Fluorogenic Labeling Strategies for Biological Imaging
Abstract
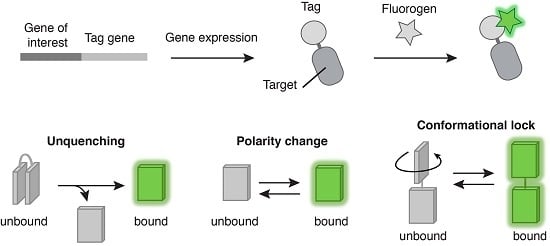
:1. Introduction
2. Covalent Versus Non-Covalent Labeling
3. Covalent Fluorogenic Labeling
4. Non-Covalent Fluorogenic Labeling
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Klymchenko, A.S. Solvatochromic and fluorogenic dyes as environment-sensitive probes: Design and biological applications. Acc. Chem. Res. 2017, 50, 366–375. [Google Scholar] [CrossRef] [PubMed]
- Bruchez, M.P. Dark dyes-bright complexes: Fluorogenic protein labeling. Curr. Opin. Chem. Biol. 2015, 27, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Jullien, L.; Gautier, A. Fluorogen-based reporters for fluorescence imaging: A review. Methods Appl. Fluoresc. 2015, 3, 042007. [Google Scholar] [CrossRef]
- O’Hare, H.; Johnsson, K.; Gautier, A. Chemical probes shed light on protein function. Curr. Opin. Struct. Biol. 2007, 17, 488–494. [Google Scholar] [CrossRef] [PubMed]
- Hinner, M.J.; Johnsson, K. How to obtain labeled proteins and what to do with them. Curr. Opin. Biotechnol. 2010, 21, 766–776. [Google Scholar] [CrossRef] [PubMed]
- Griffin, B.; Adams, S.; Tsien, R. Specific covalent labeling of recombinant protein molecules inside live cells. Science 1998, 281, 269–272. [Google Scholar] [CrossRef] [PubMed]
- Andresen, M.; Schmitz-Salue, R.; Jakobs, S. Short tetracysteine tags to beta-tubulin demonstrate the significance of small labels for live cell imaging. Mol. Biol. Cell 2004, 15, 5616–5622. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, C.; Gaietta, G.; Bunemann, M.; Adams, S.; Oberdorff-Maass, S.; Behr, B.; Vilardaga, J.; Tsien, R.; Eisman, M.; Lohse, M. A FlAsH-based FRET approach to determine G protein - coupled receptor activation in living cells. Nat. Meth. 2005, 2, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Dyachok, O.; Isakov, Y.; Sagetorp, J.; Tengholm, A. Oscillations of cyclic AMP in hormone-stimulated insulin-secreting beta-cells. Nature 2006, 439, 349–352. [Google Scholar] [CrossRef] [PubMed]
- Martin, B.; Giepmans, B.; Adams, S.; Tsien, R. Mammalian cell-based optimization of the biarsenical-binding tetracysteine motif for improved fluorescence and affinity. Nat. Biotechnol. 2005, 23, 1308–1314. [Google Scholar] [CrossRef] [PubMed]
- Keppler, A.; Gendreizig, S.; Gronemeyer, T.; Pick, H.; Vogel, H.; Johnsson, K. A general method for the covalent labeling of fusion proteins with small molecules in vivo. Nat. Biotechnol. 2003, 21, 86–89. [Google Scholar] [CrossRef] [PubMed]
- Keppler, A.; Pick, H.; Arrivoli, C.; Vogel, H.; Johnsson, K. Labeling of fusion proteins with synthetic fluorophores in live cells. Proc. Natl. Acad. Sci. USA 2004, 101, 9955–9959. [Google Scholar] [CrossRef] [PubMed]
- Juillerat, A.; Gronemeyer, T.; Keppler, A.; Gendreizig, S.; Pick, H.; Vogel, H.; Johnsson, K. Directed evolution of O6-alkylguanine-DNA alkyltransferase for efficient labeling of fusion proteins with small molecules in vivo. Chem. Biol. 2003, 10, 313–317. [Google Scholar] [CrossRef]
- Juillerat, A.; Heinis, C.; Sielaff, I.; Barnikow, J.; Jaccard, H.; Kunz, B.; Terskikh, A.; Johnsson, K. Engineering substrate specificity of O6-alkylguanine-DNA alkyltransferase for specific protein labeling in living cells. ChemBioChem 2005, 6, 1263–1269. [Google Scholar] [CrossRef] [PubMed]
