, E.; Giachetti, A.; Ziche, M.; Donnini, S. Hydroxytyrosol, a product from olive oil, reduces colon cancer growth by enhancing epidermal growth factor receptor degradation. Mol. Nutr. Food Res. 2016, 60, 519–529. [Google Scholar] [CrossRef] [PubMed]
- Notarnicola, M.; Pisanti, S.; Tutino, V.; Bocale, D.; Rotelli, M.T.; Gentile, A.; Memeo, V.; Bifulco, M.; Perri, E.; Caruso, M.G. Effects of olive oil polyphenols on fatty acid synthase gene expression and activity in human colorectal cancer cells. Genes Nutr. 2011, 6, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Talorete, T.P.N.; Yamada, P.; Isoda, H. Anti-proliferative and apoptotic effects of oleuropein and hydroxytyrosol on human breast cancer MCF-7 cells. Cytotechnology 2009, 59, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Valls, R.M.; Farràs, M.; Suárez, M.; Fernández-Castillejo, S.; Fitó, M.; Konstantinidou, V.; Fuentes, F.; López-Miranda, J.; Giralt, M.; Covas, M.I.; et al. Effects of functional olive oil enriched with its own phenolic compounds on endothelial function in hypertensive patients. A randomised controlled trial. Food Chem. 2015, 167, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Lockyer, S.; Corona, G.; Yaqoob, P.; Spencer, J.P.; Rowland, I. Secoiridoids delivered as olive leaf extract induce acute improvements in human vascular function and reduction of an inflammatory cytokine: A randomised, double-blind, placebo-controlled, cross-over trial. Br. J. Nutr. 2015, 114, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Catalán, Ú.; Rubió, L.; López de las Hazas, M.C.; Herrero, P.; Nadal, P.; Canela, N.; Pedret, A.; Motilva, M.J.; Solà, R. Hydroxytyrosol and its complex forms (secoiridoids) modulate aorta and heart proteome in healthy rats: Potential cardio-protective effects. Mol. Nutr. Food Res. 2016, 60, 2114–2129. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Choi, Y.; Um, S.J.; Yoon, S.K.; Park, T. Oleuropein attenuates hepatic steatosis induced by high-fat diet in mice. J. Hepatol. 2011, 54, 984–993. [Google Scholar] [CrossRef] [PubMed]
- Priore, P.; Siculella, L.; Gnoni, G.V. Extra virgin olive oil phenols down-regulate lipid synthesis in primary-cultured rat-hepatocytes. J. Nutr. Biochem. 2014, 25, 683–691. [Google Scholar] [CrossRef] [PubMed]
- Malhi, H.; Kaufman, R.J. Endoplasmic reticulum stress in liver disease. J. Hepatol. 2011, 54, 795–809. [Google Scholar] [CrossRef] [PubMed]
- Giordano, E.; Dangles, O.; Rakotomanomana, N.; Baracchini, S.; Visioli, F. 3-O-Hydroxytyrosol glucuronide and 4-O-hydroxytyrosol glucuronide reduce endoplasmic reticulum stress in vitro. Food Funct. 2015, 6, 3275–3281. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Bai, L.; Yan, J.; Li, Y.; Shen, W.; Wang, Y.; Wertz, K.; Weber, P.; Zhang, Y.; Chen, Y.; et al. Mitochondrial dynamic remodeling in strenuous exercise-induced muscle and mitochondrial dysfunction: Regulatory effects of hydroxytyrosol. Free Radic. Biol. Med. 2011, 50, 1437–1446. [Google Scholar] [CrossRef] [PubMed]
- Soni, M.; Prakash, C.; Sehwag, S.; Kumar, V. Protective effect of hydroxytyrosol in arsenic-induced mitochondrial dysfunction in rat brain. J. Biochem. Mol. Toxicol. 2017, e21906. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.; Shen, W.; Yu, G.; Jia, H.; Li, X.; Feng, Z.; Wang, Y.; Weber, P.; Wertz, K.; Sharman, E.; et al. Hydroxytyrosol promotes mitochondrial biogenesis and mitochondrial function in 3T3-L1 adipocytes. J. Nutr. Biochem. 2010, 21, 634–644. [Google Scholar] [CrossRef] [PubMed]
- Hagiwara, K.; Goto, T.; Araki, M.; Miyazaki, H.; Hagiwara, H. Olive polyphenol hydroxytyrosol prevents bone loss. Eur. J. Pharmacol. 2011, 662, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Takeda, R.; Koike, T.; Taniguchi, I.; Tanaka, K. Double-blind placebo-controlled trial of hydroxytyrosol of Olea europaea on pain in gonarthrosis. Phytomedicine 2013, 20, 861–864. [Google Scholar] [CrossRef] [PubMed]
