Unconventional Secretion of Heat Shock Proteins in Cancer
Abstract
:1. Heat Shock Protein Functions and Families
2. The Discovery of Chaperone Secretion
3. DAMP vs. DAMPER—Dual Role of Extracellular HSPs?
4. Unconventional Mechanisms of HSP Secretion
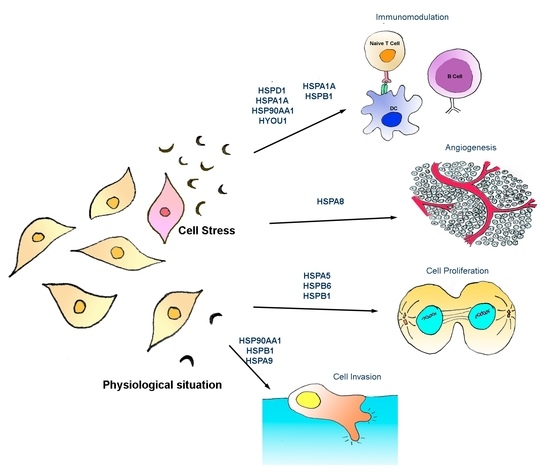
5. Functions of Extracellular HSPs in Cancer
6. Extracellular HSP-Based Cancer Therapies
7. Conclusions
Acknowledgments
Conflicts of Interest
References
- Lindquist, S.L.; Kelly, J.W. Chemical and biological approaches for adapting proteostasis to ameliorate protein misfolding and aggregation diseases: Progress and prognosis. Cold Spring Harb. Perspect. Biol. 2011, 3. [Google Scholar] [CrossRef] [PubMed]
- Richter, K.; Haslbeck, M.; Buchner, J. The heat shock response: Life on the verge of death. Mol. Cell 2010, 40, 253–266. [Google Scholar] [CrossRef] [PubMed]
- Whitesell, L.; Lindquist, S.L. HSP90 and the chaperoning of cancer. Nat. Rev. Cancer 2005, 5, 761–772. [Google Scholar] [CrossRef] [PubMed]
- HUGO Gene Nomenclature Committee. Available online: http://www.genenames.org/genefamilies/HSP (accessed on 1 January 2017).
- Kampinga, H.H.; Hageman, J.; Vos, M.J.; Kubota, H.; Tanguay, R.M.; Bruford, E.A.; Cheetham, M.E.; Chen, B.; Hightower, L.E. Guidelines for the nomenclature of the human heat shock proteins. Cell Stress Chaperones 2009, 14, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Facciponte, J.G.; Wang, X.Y.; Subjeck, J.R. HSP110 and Grp170, members of the HSP70 superfamily, bind to scavenger receptor-A and scavenger receptor expressed by endothelial cells-I. Eur. J. Immunol. 2007, 37, 2268–2279. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Sun, X.; Chen, X.; Subjeck, J.; Wang, X.Y. Secretion of stress protein grp170 promotes immune-mediated inhibition of murine prostate tumor. Cancer Immunol. Immunother. 2009, 58, 1319–1328. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Y.; Arnouk, H.; Chen, X.; Kazim, L.; Repasky, E.A.; Subjeck, J.R. Extracellular targeting of endoplasmic reticulum chaperone glucose-regulated protein 170 enhances tumor immunity to a poorly immunogenic melanoma. J. Immunol. 2006, 177, 1543–1551. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; Yi, H.; Guo, C.; Yu, X.; Zuo, D.; Chen, X.; Kane, J.M.; Repasky, E.A.; Subjeck, J.R.; Wang, X.Y. CD204 suppresses large heat shock protein-facilitated priming of tumor antigen gp100-specific T cells and chaperone vaccine activity against mouse melanoma. J. Immunol. 2011, 187, 2905–2914. [Google Scholar] [CrossRef] [PubMed]
- Berthenet, K.; Boudesco, C.; Collura, A.; Svrcek, M.; Richaud, S.; Hammann, A.; Causse, S.; Yousfi, N.; Wanherdrick, K.; Duplomb, L.; et al. Extracellular HSP110 skews macrophage polarization in colorectal cancer. Oncoimmunology 2016, 5, e1170264. [Google Scholar] [CrossRef] [PubMed]
- Nirdé, P.; Derocq, D.; Maynadier, M.; Chambon, M.; Basile, I.; Gary-Bobo, M.; Garcia, M. Heat shock cognate 70 protein secretion as a new growth arrest signal for cancer cells. Oncogene 2010, 29, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, L.S.; Barreto, A.; Franco, M.A.; Angel, J. Immunomodulators released during rotavirus infection of polarized caco-2 cells. Viral Immunol. 2009, 22, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Zou, N.; Ao, L.; Cleveland, J.C.; Yang, X.; Su, X.; Cai, G.Y.; Banerjee, A.; Fullerton, D.A.; Meng, X. Critical role of extracellular heat shock cognate protein 70 in the myocardial inflammatory response and cardiac dysfunction after global ischemia-reperfusion. Am. J. Physiol. Heart Circ. Physiol. 2008, 294, H2805–H2813. [Google Scholar] [CrossRef] [PubMed]
- Kern, J.; Untergasser, G.; Zenzmaier, C.; Sarg, B.; Gastl, G.; Gunsilius, E.; Steurer, M. GRP-78 secreted by tumor cells blocks the antiangiogenic activity of bortezomib. Blood 2009, 114, 3960–3967. [Google Scholar] [CrossRef] [PubMed]
- Tsuneki, M.; Maruyama, S.; Yamazaki, M.; Xu, B.; Essa, A.; Abé, T.; Babkair, H.; Cheng, J.; Yamamoto, T.; Saku, T. Extracellular heat shock protein A9 is a novel interaction partner of podoplanin in oral squamous cell carcinoma cells. Biochem. Biophys. Res. Commun. 2013, 434, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Pei, W.; Tanaka, K.; Huang, S.C.; Xu, L.; Liu, B.; Sinclair, J.; Idol, J.; Varshney, G.K.; Huang, H.; Lin, S.; et al. Extracellular HSP60 triggers tissue regeneration and wound healing by regulating inflammation and cell proliferation. Npj Regen. Med. 2016, 1, 16013. [Google Scholar] [CrossRef]
- Sarikonda, G.; Sachithanantham, S.; Miller, J.F.; Pagni, P.P.; Coppieters, K.T.; von Herrath, M. The HSP60 peptide p277 enhances anti-CD3 mediated diabetes remission in non-obese diabetic mice. J. Autoimmun. 2015, 59, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Breloer, M.; Dorner, B.; Moré, S.H.; Roderian, T.; Fleischer, B.; von Bonin, A. Heat shock proteins as “danger signals”: Eukaryotic HSP60 enhances and accelerates antigen-specific IFN-γ production in T cells. Eur. J. Immunol. 2001, 31, 2051–2059. [Google Scholar] [CrossRef]
- Bohonowych, J.E.; Hance, M.W.; Nolan, K.D.; Defee, M.; Parsons, C.H.; Isaacs, J.S. Extracellular HSP90 mediates an NF-κB dependent inflammatory stromal program: Implications for the prostate tumor microenvironment. Prostate 2014, 74, 395–407. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Zou, M.; Bhatia, A.; Jayaprakash, P.; Hofman, F.; Ying, Q.; Chen, M.; Woodley, D.T.; Li, W. Breast cancer MDA-MB-231 cells use secreted heat shock protein-90α (HSP90α) to survive a hostile hypoxic environment. Sci. Rep. 2016, 6, 20605. [Google Scholar] [CrossRef] [PubMed]
- Berwin, B.; Hart, J.P.; Rice, S.; Gass, C.; Pizzo, S.V.; Post, S.R.; Nicchitta, C.V. Scavenger receptor-A mediates gp96/GRP94 and calreticulin internalization by antigen-presenting cells. EMBO J. 2003, 22, 6127–6136. [Google Scholar] [CrossRef] [PubMed]