- Gronemeyer, T.; Chidley, C.; Juillerat, A.; Heinis, C.; Johnsson, K. Directed evolution of O6-alkylguanine-DNA alkyltransferase for applications in protein labeling. Protein Eng. Des. Sel. 2006, 19, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Gautier, A.; Juillerat, A.; Heinis, C.; Corrêa, I.R., Jr.; Kindermann, M.; Beaufils, F.; Johnsson, K. An engineered protein tag for multiprotein labeling in living cells. Chem. Biol. 2008, 15, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Los, G.; Encell, L.; McDougall, M.; Hartzell, D.; Karassina, N.; Zimprich, C.; Wood, M.; Learish, R.; Ohana, R.; Urh, M.; et al. HaloTag: A novel protein labeling technology for cell imaging and protein analysis. ACS Chem. Biol. 2008, 3, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, T.; Johnsson, K.; Okuno, H.; Bito, H.; Inoue, T.; Nagano, T.; Urano, Y. Real-time measurements of protein dynamics using fluorescence activation-coupled protein labeling method. J. Am. Chem. Soc. 2011, 133, 6745–6751. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Zhang, A.; Baker, B.; Sun, L.; Howard, A.; Buswell, J.; Maurel, D.; Masharina, A.; Johnsson, K.; Noren, C.J.; et al. Development of SNAP-tag fluorogenic probes for wash-free fluorescence imaging. ChemBioChem 2011, 12, 2217–2226. [Google Scholar] [CrossRef] [PubMed]
- Jing, C.; Cornish, V.W. A fluorogenic TMP-tag for high signal-to-background intracellular live cell imaging. ACS Chem. Biol. 2013, 8, 1704–1712. [Google Scholar] [CrossRef] [PubMed]
- Lukinavicius, G.; Umezawa, K.; Olivier, N.; Honigmann, A.; Yang, G.; Plass, T.; Mueller, V.; Reymond, L.; Corrêa, I.R., Jr.; Luo, Z.-G.; et al. A near-infrared fluorophore for live-cell super-resolution microscopy of cellular proteins. Nat. Chem. 2013, 5, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Grimm, J.B.; English, B.P.; Chen, J.; Slaughter, J.P.; Zhang, Z.; Revyakin, A.; Patel, R.; Macklin, J.J.; Normanno, D.; Singer, R.H.; et al. A general method to improve fluorophores for live-cell and single-molecule microscopy. Nat. Meth. 2015, 12, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Lukinavicius, G.; Reymond, L.; D'Este, E.; Masharina, A.; Göttfert, F.; Ta, H.; Güther, A.; Fournier, M.; Rizzo, S.; Waldmann, H.; et al. Fluorogenic probes for live-cell imaging of the cytoskeleton. Nat. Meth. 2014, 11, 731–733. [Google Scholar] [CrossRef] [PubMed]
- Lukinavicius, G.; Reymond, L.; Umezawa, K.; Sallin, O.; D'Este, E.; Göttfert, F.; Ta, H.; Hell, S.W.; Urano, Y.; Johnsson, K. Fluorogenic probes for multicolor imaging in living cells. J. Am. Chem. Soc. 2016, 138, 9365–9368. [Google Scholar] [CrossRef] [PubMed]
- Prifti, E.; Reymond, L.; Umebayashi, M.; Hovius, R.; Riezman, H.; Johnsson, K. A fluorogenic probe for SNAP-tagged plasma membrane proteins based on the solvatochromic molecule nile red. ACS Chem. Biol. 2014, 9, 606–612. [Google Scholar] [CrossRef] [PubMed]
- Hori, Y.; Ueno, H.; Mizukami, S.; Kikuchi, K. Photoactive yellow protein-based protein labeling system with turn-on fluorescence intensity. J. Am. Chem. Soc. 2009, 131, 16610–16611. [Google Scholar] [CrossRef] [PubMed]