- Zheng, A.; Li, H.; Cao, K.; Xu, J.; Zou, X.; Li, Y.; Chen, C.; Liu, J.; Feng, Z. Maternal hydroxytyrosol administration improves neurogenesis and cognitive function in prenatally stressed offspring. J. Nutr. Biochem. 2015, 26, 190–199. [Google Scholar] [CrossRef] [PubMed]

| Properties | Reference | Model | Intervention | Results/Findings |
|---|---|---|---|---|
| Antioxidant | Giordano et al., 2014 [37] | Animal/cellular | C57BL/6 mice were randomly assigned to a standard diet without HT or the same diet supplemented with HT (0.03 gm%) for 8 weeks. Murine 3T3-L1 pre-adipocytes, after differentiation, were cultured with H2O2 and 1 or 5 μM HT. | Modulated gene expression of pathways related to oxidative stress (OS) in adipose tissue. Decreased GSSG/GSH ratio in cultured adipocytes. |
| Granados-Principal et al., 2014 [38] | Animal | Thirty-six female Sprague–Dawley rats with induced mammary tumors were divided into four groups: control, HT (0.5 mg/kg, 5 days/week), doxorubicin (1 mg/kg/week), and doxorubicin + HT. | Improved drug-induced cardiac alterations induced by doxorubicin by reducing mitochondrial damage and OS. | |
| Zhu et al., 2010 [40] | Cellular | Cultured ARPE-19 cells were pretreated with HT dissolved in dimethylsulfoxide (DMSO; final DMSO concentration ≤ 0.025%) and treated with acrolein to induce OS. | Increased translocation of Nrf2 to the nucleus in cells not challenged with acrolein. Increased expression of phase II detoxifying enzymes (GCL, GR, GSH GPx, HO-1, NQO1) and of genes involved in oxidative defense (indirect effect). Increased expression and activity of CAT. | |
| Zrelli et al., 2011 [41] | Cellular | VECs with OS induced by H2O2 were incubated with HT (10, 30 and 50 μM). | Prevented intracellular increase of ROS levels. Increased CAT mRNA expression levels. Increased expression and activity of FOXO3a. | |
| Anti-inflammatory | Richard et al., 2011 [9] | Cellular | Murine macrophages (RAW264.7 cells) were stimulated with lipopolysaccharide (LPS) and treated with olive vegetation water containing 2.5% HT. | Inhibited of NO and PGE2 production. Decreased secretion of cytokines (interleukin (IL)-1α, IL-1β, IL-6, IL-12, TNF-α, and chemokines (CXCL10/IP-10, CCL2/MPC-1)). Decreased gene expression of iNOS, IL-1α, CXCL10/IP-10, MIP-1β, matrix metalloproteinase-9 and PGES. |
| Pirozzi et al., 2016 [42] | Animal | Rats with NAFLD were divided into three groups: Control diet, HFD and HFD + HT (10 mg/kg/d) for 5 weeks. | Increased levels of PPAR-α. Reduced the expression of pro-inflammatory cytokines TNF-α and IL-6. | |
| Lopez et al., 2017 [46] | Cellular | hECs were cultured in the presence of HT and HT metabolites and treated with TNF-α. | HT and its metabolites suppressed production of ROS induced by TNF-α. Down-regulated TNF-α-induced phosphorylation of NF-κB signaling proteins (IKKαβ, IκBα, and p65) in hECs. | |
| Anticancer | Warleta et al., 2011 [47] | Cellular | Three human breast cell lines were treated with HT and tyrosol: mammary epithelial cells (MCF10A) and breast cancer cells (MDA-MB-231, MCF7). | Decreased ROS production in a dose-dependent manner in MCF10A cells. Decreased H2O2 induced ROS level in breast cancer cells. Reduced DNA damage significantly in MCF7, MDA-MB-231 and MCF10A (in unexposed cells). |
| Terzuoli et al., 2016 [48] | Cellular | Human colorectal adenocarcinoma cells (HT-29, CaCo2, WiDr) or human colon fibroblast cells (CCD18Co) treated with HT. | Down-regulated EGFR expression in colon tumor cells by promoting its degradation via lysosomal and proteasomal mechanisms. | |
| Notarnicola et al., 2011 [49] | Cellular | Human colon adenocarcinoma cell lines (HT-29 and SW-620) were treated with HT and oleuropein at different concentrations (10, 25, 50 and 100 μM) for 24 and 72 h. | Reduced both gene expression and activity of FAS (starting from 10 μM). Showed an anti-proliferative effect in SW-620 both cell lines Had a pro-apoptotic effect in HT-29 cells. | |