- Genereux, J.C.; Qu, S.; Zhou, M.; Ryno, L.M.; Wang, S.; Shoulders, M.D.; Kaufman, R.J.; Lasmézas, C.I.; Kelly, J.W.; Wiseman, R.L. Unfolded protein response-induced ERdj3 secretion links ER stress to extracellular proteostasis. EMBO J. 2015, 34, 4–19. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Lin, C.F.L.; Skinner, K.A.; Schiffhauer, L.M.; Peacock, J.; Hicks, D.G.; Redmond, E.M.; Morrow, D.; Huston, A.; Shayne, M.; et al. Heat Shock Protein 27 Differentiates Tolerogenic Macrophages That May Support Human Breast Cancer Progression. Cancer Res. 2011, 71, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Batulan, Z.; Pulakazhi Venu, V.K.; Li, Y.; Koumbadinga, G.; Alvarez-Olmedo, D.G.; Shi, C.; O’Brien, E.R. Extracellular release and signaling by heat shock protein 27: Role in modifying vascular inflammation. Front. Immunol. 2016, 7, 285. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, X.; Zhu, H.; Kranias, E.G.; Tang, Y.; Peng, T.; Chang, J.; Fan, G.C. HSP20 functions as a novel cardiokine in promoting angiogenesis via activation of VEGFR2. PLoS ONE 2012, 7, e32765. [Google Scholar] [CrossRef] [PubMed]
- Kozawa, O.; Matsuno, H.; Niwa, M.; Hatakeyama, D.; Oiso, Y.; Kato, K.; Uematsu, T. HSP20, low-molecular-weight heat shock-related protein, acts extracellularly as a regulator of platelet functions: A novel defense mechanism. Life Sci. 2002, 72, 113–124. [Google Scholar] [CrossRef]
- Bhat, S.P.; Gangalum, R.K. Secretion of αB-Crystallin via exosomes: New clues to the function of human retinal pigment epithelium. Commun. Integr. Biol. 2011, 4, 739–741. [Google Scholar] [CrossRef] [PubMed]
- Sreekumar, P.G.; Kannan, R.; Kitamura, M.; Spee, C.; Barron, E.; Ryan, S.J.; Hinton, D.R. αB crystallin is apically secreted within exosomes by polarized human retinal pigment epithelium and provides neuroprotection to adjacent cells. PLoS ONE 2010, 5, e12578. [Google Scholar] [CrossRef] [PubMed]
- Cortesi, L.; Barchetti, A.; De Matteis, E.; Rossi, E.; Della Casa, L.; Marcheselli, L.; Tazzioli, G.; Lazzaretti, M.G.; Ficarra, G.; Federico, M.; et al. Identification of protein clusters predictive of response to chemotherapy in breast cancer patients. J. Proteome Res. 2009, 8, 4916–4933. [Google Scholar] [CrossRef] [PubMed]
- Doyle, S.M.; Genest, O.; Wickner, S. Protein rescue from aggregates by powerful molecular chaperone machines. Nat. Rev. Mol. Cell Biol. 2013, 14, 617–629. [Google Scholar] [CrossRef] [PubMed]
- Hightower, L.E.; Guidon, P.T. Selective release from cultured mammalian cells of heat-shock (stress) proteins that resemble glia-axon transfer proteins. J. Cell. Physiol. 1989, 138, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Mathew, A.; Bell, A.; Johnstone, R.M. HSP-70 is closely associated with the transferrin receptor in exosomes from maturing reticulocytes. Biochem. J. 1995, 308 Pt 3, 823–830. [Google Scholar] [CrossRef] [PubMed]
- Pockley, A.G.; Bulmer, J.; Hanks, B.M.; Wright, B.H. Identification of human heat shock protein 60 (HSP60) and anti-HSP60 antibodies in the peripheral circulation of normal individuals. Cell Stress Chaperones 1999, 4, 29–35. [Google Scholar] [CrossRef]
- Pockley, A.G.; Shepherd, J.; Corton, J.M. Detection of heat shock protein 70 (HSP70) and anti-HSP70 antibodies in the serum of normal individuals. Immunol. Investig. 1998, 27, 367–377. [Google Scholar] [CrossRef]
- Ferrarini, M.; Heltai, S.; Zocchi, M.R.; Rugarli, C. Unusual expression and localization of heat-shock proteins in human tumor cells. Int. J. Cancer 1992, 51, 613–619. [Google Scholar] [CrossRef] [PubMed]
- Multhoff, G.; Botzler, C.; Wiesnet, M.; Müller, E.; Meier, T.; Wilmanns, W.; Issels, R.D. A stress-inducible 72-kDa heat-shock protein (HSP72) is expressed on the surface of human tumor cells, but not on normal cells. Int. J. Cancer 1995, 61, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Multhoff, G.; Botzler, C.; Jennen, L.; Schmidt, J.; Ellwart, J.; Issels, R. Heat shock protein 72 on tumor cells: A recognition structure for natural killer cells. J. Immunol. 1997, 158, 4341–4350. [Google Scholar] [PubMed]
- Théry, C.; Regnault, A.; Garin, J.; Wolfers, J.; Zitvogel, L.; Ricciardi-Castagnoli, P.; Raposo, G.; Amigorena, S. Molecular characterization of dendritic cell-derived exosomes. Selective accumulation of the heat shock protein HSC73. J. Cell Biol. 1999, 147, 599–610. [Google Scholar] [CrossRef] [PubMed]
- Asea, A.; Kraeft, S.K.; Kurt-Jones, E.A.; Stevenson, M.A.; Chen, L.B.; Finberg, R.W.; Koo, G.C.; Calderwood, S.K. HSP70 stimulates cytokine production through a CD14-dependant pathway, demonstrating its dual role as a chaperone and cytokine. Nat. Med. 2000, 6, 435–442. [Google Scholar] [PubMed]
- Basu, S. Necrotic but not apoptotic cell death releases heat shock proteins, which deliver a partial maturation signal to dendritic cells and activate the NF-κB pathway. Int. Immunol. 2000, 12, 1539–1546. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, E.J.; Matzinger, P. Is cancer dangerous to the immune system? Semin. Immunol. 1996, 8, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Liston, A.; Masters, S.L. Homeostasis-altering molecular processes as mechanisms of inflammasome activation. Nat. Rev. Immunol. 2017, 17, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Kono, H.; Rock, K.L. How dying cells alert the immune system to danger. Nat. Rev. Immunol. 2008, 8, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Van Eden, W.; Spiering, R.; Broere, F.; van der Zee, R. A case of mistaken identity: HSPs are no DAMPs but DAMPERs. Cell Stress Chaperones 2012, 17, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Broere, F.; van der Zee, R.; van Eden, W. Heat shock proteins are no DAMPs, rather “DAMPERs”. Nat. Rev. Immunol. 2011, 11, 565. [Google Scholar] [CrossRef] [PubMed]
- Motta, A.; Schmitz, C.; Rodrigues, L.; Ribeiro, F.; Teixeira, C.; Detanico, T.; Bonan, C.; Zwickey, H.; Bonorino, C. Mycobacterium tuberculosis heat-shock protein 70 impairs maturation of dendritic cells from bone marrow precursors, induces interleukin-10 production and inhibits T-cell proliferation in vitro. Immunology 2007, 121, 462–472. [Google Scholar] [CrossRef] [PubMed]
- Bendz, H.; Marincek, B.C.; Momburg, F.; Ellwart, J.W.; Issels, R.D.; Nelson, P.J.; Noessner, E. Calcium signaling in dendritic cells by human or mycobacterial HSP70 is caused by contamination and is not required for HSP70-mediated enhancement of cross-presentation. J. Biol. Chem. 2008, 283, 26477–26483. [Google Scholar] [CrossRef] [PubMed]