- Hori, Y.; Nakaki, K.; Sato, M.; Mizukami, S.; Kikuchi, K. Development of protein-labeling probes with a redesigned fluorogenic switch based on intramolecular association for no-wash live-cell imaging. Angew. Chem. Int. Ed. 2012, 51, 5611–5614. [Google Scholar] [CrossRef] [PubMed]
- Hori, Y.; Norinobu, T.; Sato, M.; Arita, K.; Shirakawa, M.; Kikuchi, K. Development of fluorogenic probes for quick no-wash live-cell imaging of intracellular proteins. J. Am. Chem. Soc. 2013, 135, 12360–12365. [Google Scholar] [CrossRef] [PubMed]
- Hori, Y.; Hirayama, S.; Sato, M.; Kikuchi, K. Redesign of a fluorogenic labeling system to improve surface charge, brightness, and binding kinetics for imaging the functional localization of bromodomains. Angew. Chem. Int. Ed. 2015, 127, 14576–14579. [Google Scholar] [CrossRef]
- Kamikawa, Y.; Hori, Y.; Yamashita, K.; Jin, L.; Hirayama, S.; Standley, D.M.; Kikuchi, K. Design of a protein tag and fluorogenic probe with modular structure for live-cell imaging of intracellular proteins. Chem. Sci. 2016, 7, 308–314. [Google Scholar] [CrossRef]
- Hirayama, S.; Hori, Y.; Benedek, Z.; Suzuki, T.; Kikuchi, K. Fluorogenic probes reveal a role of GLUT4 N-glycosylation in intracellular trafficking. Nat. Chem. Biol. 2016, 12, 853–859. [Google Scholar] [CrossRef] [PubMed]
- Yapici, I.; Lee, K.S.S.; Berbasova, T.; Nosrati, M.; Jia, X.; Vasileiou, C.; Wang, W.; Santos, E.M.; Geiger, J.H.; Borhan, B. “Turn-on” protein fluorescence: in situ formation of cyanine dyes. J. Am. Chem. Soc. 2015, 137, 1073–1080. [Google Scholar] [CrossRef] [PubMed]
- Herwig, L.; Rice, A.J.; Bedbrook, C.N.; Zhang, R.K.; Lignell, A.; Cahn, J.K.B.; Renata, H.; Dodani, S.C.; Cho, I.; Cai, L.; et al. Directed evolution of a bright near-infrared fluorescent rhodopsin using a synthetic chromophore. Cell Chem. Biol. 2017, 24, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Iwaki, T.; Torigoe, C.; Noji, M.; Nakanishi, M. Antibodies for fluorescent molecular rotors. Biochemistry 1993, 32, 7589–7592. [Google Scholar] [CrossRef] [PubMed]
- Simeonov, A.; Matsushita, M.; Juban, E.A.; Thompson, E.H.; Hoffman, T.Z.; Beuscher, A.E.; Taylor, M.J.; Wirsching, P.; Rettig, W.; McCusker, J.K.; et al. Blue-fluorescent antibodies. Science 2000, 290, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Debler, E.W.; Kaufmann, G.F.; Meijler, M.M.; Heine, A. Deeply inverted electron-hole recombination in a luminescent antibody-stilbene complex. Science 2008, 319, 1232–1235. [Google Scholar] [CrossRef] [PubMed]
- Szent-Gyorgyi, C.; Schmidt, B.A.; Creeger, Y.; Fisher, G.W.; Zakel, K.L.; Adler, S.; Fitzpatrick, J.A.J.; Woolford, C.A.; Yan, Q.; Vasilev, K.V.; et al. Fluorogen-activating single-chain antibodies for imaging cell surface proteins. Nat. Biotechnol. 2008, 26, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Yates, B.P.; Peck, M.A.; Berget, P.B. Directed evolution of a fluorogen-activating single chain antibody for function and enhanced brightness in the cytoplasm. Mol. Biotechnol. 2012, 54, 829–841. [Google Scholar] [CrossRef] [PubMed]
- Telmer, C.A.; Verma, R.; Teng, H.; Andreko, S.; Law, L.; Bruchez, M.P. Rapid, specific, no-wash, far-red fluorogen activation in subcellular compartments by targeted fluorogen activating proteins. ACS Chem. Biol. 2015, 10, 1239–1246. [Google Scholar] [CrossRef] [PubMed]
