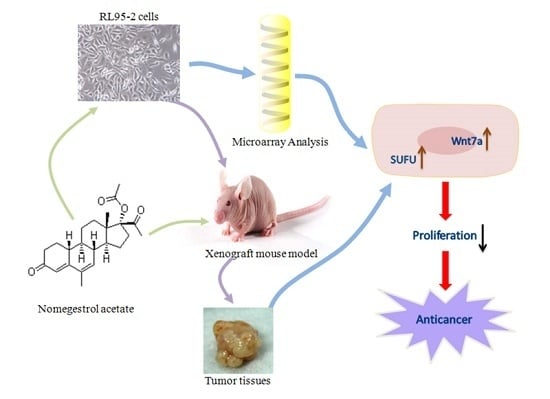
Nomegestrol Acetate Suppresses Human Endometrial Cancer RL95-2 Cells Proliferation In Vitro and In Vivo Possibly Related to Upregulating Expression of SUFU and Wnt7a
Abstract
:1. Introduction
2. Results
2.1. NOMAC Inhibited Cell Viability in a Dose-Dependent Manner
2.2. Effect of NOMAC on Cell Proliferation of RL95-2 and KLE
2.3. Effect of NOMAC on cDNA Microarray
2.4. Effects of NOMAC on the mRNA Levels of SUFU and Wnt7a in RL95-2 and KLE Cells
2.5. Effects of NOMAC on the Protein Levels of SUFU and Wnt7a in RL95-2 and KLE Cells
2.6. NOMAC Inhibits Tumor Growth In Vivo
2.7. NOMAC Suppresses the Expression of SUFU and Wnt7a in Tumor Tissues
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Cell Culture
4.3. Cell Viability Assay
4.4. EdU Cell Proliferation Assay
4.5. Microarray Analysis
4.6. RT-qPCR Analysis
4.7. Western Blot Analysis
4.8. Xenograft Model and Treatment
4.9. Hematoxylin and Eosin Staining
4.10. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- De Boer, S.M.; Powell, M.E.; Mileshkin, L.; Katsaros, D.; Bessette, P.; Haie-Meder, C.; Ottevanger, P.B.; Ledermann, J.A.; Khaw, P.; Colombo, A.; et al. Toxicity and quality of life after adjuvant chemoradiotherapy versus radiotherapy alone for women with high-risk endometrial cancer (PORTEC-3): An open-label, multicentre, randomised, phase 3 trial. Lancet Oncol. 2016, 17, 1114–1126. [Google Scholar] [CrossRef]
- Vanderstichele, A.; Neven, P.; Vergote, I. Combined modality adjuvant therapy for high-risk endometrial cancer. Lancet Oncol. 2016, 17, 1029–1030. [Google Scholar] [CrossRef]
- Rauh-Hain, J.A.; del Carmen, M.G. Treatment for advanced and recurrent endometrial carcinoma: Combined modalities. Oncologist 2010, 15, 852–861. [Google Scholar] [CrossRef] [PubMed]
- Morice, P.; Leary, A.; Creutzberg, C.; Abu-Rustum, N.; Darai, E. Endometrial cancer. Lancet 2016, 387, 1094–1108. [Google Scholar] [CrossRef]
- Baek, J.S.; Lee, W.H.; Kang, W.D.; Kim, S.M. Fertility-preserving treatment in complex atypical hyperplasia and early endometrial cancer in young women with oral progestin: Is it effective? Obstet. Gynecol. Sci. 2016, 59, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Xiao, M.; Bai, H.; Zhang, Z. Sexual function and quality of life among patients with endometrial cancer after surgery. Int. J. Gynecol. Cancer 2017, 27, 608–612. [Google Scholar] [CrossRef] [PubMed]
- Davies, C.; Pan, H.; Godwin, J.; Gray, R.; Arriagada, R.; Raina, V.; Abraham, M.; Medeiros, A.V.; Badran, A.; Bonfill, X.; et al. Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet 2013, 381, 805–816. [Google Scholar] [CrossRef]
- Burki, T.K. New risk loci for endometrial cancer identified. Lancet Oncol. 2016, 17, e229. [Google Scholar] [CrossRef]
- Hahn, H.S.; Yoon, S.G.; Hong, J.S.; Hong, S.R.; Park, S.J.; Lim, J.Y.; Kwon, Y.S.; Lee, I.H.; Lim, K.T.; Lee, K.H.; et al. Conservative treatment with progestin and pregnancy outcomes in endometrial cancer. Int. J. Gynecol. Cancer 2009, 19, 1068–1073. [Google Scholar] [CrossRef] [PubMed]
- Arteaga, G.A.; Castellanos, B.G.; Marquez, A.G. Conservative treatment of endometrial cancer as a way to preserve fertility. Five-year experience at Instituto Nacional de Perinatilogia Isidro Espinoza de los Reyes. Ginecol. Obstet. Mex. 2012, 80, 394–399. [Google Scholar]
- Kim, M.K.; Seong, S.J.; Kim, Y.S.; Song, T.; Kim, M.L.; Yoon, B.S.; Jun, H.S.; Lee, Y.H. Combined medroxyprogesterone acetate/levonorgestrel-intrauterine system treatment in young women with early-stage endometrial cancer. Am. J. Obstet. Gynecol. 2013, 209, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Nishio, S.; Koyanagi, T.; Miyabe, K.; Kuromatsu, H. Two cases of multidrug-resistant recurrent endometrial cancer successfully treated with medroxyprogesterone acetate (MPA). Gan Kagaku Ryoho Cancer Chem. 2010, 37, 735–738. [Google Scholar]