- Wieten, L.; van der Zee, R.; Spiering, R.; Wagenaar-Hilbers, J.; van Kooten, P.; Broere, F.; van Eden, W. A novel heat-shock protein coinducer boosts stress protein HSP70 to activate T cell regulation of inflammation in autoimmune arthritis. Arthritis Rheum. 2010, 62, 1026–1035. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.I.; Namba, T.; Arai, Y.; Fujimoto, M.; Adachi, H.; Sobue, G.; Takeuchi, K.; Nakai, A.; Mizushima, T. Genetic evidence for a protective role for heat shock factor 1 and heat shock protein 70 against colitis. J. Biol. Chem. 2007, 282, 23240–23252. [Google Scholar] [CrossRef] [PubMed]
- Kovalchin, J.T.; Mendonca, C.; Wagh, M.S.; Wang, R.; Chandawarkar, R.Y. In vivo treatment of mice with heat shock protein, gp 96, improves survival of skin grafts with minor and major antigenic disparity. Transpl. Immunol. 2006, 15, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Hunter-Lavin, C.; Davies, E.L.; Bacelar, M.M.F.V.G.; Marshall, M.J.; Andrew, S.M.; Williams, J.H.H. HSP70 release from peripheral blood mononuclear cells. Biochem. Biophys. Res. Commun. 2004, 324, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Mambula, S.S.; Calderwood, S.K. Heat shock protein 70 is secreted from tumor cells by a nonclassical pathway involving lysosomal endosomes. J. Immunol. 2006, 177, 7849–7857. [Google Scholar] [CrossRef] [PubMed]
- Mambula, S.S.; Stevenson, M.A.; Ogawa, K.; Calderwood, S.K. Mechanisms for HSP70 secretion: Crossing membranes without a leader. Methods 2007, 43, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Broquet, A.H.; Thomas, G.; Masliah, J.; Trugnan, G.; Bachelet, M. Expression of the molecular chaperone HSP70 in detergent-resistant microdomains correlates with its membrane delivery and release. J. Biol. Chem. 2003, 278, 21601–21606. [Google Scholar] [CrossRef] [PubMed]
- Evdokimovskaya, Y.; Skarga, Y.; Vrublevskaya, V.; Morenkov, O. Secretion of the heat shock proteins HSP70 and HSC70 by baby hamster kidney (BHK-21) cells. Cell Biol. Int. 2010, 34, 985–990. [Google Scholar] [CrossRef] [PubMed]
- Mambula, S.S.; Calderwood, S.K. Heat induced release of HSP70 from prostate carcinoma cells involves both active secretion and passive release from necrotic cells. Int. J. Hyperth. 2006, 22, 575–585. [Google Scholar] [CrossRef] [PubMed]
- Lancaster, G.I.; Febbraio, M.A. Exosome-dependent trafficking of HSP70: A novel secretory pathway for cellular stress proteins. J. Biol. Chem. 2005, 280, 23349–23355. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.R.; Robinson, M.B.; Gifondorwa, D.J.; Tytell, M.; Milligan, C.E. Regulation of heat shock protein 70 release in astrocytes: Role of signaling kinases. Dev. Neurobiol. 2007, 67, 1815–1829. [Google Scholar] [CrossRef] [PubMed]
- Zhan, R.; Leng, X.; Liu, X.; Wang, X.; Gong, J.; Yan, L.; Wang, L.; Wang, Y.; Wang, X.; Qian, L.J. Heat shock protein 70 is secreted from endothelial cells by a non-classical pathway involving exosomes. Biochem. Biophys. Res. Commun. 2009, 387, 229–233. [Google Scholar] [CrossRef] [PubMed]
- Hegmans, J.P.J.J.; Bard, M.P.L.; Hemmes, A.; Luider, T.M.; Kleijmeer, M.J.; Prins, J.B.; Zitvogel, L.; Burgers, S.A.; Hoogsteden, H.C.; Lambrecht, B.N. Proteomic analysis of exosomes secreted by human mesothelioma cells. Am. J. Pathol. 2004, 164, 1807–1815. [Google Scholar] [CrossRef]
- Takeuchi, T.; Suzuki, M.; Fujikake, N.; Popiel, H.A.; Kikuchi, H.; Futaki, S.; Wada, K.; Nagai, Y. Intercellular chaperone transmission via exosomes contributes to maintenance of protein homeostasis at the organismal level. Proc. Natl. Acad. Sci. USA 2015, 112, E2497–E2506. [Google Scholar] [CrossRef] [PubMed]
- Barreto, A.; Gonzalez, J.M.; Kabingu, E.; Asea, A.; Fiorentino, S. Stress-induced release of HSC70 from human tumors. Cell. Immunol. 2003, 222, 97–104. [Google Scholar] [CrossRef]
- Evdonin, A.L.; Guzhova, I.V.; Margulis, B.A.; Medvedeva, N.D. Phospholipse c inhibitor, u73122, stimulates release of HSP-70 stress protein from A431 human carcinoma cells. Cancer Cell Int. 2004, 4, 2. [Google Scholar] [CrossRef] [PubMed]
- Evdonin, A.L.; Martynova, M.G.; Bystrova, O.A.; Guzhova, I.V.; Margulis, B.A.; Medvedeva, N.D. The release of HSP70 from A431 carcinoma cells is mediated by secretory-like granules. Eur. J. Cell Biol. 2006, 85, 443–455. [Google Scholar] [CrossRef] [PubMed]
- Pittet, J.F.; Lee, H.; Morabito, D.; Howard, M.B.; Welch, W.J.; Mackersie, R.C. Serum levels of HSP72 measured early after trauma correlate with survival. J. Trauma 2002, 52, 611–617. [Google Scholar] [PubMed]
- Chan, Y.C.; Shukla, N.; Abdus-Samee, M.; Berwanger, C.S.; Stanford, J.; Singh, M.; Mansfield, A.O.; Stansby, G. Anti-heat-shock protein 70 kDa antibodies in vascular patients. Eur. J. Vasc. Endovasc. Surg. 1999, 18, 381–385. [Google Scholar] [CrossRef] [PubMed]
- Ganter, M.T. Extracellular heat shock protein 72 is a marker of the stress protein response in acute lung injury. AJP Lung Cell. Mol. Physiol. 2006, 291, L354–L361. [Google Scholar] [CrossRef] [PubMed]
- Hurwitz, M.D.; Kaur, P.; Nagaraja, G.M.; Bausero, M.A.; Manola, J.; Asea, A. Radiation therapy induces circulating serum HSP72 in patients with prostate cancer. Radiother. Oncol. 2010, 95, 350–358. [Google Scholar] [CrossRef] [PubMed]
- Kimura, F.; Itoh, H.; Ambiru, S.; Shimizu, H.; Togawa, A.; Yoshidome, H.; Ohtsuka, M.; Shimamura, F.; Kato, A.; Nukui, Y.; et al. Circulating heat-shock protein 70 is associated with postoperative infection and organ dysfunction after liver resection. Am. J. Surg. 2004, 187, 777–784. [Google Scholar] [CrossRef] [PubMed]
- Pagetta, A.; Folda, A.; Brunati, A.M.; Finotti, P. Identification and purification from the plasma of Type 1 diabetic subjects of a proteolytically active Grp94. Diabetologia 2003, 46, 996–1006. [Google Scholar] [CrossRef] [PubMed]
- Walsh, R.C.; Koukoulas, I.; Garnham, A.; Moseley, P.L.; Hargreaves, M.; Febbraio, M.A. Exercise increases serum HSP72 in humans. Cell Stress Chaperones 2001, 6, 386–393. [Google Scholar] [CrossRef]