- Ozhalici-Unal, H.; Pow, C.L.; Marks, S.A.; Jesper, L.D.; Silva, G.L.; Shank, N.I.; Jones, E.W.; Burnette, J.M.; Berget, P.B.; Armitage, B.A. A rainbow of fluoromodules: A promiscuous scFv protein binds to and activates a diverse set of fluorogenic cyanine dyes. J. Am. Chem. Soc. 2008, 130, 12620–12621. [Google Scholar] [PubMed]
- Zanotti, K.J.; Silva, G.L.; Creeger, Y.; Robertson, K.L.; Waggoner, A.S.; Berget, P.B.; Armitage, B.A. Blue fluorescent dye-protein complexes based on fluorogenic cyanine dyes and single chain antibody fragments. Org. Biomol. Chem. 2011, 9, 1012–1020. [Google Scholar] [CrossRef] [PubMed]
- Holleran, J.; Brown, D.; Fuhrman, M.H.; Adler, S.A.; Fisher, G.W.; Jarvik, J.W. Fluorogen-activating proteins as biosensors of cell-surface proteins in living cells. Cytometry A 2010, 77, 776–782. [Google Scholar] [CrossRef] [PubMed]
- Fisher, G.W.; Adler, S.A.; Fuhrman, M.H.; Waggoner, A.S.; Bruchez, M.P.; Jarvik, J.W. Detection and quantification of 2AR internalization in living cells using FAP-based biosensor technology. J. Biomol. Screen. 2010, 15, 703–709. [Google Scholar] [CrossRef] [PubMed]
- Yan, Q.; Schmidt, B.F.; Perkins, L.A.; Naganbabu, M.; Saurabh, S.; Andreko, S.K.; Bruchez, M.P. Near-instant surface-selective fluorogenic protein quantification using sulfonated triarylmethane dyes and fluorogen activating proteins. Org. Biomol. Chem. 2015, 13, 2078–2086. [Google Scholar] [CrossRef] [PubMed]
- Holleran, J.P.; Glover, M.L.; Peters, K.W.; Bertrand, C.A.; Watkins, S.C.; Jarvik, J.W.; Frizzell, R.A. Pharmacological rescue of the mutant cystic fibrosis transmembrane conductance regulator (CFTR) detected by use of a novel fluorescence platform. Mol. Med. 2012, 18, 685–696. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Tapia, P.H.; Fisher, G.W.; Waggoner, A.S.; Jarvik, J.; Sklar, L.A. High-throughput flow cytometry compatible biosensor based on fluorogen activating protein technology. Cytometry A 2013, 83, 220–226. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Tapia, P.H.; Fisher, G.W.; Simons, P.C.; Strouse, J.J.; Foutz, T.; Waggoner, A.S.; Jarvik, J.; Sklar, L.A. Discovery of regulators of receptor internalization with high-throughput flow cytometry. Mol. Pharmacol. 2012, 82, 645–657. [Google Scholar] [CrossRef] [PubMed]
- Fisher, G.W.; Fuhrman, M.H.; Adler, S.A.; Szent-Gyorgyi, C.; Waggoner, A.S.; Jarvik, J.W. Self-checking cell-based assays for GPCR desensitization and resensitization. J. Biomol. Screen. 2014, 19, 1220–1226. [Google Scholar] [CrossRef] [PubMed]
- Snyder, J.C.; Pack, T.F.; Rochelle, L.K.; Chakraborty, S.K.; Zhang, M.; Eaton, A.W.; Bai, Y.; Ernst, L.A.; Barak, L.S.; Waggoner, A.S.; et al. A rapid and affordable screening platform for membrane protein trafficking. BMC Biol. 2015, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Pratt, C.P.; He, J.; Wang, Y.; Barth, A.L.; Bruchez, M.P. Fluorogenic green-inside red-outside (GIRO) labeling approach reveals adenylyl cyclase-dependent control of BKα surface expression. Bioconjug Chem. 2015, 26, 1963–1971. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, J.A.J.; Yan, Q.; Sieber, J.J.; Dyba, M.; Schwarz, U.; Szent-Gyorgyi, C.; Woolford, C.A.; Berget, P.B.; Waggoner, A.S.; Bruchez, M.P. STED nanoscopy in living cells using fluorogen activating proteins. Bioconjug Chem. 2009, 20, 1843–1847. [Google Scholar] [CrossRef] [PubMed]