| Han et al., 2009 [50] | Cellular | MCF-7 human breast cancer cells were treated with HT (6.25, 12.5, 25, 50 μg/mL) and oleuropein for 12 h. | Inhibited cell proliferation, reducing the cell viability in a time and concentration-dependent manner. Induced cell apoptosis by caspases activation and also blocked the cell cycle in G1 phase. | |
| Endothelial and vascular function | Valls et al., 2015 [51] | Human | Thirteen pre- and stage-1 hypertensive patients received a single dose of 30 ml of functional virgin olive oil (FVOO) (phenolic content = 961 mg/kg) or regular virgin olive oil (VOO) (phenolic content = 289 mg/kg) in a postprandial randomized, double blind, crossover trial. | FVOO improved human endothelial function (ischemic reactive hyperemia values were higher with FVOO than with VOO). Postprandial values of PAI-I, and hsCRP were lower after FVOO versus VOO. FVOO reduced oxidized LDL. |
| Lockyer et al., 2015 [52] | Human | Eighteen healthy volunteers who consumed either OLE (51 mg oleuropein; 10 mg HT), or a matched control (separated by a 4-week wash out) on a single occasion were studied in a randomized, double-blind, placebo-controlled, cross-over, acute intervention trial. | OLE reduced arterial stiffness. OLE reduced IL-8 production. | |
| Catalán et al., 2015 [11] | Cellular | Human aortic endothelial cells (HAEC) were treated with HT and the mixture of its metabolites (1, 2, 5, and 10 μM) and co-incubated with TNF-α for 18 and 24 h. | HT and its metabolites reduced the secretion of E-selectin, ICAM-1, and VCAM-1. Free HT and HT metabolites were effective in the reduction of the endothelial dysfunction biomarkers. | |
| Catalán et al., 2016 [53] | Animal | Twelve female Wistar rats were separated in three groups: standard diet, diet supplemented with HT or diet supplemented with secoroids (SEC) in doses of 5 mg/kg/d for 21 days. | HT was detected in heart tissue mainly in its free form after supplementation with HT or SEC. Supplementation changed heart and aorta proteome. These proteins are related to cardiovascular function, improving endothelial and vascular function. | |
| Anti-steatotic | Park et al., 2011 [54] | Animal | Male C57BL and 6N mice were separated into three groups: normal diet, HFD, HFD supplemented with oleuropein (the precursor of HT), for 10 weeks. | Had a protective effect in reversing the negative effects induced by a HFD, regularizing: hepatic steatosis, increased plasma lipids, and increased body weight and liver. Down-regulated transcription factors and their target genes involved in adipogenesis. |
| Priore et al., 2014 [55] | Cellular | Rat-liver cells were treated with HT, tyrosol and oleuropein (EVOO phenols). | Cholesterol synthesis and fatty acids (FA) synthesis were inhibited by the treatment. Reduced the activity of ACC. | |
| Pirozzi et al., 2016 [42] | Animal | Male rats were divided into three groups: standard diet, HFD, HFD + HT (10 mg/kg/d) for 5 weeks. | Reduced AST, ALT and cholesterol levels in serum, and reduced liver steatosis. Increased the activity of PPAR-α. Increased phosphorylation of ACC, increasing hepatic metabolism and oxidation of FA. | |
| ER stress and autophagy | Giordano et al., 2014 [13] | Cellular | Human hepatocarcinoma cells (HepG2) were treated with HT (1 μM and 5 μM) and 100 μM lipoic acid (LA) and glutathione-ethyl ester (GSH), for 24 h. UPR was induced tunicamycin for 4 h. | Reduced mRNA levels of CHOP and BiP compared with LA and GSH and with tunicamycin alone. Reduced protein levels of BiP and levels of eIF2a. Modulated the antiapoptotic Bcl2 protein levels. Prevented ER stress in hepatic cells. |