- Febbraio, M.A.; Ott, P.; Nielsen, H.B.; Steensberg, A.; Keller, C.; Krustrup, P.; Secher, N.H.; Pedersen, B.K. Exercise induces hepatosplanchnic release of heat shock protein 72 in humans. J. Physiol. 2002, 544, 957–962. [Google Scholar] [CrossRef] [PubMed]
- Schilling, D.; Gehrmann, M.; Steinem, C.; de Maio, A.; Pockley, A.G.; Abend, M.; Molls, M.; Multhoff, G. Binding of heat shock protein 70 to extracellular phosphatidylserine promotes killing of normoxic and hypoxic tumor cells. FASEB J. 2009, 23, 2467–2477. [Google Scholar] [CrossRef] [PubMed]
- Hantschel, M.; Pfister, K.; Jordan, A.; Scholz, R.; Andreesen, R.; Schmitz, G.; Schmetzer, H.; Hiddemann, W.; Multhoff, G. HSP70 plasma membrane expression on primary tumor biopsy material and bone marrow of leukemic patients. Cell Stress Chaperones 2000, 5, 438–442. [Google Scholar] [CrossRef]
- Kaur, J.; Das, S.N.; Srivastava, A.; Ralhan, R. Cell surface expression of 70 kDa heat shock protein in human oral dysplasia and squamous cell carcinoma: Correlation with clinicopathological features. Oral Oncol. 1998, 34, 93–98. [Google Scholar] [CrossRef]
- Kleinjung, T.; Arndt, O.; Feldmann, H.J.; Bockmühl, U.; Gehrmann, M.; Zilch, T.; Pfister, K.; Schönberger, J.; Marienhagen, J.; Eilles, C.; et al. Heat shock protein 70 (HSP70) membrane expression on head-and-neck cancer biopsy-a target for natural killer (NK) cells. Int. J. Radiat. Oncol. Biol. Phys. 2003, 57, 820–826. [Google Scholar] [CrossRef]
- Vega, V.L.; Rodriguez-Silva, M.; Frey, T.; Gehrmann, M.; Diaz, J.C.; Steinem, C.; Multhoff, G.; Arispe, N.; de Maio, A. HSP70 Translocates into the plasma membrane after stress and is released into the extracellular environment in a membrane-associated form that activates macrophages. J. Immunol. 2008, 180, 4299–4307. [Google Scholar] [CrossRef] [PubMed]
- Alder, G.M.; Austen, B.M.; Bashford, C.L.; Mehlert, A.; Pasternak, C.A. Heat shock proteins induce pores in membranes. Biosci. Rep. 1990, 10, 509–518. [Google Scholar] [CrossRef] [PubMed]
- Negulyaev, Y.A.; Vedernikova, E.A.; Kinev, A.V.; Voronin, A.P. Exogenous heat shock protein HSP70 activates potassium channels in U937 cells. Biochim. Biophys. Acta 1996, 1282, 156–162. [Google Scholar] [CrossRef]
- Arispe, N.; de Maio, A. ATP and ADP modulate a cation channel formed by HSC70 in acidic phospholipid membranes. J. Biol. Chem. 2000, 275, 30839–30843. [Google Scholar] [CrossRef] [PubMed]
- Arispe, N.; Doh, M.; de Maio, A. Lipid interaction differentiates the constitutive and stress-induced heat shock proteins HSC70 and HSP70. Cell Stress Chaperones 2002, 7, 330–338. [Google Scholar] [CrossRef]
- Bassan, M.; Zamostiano, R.; Giladi, E.; Davidson, A.; Wollman, Y.; Pitman, J.; Hauser, J.; Brenneman, D.E.; Gozes, I. The identification of secreted heat shock 60-like protein from rat glial cells and a human neuroblastoma cell line. Neurosci. Lett. 1998, 250, 37–40. [Google Scholar] [CrossRef]
- Hayoun, D.; Kapp, T.; Edri-Brami, M.; Ventura, T.; Cohen, M.; Avidan, A.; Lichtenstein, R.G. HSP60 is transported through the secretory pathway of 3-MCA-induced fibrosarcoma tumour cells and undergoes N-glycosylation. FEBS J. 2012, 279, 2083–2095. [Google Scholar] [CrossRef] [PubMed]
- Merendino, A.M.; Bucchieri, F.; Campanella, C.; Marcianò, V.; Ribbene, A.; David, S.; Zummo, G.; Burgio, G.; Corona, D.F.V.; Conway de Macario, E.; et al. HSP60 is actively secreted by human tumor cells. PLoS ONE 2010, 5, e9247. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Knowlton, A.A. HSP60 trafficking in adult cardiac myocytes: Role of the exosomal pathway. AJP Hear. Circ. Physiol. 2007, 292, H3052–H3056. [Google Scholar] [CrossRef] [PubMed]
- Campanella, C.; Bucchieri, F.; Merendino, A.M.; Fucarino, A.; Burgio, G.; Corona, D.F.V.; Barbieri, G.; David, S.; Farina, F.; Zummo, G.; et al. The odyssey of HSP60 from tumor cells to other destinations includes plasma membrane-associated stages and golgi and exosomal protein-trafficking modalities. PLoS ONE 2012, 7, e42008. [Google Scholar] [CrossRef] [PubMed]
- Lewthwaite, J.; Owen, N.; Coates, A.; Henderson, B.; Steptoe, A. Circulating human heat shock protein 60 in the plasma of British civil servants: Relationship to physiological and psychosocial stress. Circulation 2002, 106, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Pockley, A.G.; Wu, R.; Lemne, C.; Kiessling, R.; de Faire, U.; Frostegård, J. Circulating heat shock protein 60 is associated with early cardiovascular disease. Hypertens 2000, 36, 303–307. [Google Scholar] [CrossRef]
- Cechetto, J.D.; Soltys, B.J.; Gupta, R.S. Localization of mitochondrial 60-kD heat shock chaperonin protein (HSP60) in pituitary growth hormone secretory granules and pancreatic zymogen granules. J. Histochem. Cytochem. 2000, 48, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Brudzynski, K.; Martinez, V.; Gupta, R.S. Immunocytochemical localization of heat-shock protein 60-related protein in β-cell secretory granules and its altered distribution in non-obese diabetic mice. Diabetologia 1992, 35, 316–324. [Google Scholar] [CrossRef] [PubMed]
- Campanella, C.; Rappa, F.; Sciumè, C.; Marino Gammazza, A.; Barone, R.; Bucchieri, F.; David, S.; Curcurù, G.; Caruso Bavisotto, C.; Pitruzzella, A.; et al. Heat shock protein 60 levels in tissue and circulating exosomes in human large bowel cancer before and after ablative surgery. Cancer 2015, 121, 3230–3239. [Google Scholar] [CrossRef] [PubMed]
- McCready, J.; Sims, J.D.; Chan, D.; Jay, D.G. Secretion of extracellular HSP90α via exosomes increases cancer cell motility: A role for plasminogen activation. BMC Cancer 2010, 10, 294. [Google Scholar] [CrossRef] [PubMed]
- Clayton, A.; Turkes, A.; Navabi, H.; Mason, M.D.; Tabi, Z. Induction of heat shock proteins in B-cell exosomes. J. Cell Sci. 2005, 118, 3631–3638. [Google Scholar] [CrossRef] [PubMed]
- Weiss, M.; Stope, M.; Klinkmann, G.; Könsgen, D.; Brucker, S.; Wallwiener, D.; Burchardt, M.; Mustea, A. Induction and secretion of pro-oncogenic heat shock protein 27 in ovarian cancer cells. Geburtshilfe Frauenheilkd. 2016, 76. [Google Scholar] [CrossRef]