- Saurabh, S.; Perez, A.M.; Comerci, C.J.; Shapiro, L.; Moerner, W.E. Super-resolution imaging of live bacteria cells using a genetically directed, highly photostable fluoromodule. J. Am. Chem. Soc. 2016, 138, 10398–10401. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, S.L.; Yan, Q.; Telmer, C.A.; Lidke, K.A.; Bruchez, M.P.; Lidke, D.S. Fluorogen-activating proteins provide tunable labeling densities for tracking FcεRI independent of IgE. ACS Chem. Biol. 2014, 2, 539–546. [Google Scholar] [CrossRef] [PubMed]
- Yan, Q.; Schwartz, S.L.; Maji, S.; Huang, F.; Szent-Gyorgyi, C.; Lidke, D.S.; Lidke, K.A.; Bruchez, M.P. Localization microscopy using noncovalent fluorogen activation by genetically encoded fluorogen-activating proteins. Chem. Phys. Chem. 2014, 15, 687–695. [Google Scholar] [CrossRef] [PubMed]
- Plamont, M.-A.; Billon-Denis, E.; Maurin, S.; Gauron, C.; Pimenta, F.M.; Specht, C.G.; Shi, J.; Querard, J.; Pan, B.; Rossignol, J.; et al. Small fluorescence-activating and absorption-shifting tag for tunable protein imaging in vivo. Proc. Natl. Acad. Sci. USA 2016, 113, 497–502. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Plamont, M.-A.; Sladitschek, H.L.; Rodrigues, V.; Aujard, I.; Neveu, P.; Le Saux, T.; Jullien, L.; Gautier, A. Dynamic multi-color protein labeling in living cells. Chem. Sci. 2017. [Google Scholar] [CrossRef]
- Babendure, J.R.; Adams, S.R.; Tsien, R.Y. Aptamers switch on fluorescence of triphenylmethane dyes. J. Am. Chem. Soc. 2003, 125, 14716–14717. [Google Scholar] [CrossRef] [PubMed]
- Stojanovic, M.N.; Kolpashchikov, D.M. Modular aptameric sensors. J. Am. Chem. Soc. 2004, 126, 9266–9270. [Google Scholar] [CrossRef] [PubMed]
- Sparano, B.A.; Koide, K. A strategy for the development of small-molecule-based sensors that strongly fluoresce when bound to a specific RNA. J. Am. Chem. Soc. 2005, 127, 14954–14955. [Google Scholar] [CrossRef] [PubMed]
- Sando, S.; Narita, A.; Hayami, M.; Aoyama, Y. Transcription monitoring using fused RNA with a dye-binding light-up aptamer as a tag: A blue fluorescent RNA. Chem. Commun. (Camb) 2008, 33, 3858–3860. [Google Scholar] [CrossRef] [PubMed]
- Constantin, T.P.; Silva, G.L.; Robertson, K.L.; Hamilton, T.P.; Fague, K.; Waggoner, A.S.; Armitage, B.A. Synthesis of new fluorogenic cyanine dyes and incorporation into RNA fluoromodules. Org. Lett. 2008, 10, 1561–1564. [Google Scholar] [CrossRef] [PubMed]
- Pei, R.; Rothman, J.; Xie, Y.; Stojanovic, M.N. Light-up properties of complexes between thiazole orange-small molecule conjugates and aptamers. Nucleic. Acids. Res. 2009, 37, e59. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Lee, K.H.; Jeon, J.; Dragulescu-Andrasi, A.; Xiao, F.; Rao, J. Combining SELEX screening and rational design to develop light-up fluorophore-RNA aptamer pairs for RNA tagging. ACS Chem. Biol. 2010, 5, 1065–1074. [Google Scholar] [CrossRef] [PubMed]
- Famulok, M. Chemical biology: Green fluorescent RNA. Nature 2004, 430, 976–977. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, S. Imaging intracellular RNA distribution and dynamics in living cells. Nat. Meth. 2009, 6, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Armitage, B.A. Imaging of RNA in live cells. Curr. Opin. Chem. Biol. 2011, 15, 806–812. [Google Scholar] [CrossRef] [PubMed]