- Saito, F.; Tashiro, H.; Yamaguchi, M.; Honda, R.; Ohba, T.; Suzuki, A.; Katabuchi, H. Development of a mouse model for testing therapeutic agents: The anticancer effect of dienogest on endometrial neoplasms. Gynecol. Endocrinol. 2016, 32, 403–407. [Google Scholar] [CrossRef] [PubMed]
- Asi, N.; Mohammed, K.; Haydour, Q.; Gionfriddo, M.R.; Vargas, O.L.M.; Prokop, L.J.; Faubion, S.S.; Murad, M.H. Progesterone vs. synthetic progestins and the risk of breast cancer: A systematic review and meta-analysis. Syst. Rev. 2016, 5, 121. [Google Scholar] [CrossRef] [PubMed]
- Christin-Maitre, S.; Laroche, E.; Bricaire, L. A new contraceptive pill containing 17β-estradiol and nomegestrol acetate. Womens Health 2013, 9, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Sun, X.; Wang, J.; Wang, Y. Mechanism of thioridazine plus medroxyprogesterone in the treatment of endometrial cancer. Zhonghua Yi Xue Za Zhi 2015, 95, 1540–1543. (In Chinese) [Google Scholar] [PubMed]
- Shields-Botella, J.; Chetrite, G.; Meschi, S.; Pasqualini, J.R. Effect of nomegestrol acetate on estrogen biosynthesis and transformation in MCF-7 and T47-D breast cancer cells. J. Steroid Biochem. Mol. Biol. 2005, 93, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Ruan, X.; Schneck, H.; Schultz, S.; Fehm, T.; Cahill, M.A.; Seeger, H.; Chen, R.; Yu, Q.; Mueck, A.O.; Neubauer, H. Nomegestrol acetate sequentially or continuously combined to estradiol did not negatively affect membrane-receptor associated progestogenic effects in human breast cancer cells. Gynecol. Endocrinol. 2012, 28, 863–866. [Google Scholar] [CrossRef] [PubMed]
- Del, P.L.; Berretta, M.; Di Francia, R.; Cavaliere, C.; Di Napoli, M.; Facchini, G.; Fiorica, F.; Mileto, M.; Schindler, A.E. Nomegestrol acetate/estradiol hormonal oral contraceptive and breast cancer risk. Anticancer Drugs 2014, 25, 745–750. [Google Scholar]
- Zhang, J.; Zhu, Y.; Zhou, X.; Hao, S.; Xie, S.; Zhou, J.; Guo, X.; Li, Z.; Huang, Y.; Chen, Q. Evaluation of biodegradable microspheres containing nomegestrol acetate in a rat model of endometriosis. Eur. J. Pharm. Sci. 2014, 65, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Andre, G. Actions of a 19-norprogesterone derivative on mammary gland: Nomegestrol acetate. J. Gynecol. Obstet. Biol. Reprod 2005, 34, 69–84. [Google Scholar] [CrossRef]
- Skrzypczak, M.; Merx, I.; Schuler-Toprak, S.; Weber, F.; Inwald, E.C.; Ortmann, O.; Treeck, O. Molecular profiling of estrogen receptor α and progesterone receptor transcript variants in endometrial cancer. Steroids 2015, 104, 122–128. [Google Scholar] [CrossRef] [PubMed]
- De Leo, V.; Musacchio, M.C.; Cappelli, V.; Piomboni, P.; Morgante, G. Hormonal contraceptives: Pharmacology tailored to women’s health. Hum. Reprod Update 2016, 22, 634–646. [Google Scholar] [CrossRef] [PubMed]
- Mansour, D.; Westhoff, C.; Kher, U.; Korver, T. Pooled analysis of two randomized, open-label studies comparing the effects of nomegestrol acetate/17β-estradiol and drospirenone/ethinyl estradiol on bleeding patterns in healthy women. Contraception 2016, 95, 390–397. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Chen, X.; Zhu, Y.; Cao, L.; Riviere, J.E. Pharmacokinetics, tissue distribution, and excretion of nomegestrol acetate in female rats. Eur. J. Drug Metab. Pharmacokinet. 2015, 40, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Stone, D.M.; Murone, M.; Luoh, S.; Ye, W.; Armanini, M.P.; Gurney, A.; Phillips, H.; Brush, J.; Goddard, A.; De Sauvage, F.J.; et al. Characterization of the human suppressor of fused, a negative regulator of the zinc-finger transcription factor Gli. J. Cell Sci. 1999, 112, 4437–4448. [Google Scholar] [PubMed]