- Liao, W.C.; Wu, M.S.; Wang, H.P.; Tien, Y.W.; Lin, J.T. Serum heat shock protein 27 Is increased in chronic pancreatitis and pancreatic carcinoma. Pancreas 2009, 38, 422–426. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.T.; Liu, Y.K.; Song, H.Y.; Dai, Z.; Qin, L.X.; Almofti, M.R.; Fang, C.Y.; Lu, H.J.; Yang, P.Y.; Tang, Z.Y. Heat-shock protein 27: A potential biomarker for hepatocellular carcinoma identified by serum proteome analysis. Proteomics 2005, 5, 4581–4588. [Google Scholar] [CrossRef] [PubMed]
- Fanelli, M.A.; Cuello Carrión, F.D.; Dekker, J.; Schoemaker, J.; Ciocca, D.R. Serological detection of heat shock protein HSP27 in normal and breast cancer patients. Cancer Epidemiol. Biomark. Prev. 1998, 7, 791–795. [Google Scholar]
- Huang, Q.; Ye, J.; Huang, Q.; Chen, W.; Wang, L.; Lin, W.; Lin, J.; Lin, X. Heat shock protein 27 is over-expressed in tumor tissues and increased in sera of patients with gastric adenocarcinoma. Clin. Chem. Lab. Med. 2010, 48, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Gangalum, R.K.; Bhat, A.M.; Kohan, S.A.; Bhat, S.P. Inhibition of the expression of the small heat shock protein αb-crystallin inhibits exosome secretion in human retinal pigment epithelial cells in culture. J. Biol. Chem. 2016, 291, 12930–12942. [Google Scholar] [CrossRef] [PubMed]
- Gangalum, R.K.; Atanasov, I.C.; Zhou, Z.H.; Bhat, S.P. B-Crystallin Is Found in detergent-resistant membrane microdomains and is secreted via exosomes from human retinal pigment epithelial cells. J. Biol. Chem. 2011, 286, 3261–3269. [Google Scholar] [CrossRef] [PubMed]
- Smith, V.L.; Jackson, L.; Schorey, J.S. Ubiquitination as a mechanism to transport soluble mycobacterial and eukaryotic proteins to exosomes. J. Immunol. 2015, 195, 2722–2730. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Gonzalo, O.; Villarroya-Beltri, C.; Sánchez-Madrid, F. Post-translational modifications of exosomal proteins. Front. Immunol. 2014, 5, 383. [Google Scholar] [CrossRef] [PubMed]
- Ploper, D.; Taelman, V.F.; Robert, L.; Perez, B.S.; Titz, B.; Chen, H.W.; Graeber, T.G.; von Euw, E.; Ribas, A.; de Robertis, E.M. MITF drives endolysosomal biogenesis and potentiates Wnt signaling in melanoma cells. Proc. Natl. Acad. Sci. USA 2015, 112, E420–E429. [Google Scholar] [CrossRef] [PubMed]
- Bunney, T.D.; Katan, M. Phosphoinositide signalling in cancer: Beyond PI3K and PTEN. Nat. Rev. Cancer 2010, 10, 342–352. [Google Scholar] [CrossRef] [PubMed]
- Kurrle, N.; Ockenga, W.; Meister, M.; Völlner, F.; Kühne, S.; John, B.A.; Banning, A.; Tikkanen, R. Phosphatidylinositol 3-Kinase dependent upregulation of the epidermal growth factor receptor upon Flotillin-1 depletion in breast cancer cells. BMC Cancer 2013, 13, 575. [Google Scholar] [CrossRef] [PubMed]
- Tzeng, H.T.; Wang, Y.C. Rab-mediated vesicle trafficking in cancer. J. Biomed. Sci. 2016, 23, 70. [Google Scholar] [CrossRef] [PubMed]
- Fortini, M.E.; Bilder, D. Endocytic regulation of Notch signaling. Curr. Opin. Genet. Dev. 2009, 19, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z. p53 regulation of the IGF-1/AKT/mTOR pathways and the endosomal compartment. Cold Spring Harb. Perspect. Biol. 2010, 2, a001057. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zheng, W.; Guo, Z.; Ju, Q.; Zhu, L.; Gao, J.; Zhou, L.; Liu, F.; Xu, Y.; Zhan, Q.; et al. A novel TP53 pathway influences the HGS-mediated exosome formation in colorectal cancer. Sci. Rep. 2016, 6, 28083. [Google Scholar] [CrossRef] [PubMed]
- Mellman, I.; Yarden, Y. Endocytosis and cancer. Cold Spring Harb. Perspect. Biol. 2013, 5, a016949. [Google Scholar] [CrossRef] [PubMed]
- Mortaz, E.; Redegeld, F.A.; Nijkamp, F.P.; Wong, H.R.; Engels, F. Acetylsalicylic acid-induced release of HSP70 from mast cells results in cell activation through TLR pathway. Exp. Hematol. 2006, 34, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Dybdahl, B.; Wahba, A.; Lien, E.; Flo, T.H.; Waage, A.; Qureshi, N.; Sellevold, O.F.M.; Espevik, T.; Sundan, A. Inflammatory response after open heart surgery: Releas e of heat-shock protein 70 and signaling through toll-like receptor-4. Circulation 2002, 105, 685–690. [Google Scholar] [CrossRef] [PubMed]
- Vabulas, R.M.; Ahmad-Nejad, P.; Ghose, S.; Kirschning, C.J.; Issels, R.D.; Wagner, H. HSP70 as endogenous stimulus of the Toll/interleukin-1 receptor signal pathway. J. Biol. Chem. 2002, 277, 15107–15112. [Google Scholar] [CrossRef] [PubMed]
- Asea, A.; Rehli, M.; Kabingu, E.; Boch, J.A.; Bare, O.; Auron, P.E.; Stevenson, M.A.; Calderwood, S.K. Novel signal transduction pathway utilized by extracellular HSP70: Role of toll-like receptor (TLR) 2 and TLR4. J. Biol. Chem. 2002, 277, 15028–15034. [Google Scholar] [CrossRef] [PubMed]
- Bausero, M.A.; Gastpar, R.; Multhoff, G.; Asea, A. Alternative mechanism by which IFN-enhances tumor recognition: Active release of heat shock protein 72. J. Immunol. 2005, 175, 2900–2912. [Google Scholar] [CrossRef] [PubMed]
- Anand, P.K.; Anand, E.; Bleck, C.K.E.; Anes, E.; Griffiths, G. Exosomal HSP70 Induces a Pro-inflammatory response to foreign particles including mycobacteria. PLoS ONE 2010, 5, e10136. [Google Scholar] [CrossRef] [PubMed]
- Lv, L.H.; Wan, Y.L.; Lin, Y.; Zhang, W.; Yang, M.; Li, G.L.; Lin, H.M.; Shang, C.Z.; Chen, Y.J.; Min, J. Anticancer drugs cause release of exosomes with heat shock proteins from human hepatocellular carcinoma cells that elicit effective natural killer cell antitumor responses in Vitro. J. Biol. Chem. 2012, 287, 15874–15885. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Kovalchin, J.T.; Muhlenkamp, P.; Chandawarkar, R.Y. Exogenous heat shock protein 70 binds macrophage lipid raft microdomain and stimulates phagocytosis, processing, and MHC-II presentation of antigens. Blood 2006, 107, 1636–1642. [Google Scholar] [CrossRef] [PubMed]
- Aneja, R.; Odoms, K.; Dunsmore, K.; Shanley, T.P.; Wong, H.R. Extracellular heat shock protein-70 induces endotoxin tolerance in THP-1 cells. J. Immunol. 2006, 177, 7184–7192. [Google Scholar] [CrossRef] [PubMed]