- Paige, J.S.; Wu, K.Y.; Jaffrey, S.R. RNA mimics of green fluorescent protein. Science 2011, 333, 642–646. [Google Scholar] [CrossRef] [PubMed]
- Strack, R.L.; Disney, M.D.; Jaffrey, S.R. A superfolding Spinach2 reveals the dynamic nature of trinucleotide repeat–containing RNA. Nat. Meth. 2013, 10, 1219–1224. [Google Scholar] [CrossRef] [PubMed]
- Filonov, G.S.; Moon, J.D.; Svensen, N.; Jaffrey, S.R. Broccoli: Rapid selection of an RNA Mimic of green fluorescent protein by fluorescence-based selection and directed evolution. J. Am. Chem. Soc. 2014, 136, 16299–16308. [Google Scholar] [CrossRef] [PubMed]
- Weber, W.; Helms, V.; McCammon, J.A.; Langhoff, P.W. Shedding light on the dark and weakly fluorescent states of green fluorescent proteins. Proc. Natl. Acad. Sci. USA 1999, 96, 6177–6182. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Querard, J.; Maurin, S.; Nath, S.S.; Le Saux, T.; Gautier, A.; Jullien, L. Photochemical properties of Spinach and its use in selective imaging. Chem. Sci. 2013, 4, 2865–2873. [Google Scholar] [CrossRef]
- Warner, K.D.; Chen, M.C.; Song, W.; Strack, R.L.; Thorn, A.; Jaffrey, S.R.; Ferré-D'Amaré, A.R. Structural basis for activity of highly efficient RNA mimics of green fluorescent protein. Nat. Struct. Mol. Biol. 2014, 21, 658–663. [Google Scholar] [CrossRef] [PubMed]
- Paige, J.S.; Nguyen-Duc, T.; Song, W.; Jaffrey, S.R. Fluorescence imaging of cellular metabolites with RNA. Science 2012, 335, 1194. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Strack, R.L.; Jaffrey, S.R. Imaging bacterial protein expression using genetically encoded RNA sensors. Nat. Meth. 2013, 10, 873–875. [Google Scholar] [CrossRef] [PubMed]
- You, M.; Litke, J.L.; Jaffrey, S.R. Imaging metabolite dynamics in living cells using a spinach-based riboswitch. Proc. Natl. Acad. Sci. USA 2015, 112, E2756–E2765. [Google Scholar] [CrossRef] [PubMed]
- Kellenberger, C.A.; Wilson, S.C.; Sales-Lee, J.; Hammond, M.C. RNA-based fluorescent biosensors for live cell imaging of second messengers cyclic di-GMP and cyclic AMP-GMP. J. Am. Chem. Soc. 2013, 135, 4906–4909. [Google Scholar] [CrossRef] [PubMed]
- Dolgosheina, E.V.; Jeng, S.C.Y.; Panchapakesan, S.S.S.; Cojocaru, R.; Chen, P.S.K.; Wilson, P.D.; Hawkins, N.; Wiggins, P.A.; Unrau, P.J. RNA mango aptamer-fluorophore: A bright, high-affinity complex for RNA labeling and tracking. ACS Chem. Biol. 2014, 9, 2412–2420. [Google Scholar] [CrossRef] [PubMed]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.; Tebo, A.G.; Gautier, A. Fluorogenic Labeling Strategies for Biological Imaging. Int. J. Mol. Sci. 2017, 18, 1473. https://doi.org/10.3390/ijms18071473
Li C, Tebo AG, Gautier A. Fluorogenic Labeling Strategies for Biological Imaging. International Journal of Molecular Sciences. 2017; 18(7):1473. https://doi.org/10.3390/ijms18071473
Chicago/Turabian StyleLi, Chenge, Alison G. Tebo, and Arnaud Gautier. 2017. "Fluorogenic Labeling Strategies for Biological Imaging" International Journal of Molecular Sciences 18, no. 7: 1473. https://doi.org/10.3390/ijms18071473
APA StyleLi, C., Tebo, A. G., & Gautier, A. (2017). Fluorogenic Labeling Strategies for Biological Imaging. International Journal of Molecular Sciences, 18(7), 1473. https://doi.org/10.3390/ijms18071473