- Kim, K.H.; Kim, J.M.; Choi, Y.L.; Shin, Y.K.; Lee, H.C.; Seong, I.O.; Kim, B.K.; Chae, S.W.; Chung, Y.S.; Kim, S.H. Expression of sonic hedgehog signaling molecules in normal, hyperplastic and carcinomatous endometrium. Pathol. Int. 2009, 59, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.; Zhang, X.; Wang, Y.; Li, L.; Wang, Q.; Zheng, J. Expression and prognostic significance of Wnt7a in human endometrial carcinoma. Obstet. Gynecol. Int. 2012, 2012, 134962. [Google Scholar] [CrossRef] [PubMed]
- Ingaramo, P.I.; Milesi, M.M.; Schimpf, M.G.; Ramos, J.G.; Vigezzi, L.; Munoz-de-Toro, M.; Luque, E.H.; Varayoud, J. Endosulfan affects uterine development and functional differentiation by disrupting Wnt7a and β-catenin expression in rats. Mol. Cell Endocrinol. 2016, 425, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Meng, F.; Xu, Y.; Yang, S.; Xiao, M.; Chen, X.; Lou, G. Overexpression of Wnt7a is associated with tumor progression and unfavorable prognosis in endometrial cancer. Int. J. Gynecol. Cancer 2013, 23, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Mottier-Pavie, V.I.; Palacios, V.; Eliazer, S.; Scoggin, S.; Buszczak, M. The Wnt pathway limits BMP signaling outside of the germline stem cell niche in Drosophila ovaries. Dev. Biol. 2016, 417, 50–62. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Krieg, S.; Hwang, J.Y.; Dhal, S.; Kuo, C.J.; Lasley, B.L.; Brenner, R.M.; Nayak, N.R. Dynamic regulation of Wnt7a expression in the primate endometrium: Implications for postmenstrual regeneration and secretory transformation. Endocrinology 2012, 153, 1063–1069. [Google Scholar] [CrossRef] [PubMed]
- Oehler, M.K.; MacKenzie, I.Z.; Wallwiener, D.; Bicknell, R.; Rees, M.C. Wnt-7a is upregulated by norethisterone in human endometrial epithelial cells: A possible mechanism by which progestogens reduce the risk of estrogen-induced endometrial neoplasia. Cancer Lett. 2002, 186, 75–81. [Google Scholar] [CrossRef]
- Richardson, G.S.; Dickersin, G.R.; Atkins, L.; MacLaughlin, D.T.; Raam, S.; Merk, L.P.; Bradley, F.M. KLE: A cell line with defective estrogen receptor derived from undifferentiated endometrial cancer. Gynecol. Oncol. 1984, 17, 213–230. [Google Scholar] [CrossRef]
- Way, D.L.; Grosso, D.S.; Davis, J.R.; Surwit, E.A.; Christian, C.D. Characterization of a new human endometrial carcinoma (RL95–2) established in tissue culture. In Vitro 1983, 19, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Tong, H.; Ke, J.Q.; Jiang, F.Z.; Wang, X.J.; Wang, F.Y.; Li, Y.R.; Lu, W.; Wan, X.P. Tumor-associated macrophage-derived CXCL8 could induce ERα suppression via HOXB13 in endometrial cancer. Cancer Lett. 2016, 376, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Francesca, F.; Giuseppe, L.; Simona, L.; Marilena, D.N.; Vincenza, G.; Stefano, G. Fertility preserving treatment with hysteroscopic resection followed by progestin therapy in young women with early endometrial cancer. J. Gynecol. Oncol. 2017, 28, e2. [Google Scholar]
- Guy, M.S.; Qamar, L.; Behbakht, K.; Post, M.D.; Sheeder, J.; Sartorius, C.A.; Spillman, M.A. Progestin treatment decreases CD133+ cancer stem cell populations in endometrial cancer. Gynecol. Oncol. 2016, 140, 518–526. [Google Scholar] [CrossRef] [PubMed]