- Kovalchin, J.T.; Wang, R.; Wagh, M.S.; Azoulay, J.; Sanders, M.; Chandawarkar, R.Y. In vivo delivery of heat shock protein 70 accelerates wound healing by up-regulating macrophage-mediated phagocytosis. Wound Repair Regen. 2006, 14, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.J.; Kim, Y.M.; Kim, D.Y.; Jeoung, D.; Han, K.; Lee, S.T.; Lee, Y.S.; Park, K.H.; Park, J.H.; Kim, D.J.; et al. Release of heat shock protein 70 (HSP70) and the effects of extracellular HSP70 on matric metalloproteinase-9 expression in human monocytic U937 cells. Exp. Mol. Med. 2006, 38, 364–374. [Google Scholar] [CrossRef] [PubMed]
- Campisi, J.; Leem, T.H.; Fleshner, M. Stress-induced extracellular HSP72 is a functionally significant danger signal to the immune system. Cell Stress Chaperones 2003, 8, 272–286. [Google Scholar] [CrossRef]
- Chalmin, F.; Ladoire, S.; Mignot, G.; Vincent, J.; Bruchard, M.; Remy-Martin, J.P.; Boireau, W.; Rouleau, A.; Simon, B.; Lanneau, D.; et al. Membrane-associated HSP72 from tumor-derived exosomes mediates STAT3-dependent immunosuppressive function of mouse and human myeloid-derived suppressor cells. J. Clin. Investig. 2010, 120, 457–471. [Google Scholar] [CrossRef] [PubMed]
- Bausinger, H.; Lipsker, D.; Ziylan, U.; Manié, S.; Briand, J.P.; Cazenave, J.P.; Muller, S.; Haeuw, J.F.; Ravanat, C.; de la Salle, H.; et al. Endotoxin-free heat-shock protein 70 fails to induce APC activation. Eur. J. Immunol. 2002, 32, 3708–3713. [Google Scholar] [CrossRef]
- Gao, B.; Tsan, M.F. Endotoxin contamination in recombinant human heat shock protein 70 (HSP70) preparation is responsible for the induction of tumor necrosis factor α release by murine macrophages. J. Biol. Chem. 2003, 278, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Gao, B.; Tsan, M.F. Recombinant human heat shock protein 60 does not induce the release of tumor necrosis factor α from murine macrophages. J. Biol. Chem. 2003, 278, 22523–22529. [Google Scholar] [CrossRef] [PubMed]
- Gao, B.; Tsan, M.F. Induction of cytokines by heat shock proteins and endotoxin in murine macrophages. Biochem. Biophys. Res. Commun. 2004, 317, 1149–1154. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Nagaraja, G.M.; Kaur, P.; Asea, E.E.; Asea, A. Chaperokine function of recombinant HSP72 produced in insect cells using a baculovirus expression system is retained. J. Biol. Chem. 2010, 285, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Zuo, D.; Yu, X.; Guo, C.; Yi, H.; Chen, X.; Conrad, D.H.; Guo, T.L.; Chen, Z.; Fisher, P.B.; Subjeck, J.R.; et al. Molecular chaperoning by glucose-regulated protein 170 in the extracellular milieu promotes macrophage-mediated pathogen sensing and innate immunity. FASEB J. 2012, 26, 1493–1505. [Google Scholar] [CrossRef] [PubMed]
- Eustace, B.K.; Sakurai, T.; Stewart, J.K.; Yimlamai, D.; Unger, C.; Zehetmeier, C.; Lain, B.; Torella, C.; Henning, S.W.; Beste, G.; et al. Functional proteomic screens reveal an essential extracellular role for HSP90 α in cancer cell invasiveness. Nat. Cell Biol. 2004, 6, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Song, X.; Zhuo, W.; Fu, Y.; Shi, H.; Liang, Y.; Tong, M.; Chang, G.; Luo, Y. The regulatory mechanism of HSP90α secretion and its function in tumor malignancy. Proc. Natl. Acad. Sci. USA 2009, 106, 21288–21293. [Google Scholar] [CrossRef] [PubMed]
- Becker, B.; Multhoff, G.; Farkas, B.; Wild, P.J.; Landthaler, M.; Stolz, W.; Vogt, T. Induction of HSP90 protein expression in malignant melanomas and melanoma metastases. Exp. Dermatol. 2004, 13, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Tsutsumi, S.; Neckers, L. Extracellular heat shock protein 90: A role for a molecular chaperone in cell motility and cancer metastasis. Cancer Sci. 2007, 98, 1536–1539. [Google Scholar] [CrossRef] [PubMed]
- Stellas, D.; Karameris, A.; Patsavoudi, E. Monoclonal antibody 4C5 immunostains human melanomas and inhibits melanoma cell invasion and metastasis. Clin. Cancer Res. 2007, 13, 1831–1838. [Google Scholar] [CrossRef] [PubMed]
- Sidera, K.; Patsavoudi, E. Extracellular HSP90: Conquering the cell surface. Cell Cycle 2008, 7, 1564–1568. [Google Scholar] [CrossRef] [PubMed]
- Hance, M.W.; Dole, K.; Gopal, U.; Bohonowych, J.E.; Jezierska-Drutel, A.; Neumann, C.A.; Liu, H.; Garraway, I.P.; Isaacs, J.S. Secreted HSP90 Is a novel regulator of the epithelial to mesenchymal transition (EMT) in prostate cancer. J. Biol. Chem. 2012, 287, 37732–37744. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Li, Y.; Guan, S.; Fan, J.; Cheng, C.F.; Bright, A.M.; Chinn, C.; Chen, M.; Woodley, D.T. Extracellular heat shock protein-90α: Linking hypoxia to skin cell motility and wound healing. EMBO J. 2007, 26, 1221–1233. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.F.; Fan, J.; Fedesco, M.; Guan, S.; Li, Y.; Bandyopadhyay, B.; Bright, A.M.; Yerushalmi, D.; Liang, M.; Chen, M.; et al. Transforming Growth Factor (TGF)—Stimulated secretion of HSP90: Using the receptor LRP-1/CD91 to promote human skin cell migration against a TGF-rich environment during wound healing. Mol. Cell. Biol. 2008, 28, 3344–3358. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.S.; Hsu, Y.M.; Chen, C.C.; Chen, L.L.; Lee, C.C.; Huang, T.S. Secreted Heat shock protein 90 induces colorectal cancer cell invasion through CD91/LRP-1 and NF-B-mediated integrin V Expression. J. Biol. Chem. 2010, 285, 25458–25466. [Google Scholar] [CrossRef] [PubMed]
- Bausero, M.A.; Page, D.T.; Osinaga, E.; Asea, A. Surface expression of HSP25 and HSP72 differentially regulates tumor growth and metastasis. Tumour Biol. 2004, 25, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, R.; Ni, M.; Gill, P.; Lee, A.S. Cell surface relocalization of the endoplasmic reticulum chaperone and unfolded protein response regulator GRP78/BiP. J. Biol. Chem. 2010, 285, 15065–15075. [Google Scholar] [CrossRef] [PubMed]
- Delpino, A.; Castelli, M. The 78 kDa glucose-regulated protein (GRP78/BIP) is expressed on the cell membrane, is released into cell culture medium and is also present in human peripheral circulation. Biosci. Rep. 2002, 22, 407–420. [Google Scholar] [CrossRef] [PubMed]