| Giordano et al., 2015 [57] | Cellular | Human hepatocarcinoma cells (HepG2) were treated with two HT hepatic metabolites: i.e., 3-O-HT glucuronide and 4-O-HT glucuronide. UPR was induced tunicamycin. | Both metabolites reduced mRNA expression levels of CHOP and BiP. The treatment also decreased BiP and CHOP protein levels. | |
| Cetrullo et al., 2016 [14] | Cellular | Primary cultures of chondrocytes obtained from patients with knee arthroplasty were incubated in the absence or presence of 100 μM H2O2 and treated with HT. | Enhanced SIRT-1 expression, positively regulating autophagy. | |
| Feng et al., 2011 [58] | Animal | Eight-week-old male Sprague–Dawley rats were selected for the experiment by their ability to perform 1 week of running exercise at low speed. Rats were divided into four groups: sedentary with or without HT, and endurance exercise with or without HT (25 mg/kg/d). After eight weeks of exercise the analyses were done. | Reduced OS and thereby mitochondrial impairment. Reduced muscular atrophy induced by autophagy and mitochondrial fission and decreased expression of PGC-1α. Up-regulated autophagy. | |
| Mitochondrial function | Hao et al., 2010 [60] | Cellular | Murine 3T3-L1 pre-adipocytes were treated with HT 0.1–50 μmol/L. | Stimulated activation and expression of PGC1α. Increased the mRNA expression levels of Nrf1, Nrf2 and Tfam (1 μmol/L HT). Promoted protein expression of complex I, II, III and V. Promoted the activity of complexes I, II, III, IV and V, increasing oxygen consumption in adipocytes. Increased mitochondrial mass. |
| Granados-Principal et al., 2014 [38] | Animal | Thirty-six female Sprague–Dawley rats with induced mammary tumors were divided into four groups: control, HT (0.5 mg/kg, 5 d/week), doxorubicin (1 mg/kg/week), and doxorubicin + HT. | Improved the mitochondrial electron transport chain in rats with cardiotoxicity induced by doxorubicin. Increased complexes II and III protein concentrations. | |
| Zheng et al., 2015 [15] | Animal | Male db/db C57BL/6J mice were separated into three groups: control, HT (10 mg/mg/d) and HT (50 mg/kg(d) | Improved expression of complexes I, II, and IV. Increased activity of complex I. Induced phase II antioxidant systems and inhibited protein oxidation in mice brain. Increased the expression of p-AMPK/AMPK, PGC-1α and SIRT1. | |
| Zhu et al., 2010 [40] | Cellular | Human retinal pigment epithelial cells (ARPE-19) were incubated with acrolein. The protective effects of HT were studied by pre-treating cells with HT for 48 h, followed by 24-h acrolein treatment in the absence of HT. | Increased the expression of PGC1α. Increased protein expression of mitochondrial transcription factor A and uncoupling protein 2 (UCP2). Increased the expression of complexes. Increased Nrf2 nuclear protein levels and its nuclear translocation. Enhanced phase II detoxifying enzymes. |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Echeverría, F.; Ortiz, M.; Valenzuela, R.; Videla, L.A. Hydroxytyrosol and Cytoprotection: A Projection for Clinical Interventions. Int. J. Mol. Sci. 2017, 18, 930. https://doi.org/10.3390/ijms18050930
Echeverría F, Ortiz M, Valenzuela R, Videla LA. Hydroxytyrosol and Cytoprotection: A Projection for Clinical Interventions. International Journal of Molecular Sciences. 2017; 18(5):930. https://doi.org/10.3390/ijms18050930
Chicago/Turabian StyleEcheverría, Francisca, Macarena Ortiz, Rodrigo Valenzuela, and Luis A. Videla. 2017. "Hydroxytyrosol and Cytoprotection: A Projection for Clinical Interventions" International Journal of Molecular Sciences 18, no. 5: 930. https://doi.org/10.3390/ijms18050930
APA StyleEcheverría, F., Ortiz, M., Valenzuela, R., & Videla, L. A. (2017). Hydroxytyrosol and Cytoprotection: A Projection for Clinical Interventions. International Journal of Molecular Sciences, 18(5), 930. https://doi.org/10.3390/ijms18050930