- Gonthier, C.; Trefoux-Bourdet, A.; Luton, D.; Koskas, M. Fertility-sparing management of endometrial cancer and atypical hyperplasia. Gynecol. Obstet. Fertil. Senol. 2017, 45, 112–118. [Google Scholar] [PubMed]
- Ramos-Solano, M.; Meza-Canales, I.D.; Torres-Reyes, L.A.; Alvarez-Zavala, M.; Alvarado-Ruiz, L.; Rincon-Orozco, B.; Garcia-Chagollan, M.; Ochoa-Hernandez, A.B.; Ortiz-Lazareno, P.C.; Rosl, F.; et al. Expression of WNT genes in cervical cancer-derived cells: Implication of Wnt7a in cell proliferation and migration. Exp. Cell Res. 2015, 335, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, K.; Utsunomiya, H.; Miki, Y.; Hanihara, M.; Fue, M.; Takagi, K.; Nishimoto, M.; Suzuki, F.; Yaegashi, N.; Suzuki, T.; et al. Retinoic acid receptor β: A potential therapeutic target in retinoic acid treatment of endometrial cancer. Int. J. Gynecol. Cancer 2017, 27, 643–650. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Xie, S.W.; Zhang, J.F.; Zhang, T.T.; Zhou, J.Y.; Cao, Y.; Cao, L. Involvement of Bcl-2, Src, and ERα in gossypol-mediated growth inhibition and apoptosis in human uterine leiomyoma and myometrial cells. Acta Pharmacol. Sin. 2010, 31, 1593–1603. [Google Scholar] [CrossRef] [PubMed]
- Warren, M.; Puskarczyk, K.; Chapman, S.C. Chick embryo proliferation studies using EdU labeling. Dev. Dyn. 2009, 238, 944–949. [Google Scholar] [CrossRef] [PubMed]
- Isci, B.E.; Ugras, D.A.; Girgin, G.; Gungor, T.; Baydar, T.; Nuri, D.A. A new diagnostic and prognostic marker in endometrial cancer: Neopterin. Int. J. Gynecol. Cancer 2017, 27, 754–758. [Google Scholar] [CrossRef] [PubMed]









| Cell Lines | Drugs | IC50 (95% CI) µmol/L | ||
|---|---|---|---|---|
| 24 h | 48 h | 72 h | ||
| RL95-2 | NOMAC | 52.80 (35.85–77.77) | 19.88 (12.01–32.91) | 21.62 (12.62–36.17) |
| MPA | >100 (--) | >100 (--) | >100 (--) | |
| KLE | NOMAC | >100 (--) | >100 (--) | >100 (--) |
| MPA | >100 (--) | >100 (--) | >100 (--) | |
| Gene ID | Gene Symbol | p-Value | Fold Change | Regulation |
|---|---|---|---|---|
| NM_019851 | FGF20 | 0.015 | 5.937 | Up |
| NM_004625 | Wnt7a | 0.017 | 3.086 | Up |
| NM_016169 | SUFU | 0.023 | 2.738 | Up |
| NM_005270 | GLI2 | 0.024 | 2.072 | Up |
| NM_001719 | BMP7 | 0.028 | 4.676 | Down |
| NM_003810 | TNFSF10 | 0.030 | 3.278 | Down |
| NM_003999 | OSMR | 0.026 | 3.218 | Down |
| NM_201282 | EGFR | 0.014 | 2.307 | Down |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, A.-y.; Xie, S.-w.; Zhou, J.-y.; Zhu, Y. Nomegestrol Acetate Suppresses Human Endometrial Cancer RL95-2 Cells Proliferation In Vitro and In Vivo Possibly Related to Upregulating Expression of SUFU and Wnt7a. Int. J. Mol. Sci. 2017, 18, 1337. https://doi.org/10.3390/ijms18071337
Ma A-y, Xie S-w, Zhou J-y, Zhu Y. Nomegestrol Acetate Suppresses Human Endometrial Cancer RL95-2 Cells Proliferation In Vitro and In Vivo Possibly Related to Upregulating Expression of SUFU and Wnt7a. International Journal of Molecular Sciences. 2017; 18(7):1337. https://doi.org/10.3390/ijms18071337
Chicago/Turabian StyleMa, A-ying, Shu-wu Xie, Jie-yun Zhou, and Yan Zhu. 2017. "Nomegestrol Acetate Suppresses Human Endometrial Cancer RL95-2 Cells Proliferation In Vitro and In Vivo Possibly Related to Upregulating Expression of SUFU and Wnt7a" International Journal of Molecular Sciences 18, no. 7: 1337. https://doi.org/10.3390/ijms18071337
APA StyleMa, A. -y., Xie, S. -w., Zhou, J. -y., & Zhu, Y. (2017). Nomegestrol Acetate Suppresses Human Endometrial Cancer RL95-2 Cells Proliferation In Vitro and In Vivo Possibly Related to Upregulating Expression of SUFU and Wnt7a. International Journal of Molecular Sciences, 18(7), 1337. https://doi.org/10.3390/ijms18071337