- Mintz, P.J.; Kim, J.; Do, K.A.; Wang, X.; Zinner, R.G.; Cristofanilli, M.; Arap, M.A.; Hong, W.K.; Troncoso, P.; Logothetis, C.J.; et al. Fingerprinting the circulating repertoire of antibodies from cancer patients. Nat. Biotechnol. 2003, 21, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Arap, M.A.; Lahdenranta, J.; Mintz, P.J.; Hajitou, A.; Sarkis, A.S.; Arap, W.; Pasqualini, R. Cell surface expression of the stress response chaperone GRP78 enables tumor targeting by circulating ligands. Cancer Cell 2004, 6, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Guzhova, I.; Kislyakova, K.; Moskaliova, O.; Fridlanskaya, I.; Tytell, M.; Cheetham, M.; Margulis, B. In vitro studies show that HSP70 can be released by glia and that exogenous HSP70 can enhance neuronal stress tolerance. Brain Res. 2001, 914, 66–73. [Google Scholar] [CrossRef]
- Popiel, H.A.; Takeuchi, T.; Fujita, H.; Yamamoto, K.; Ito, C.; Yamane, H.; Muramatsu, S.; Toda, T.; Wada, K.; Nagai, Y. HSP40 gene therapy exerts therapeutic effects on polyglutamine disease mice via a non-cell autonomous mechanism. PLoS ONE 2012, 7, e51069. [Google Scholar] [CrossRef] [PubMed]
- Rodina, A.; Wang, T.; Yan, P.; Gomes, E.D.; Dunphy, M.P.S.; Pillarsetty, N.; Koren, J.; Gerecitano, J.F.; Taldone, T.; Zong, H.; et al. The epichaperome is an integrated chaperome network that facilitates tumour survival. Nature 2016, 538, 397–401. [Google Scholar] [CrossRef] [PubMed]
- Hajj, G.N.M.; Arantes, C.P.; Dias, M.V.S.; Roffé, M.; Costa-Silva, B.; Lopes, M.H.; Porto-Carreiro, I.; Rabachini, T.; Lima, F.R.; Beraldo, F.H.; et al. The unconventional secretion of stress-inducible protein 1 by a heterogeneous population of extracellular vesicles. Cell. Mol. Life Sci. 2013, 70, 3211–3227. [Google Scholar] [CrossRef] [PubMed]
- Dias, M.V.S.; Teixeira, B.L.; Rodrigues, B.R.; Sinigaglia-Coimbra, R.; Porto-Carreiro, I.; Roffé, M.; Hajj, G.N.M.; Martins, V.R. PRNP/prion protein regulates the secretion of exosomes modulating CAV1/caveolin-1-suppressed autophagy. Autophagy 2016, 12, 2113–2128. [Google Scholar] [CrossRef] [PubMed]
- Lopes, M.H.; Santos, T.G.; Rodrigues, B.R.; Queiroz-Hazarbassanov, N.; Cunha, I.W.; Wasilewska-Sampaio, A.P.; Costa-Silva, B.; Marchi, F.A.; Bleggi-Torres, L.F.; Sanematsu, P.I.; et al. Disruption of prion protein-HOP engagement impairs glioblastoma growth and cognitive decline and improves overall survival. Oncogene 2015, 34, 3305–3314. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Shinogle, H.E.; Garg, G.; Vielhauer, G.A.; Holzbeierlein, J.M.; Dobrowsky, R.T.; Blagg, B.S.J. HSP90 C-terminal inhibitors exhibit antimigratory activity by disrupting the HSP90α/Aha1 complex in PC3-MM2 cells. ACS Chem. Biol. 2015, 10, 577–590. [Google Scholar] [CrossRef] [PubMed]
- El Hamidieh, A.; Grammatikakis, N.; Patsavoudi, E. Cell surface Cdc37 participates in extracellular HSP90 mediated cancer cell invasion. PLoS ONE 2012, 7, e42722. [Google Scholar] [CrossRef] [PubMed]
- Tatebe, H.; Shiozaki, K. Identification of Cdc37 as a novel regulator of the stress-responsive mitogen-activated protein kinase. Mol. Cell. Biol. 2003, 23, 5132–5142. [Google Scholar] [CrossRef] [PubMed]
- Sõti, C.; Nagy, E.; Giricz, Z.; Vígh, L.; Csermely, P.; Ferdinandy, P. Heat shock proteins as emerging therapeutic targets. Br. J. Pharmacol. 2005, 146, 769–780. [Google Scholar] [CrossRef] [PubMed]
- Nahleh, Z.; Tfayli, A.; Najm, A.; El Sayed, A.; Nahle, Z. Heat shock proteins in cancer: Targeting the “chaperones”. Future Med. Chem. 2012, 4, 927–935. [Google Scholar] [CrossRef] [PubMed]
- Isaacs, J.S. HSP90 as a “Chaperone” of the epigenome: Insights and opportunities for cancer therapy. Adv. Cancer Res. 2016, 129, 107–140. [Google Scholar] [PubMed]
- Garg, G.; Khandelwal, A.; Blagg, B.S.J. Anticancer inhibitors of HSP90 function: Beyond the usual suspects. Adv. Cancer Res. 2016, 129, 51–88. [Google Scholar] [PubMed]
- Calderwood, S.K.; Gong, J. Heat shock proteins promote cancer: It’s a protection racket. Trends Biochem. Sci. 2016, 41, 311–323. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.; Lee, Y.S.; Kim, S.H.; Ko, J.K.; Kim, C.W. MHC independent anti-tumor immune responses induced by HSP70-enriched exosomes generate tumor regression in murine models. Cancer Lett. 2009, 275, 256–265. [Google Scholar] [CrossRef] [PubMed]
- Gastpar, R.; Gehrmann, M.; Bausero, M.A.; Asea, A.; Gross, C.; Schroeder, J.A.; Multhoff, G. Heat shock protein 70 surface-positive tumor exosomes stimulate migratory and cytolytic activity of natural killer cells. Cancer Res. 2005, 65, 5238–5247. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Guo, J.; Yang, M.; Zhu, X.; Cao, X. Chemokine-containing exosomes are released from heat-stressed tumor cells via lipid raft-dependent pathway and act as efficient tumor vaccine. J. Immunol. 2011, 186, 2219–2228. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.H.; Grossmann, M.E.; Young, C.Y.F. Forced expression of heat-shock protein 70 increases the secretion of HSP70 and provides protection against tumour growth. Br. J. Cancer 2004, 90, 926–931. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Wang, J.; Shao, C.; Liu, S.; Yu, Y.; Wang, Q.; Cao, X. Efficient induction of antitumor T cell immunity by exosomes derived from heat-shocked lymphoma cells. Eur. J. Immunol. 2006, 36, 1598–1607. [Google Scholar] [CrossRef] [PubMed]
- Shevtsov, M.; Multhoff, G. Heat shock protein-peptide and HSP-based immunotherapies for the treatment of cancer. Front. Immunol. 2016, 7, 171. [Google Scholar] [CrossRef] [PubMed]
- Castelli, C.; Rivoltini, L.; Rini, F.; Belli, F.; Testori, A.; Maio, M.; Mazzaferro, V.; Coppa, J.; Srivastava, P.K.; Parmiani, G. Heat shock proteins: Biological functions and clinical application as personalized vaccines for human cancer. Cancer Immunol. Immunother. 2004, 53, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Pilla, L.; Patuzzo, R.; Rivoltini, L.; Maio, M.; Pennacchioli, E.; Lamaj, E.; Maurichi, A.; Massarut, S.; Marchianò, A.; Santantonio, C.; et al. A phase II trial of vaccination with autologous, tumor-derived heat-shock protein peptide complexes Gp96, in combination with GM-CSF and interferon-α in metastatic melanoma patients. Cancer Immunol. Immunother. 2006, 55, 958–968. [Google Scholar] [CrossRef] [PubMed]
- Mazzaferro, V.; Coppa, J.; Carrabba, M.G.; Rivoltini, L.; Schiavo, M.; Regalia, E.; Mariani, L.; Camerini, T.; Marchianò, A.; Andreola, S.; et al. Vaccination with autologous tumor-derived heat-shock protein gp96 after liver resection for metastatic colorectal cancer. Clin. Cancer Res. 2003, 9, 3235–3245. [Google Scholar] [PubMed]
- Wood, C.; Srivastava, P.; Bukowski, R.; Lacombe, L.; Gorelov, A.I.; Gorelov, S.; Mulders, P.; Zielinski, H.; Hoos, A.; Teofilovici, F.; et al. An adjuvant autologous therapeutic vaccine (HSPPC-96; vitespen) versus observation alone for patients at high risk of recurrence after nephrectomy for renal cell carcinoma: A multicentre, open-label, randomised phase III trial. Lancet 2008, 372, 145–154. [Google Scholar] [CrossRef]
- Bloch, O.; Crane, C.A.; Fuks, Y.; Kaur, R.; Aghi, M.K.; Berger, M.S.; Butowski, N.A.; Chang, S.M.; Clarke, J.L.; McDermott, M.W.; et al. Heat-shock protein peptide complex-96 vaccination for recurrent glioblastoma: A phase II, single-arm trial. Neuro Oncol. 2014, 16, 274–279. [Google Scholar] [CrossRef] [PubMed]
- Janetzki, S.; Palla, D.; Rosenhauer, V.; Lochs, H.; Lewis, J.J.; Srivastava, P.K. Immunization of cancer patients with autologous cancer-derived heat shock protein gp96 preparations: A pilot study. Int. J. Cancer 2000, 88, 232–238. [Google Scholar] [CrossRef]
- Testori, A.; Richards, J.; Whitman, E.; Mann, G.B.; Lutzky, J.; Camacho, L.; Parmiani, G.; Tosti, G.; Kirkwood, J.M.; Hoos, A.; et al. Phase III comparison of vitespen, an autologous tumor-derived heat shock protein gp96 peptide complex vaccine, with physician’s choice of treatment for stage IV melanoma: The C-100–21 Study Group. J. Clin. Oncol. 2008, 26, 955–962. [Google Scholar] [CrossRef] [PubMed]
- Clinical Trials. Available online: https://clinicaltrials.gov/ (accessed on 1 February 2017).
- Krause, S.W.; Gastpar, R.; Andreesen, R.; Gross, C.; Ullrich, H.; Thonigs, G.; Pfister, K.; Multhoff, G. Treatment of colon and lung cancer patients with ex vivo heat shock protein 70-peptide-activated, autologous natural killer cells: A clinical phase I trial. Clin. Cancer Res. 2004, 10, 3699–3707. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Qiao, Y.; Liu, B.; Laska, E.J.; Chakravarthi, P.; Kulko, J.M.; Bona, R.D.; Fang, M.; Hegde, U.; Moyo, V.; et al. Combination of imatinib mesylate with autologous leukocyte-derived heat shock protein and chronic myelogenous leukemia. Clin. Cancer Res. 2005, 11, 4460–4468. [Google Scholar] [CrossRef] [PubMed]

| Family | HUGO Symbol | Synonyms | Intracellular Function (Gene Cards) | Extracellular Role |
|---|---|---|---|---|
| HSP70 | HYOU1 | HSP12A, Grp170 | Endoplasmic reticulum (ER)-associated protein involved in stress responses promoted by hypoxia | Mediates cross-presentation in macrophages [6]; enhances immunogenicity [7,8]; potentiates TLR9 activation [9] |
| HSPH1 | HSP110 | Prevents the aggregation of denatured proteins, inhibits HSPA8/HSC70 | Binds to scavenger receptors on macrophages and mediates cross-presentation [6]; affects macrophage polarization [10] | |
| HSPA8 | HSC71, HSP73, HSC70 | Facilitates peptide folding; ATPase in clathrin-coated vesicle disassembly | Inhibits cell proliferation [11]; dual role in inflammatory response [12,13] | |
| HSPA1A | HSP70, HSP72 | Stabilizes proteins and prevents aggregation; mediates protein folding; involved in the ubiquitin-proteasome pathway | Induces antitumor immune responses [12] | |
| HSPA5 | GRP78, BiP | Involved in the folding and assembly of proteins in the ER | Resistance to antiangiogenic agents [14] | |
| HSPA9 | GRP75 | Localized to the mitochondria, ER, and plasma membrane. Role in cell proliferation and stress response | Interacts to adhesion molecule podoplanin and regulates cell growth and metastasis in oral squamous cell carcinoma [15] | |
| Chaperonin | HSPD1 | HSP60 | Folding and assembly of newly imported proteins in the mitochondria | Tissue regeneration 15[16]; Modulates innate and adaptive immune system 16[17]; Induction of cytokine release [18] |
| HSPC | HSP90AA1 | HSP90, HSP90α | Promotes maturation and structural maintenance of target proteins involved in cell cycle control and signal transduction | Increased in cell mobility and cancer invasiveness; Increase cytokine production, STAT3 activation and MMP9 expression in prostate tumor [19]; Protection against hypoxia via LRP1 [20] |
| HSP90B1 | GRP94, GP96 | Molecular chaperone that functions in the processing and transport of secreted proteins | Antigen-presenting activity [21] | |
| DNAJ | DNAJB1 | HSP40 | Interacts with HSP70 and stimulates ATPase activity | Binds misfolded protein and inhibits protein aggregation, alleviating toxicity [22] |
| HSPB | HSPB1 | HSP27, Hsp25 | Involved in stress resistance and actin organization | Induces macrophage differentiation to M2 [23]; interacts with plasma membrane proteins, altering cell signaling [24] |
| HSPB6 | HSP20 | Heat shock protein that likely plays a role in smooth muscle relaxation | Induces proliferation, migration and tube formation in endothelial cells [25]; Regulator of platelets functions [26] | |
| CRYAB | HSPB5, αB-crystallin | Hold client proteins in large soluble aggregates; autokinase activity; participation in intracellular architecture | Increased levels associated with photoreceptor neurons death in age-related macular degeneration [27,28]; potential circulating biomarker to predict response to chemotherapy [29] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, T.G.; Martins, V.R.; Hajj, G.N.M. Unconventional Secretion of Heat Shock Proteins in Cancer. Int. J. Mol. Sci. 2017, 18, 946. https://doi.org/10.3390/ijms18050946
Santos TG, Martins VR, Hajj GNM. Unconventional Secretion of Heat Shock Proteins in Cancer. International Journal of Molecular Sciences. 2017; 18(5):946. https://doi.org/10.3390/ijms18050946
Chicago/Turabian StyleSantos, Tiago Góss, Vilma Regina Martins, and Glaucia Noeli Maroso Hajj. 2017. "Unconventional Secretion of Heat Shock Proteins in Cancer" International Journal of Molecular Sciences 18, no. 5: 946. https://doi.org/10.3390/ijms18050946
APA StyleSantos, T. G., Martins, V. R., & Hajj, G. N. M. (2017). Unconventional Secretion of Heat Shock Proteins in Cancer. International Journal of Molecular Sciences, 18(5), 946. https://doi.org/10.3390/ijms18050946