Therapeutic Efficacy of the Novel Stimuli-Sensitive Nano-Ferritins Containing Doxorubicin in a Head and Neck Cancer Model
Abstract
:1. Introduction
2. Results and Discussion
2.1. Dox Encapsulation in HFt-MP-PAS40
2.2. In Vitro Antiproliferative Effects of HFt-MP-PAS40-Dox Nanocarriers
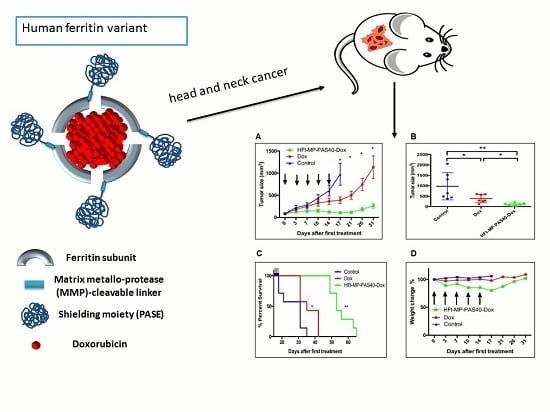
2.3. Therapeutic Evaluation of HFt-MP-PAS40-Dox Efficacy In Vivo
2.4. Tolerability of HFt-MP-PAS40-Dox
3. Materials and Methods
3.1. Cloning, Overexpression, and Purification of HFt-MP-PAS40-Dox Construct
3.2. Dox Encapsulation in HFt-Based Nanocarriers
3.3. Antiproliferative Effects of HFt-MP-PAS40-Dox In Vitro
3.4. Western Blot Analysis
3.5. Therapeutic Evaluation of HFt-MP-PAS40-Dox In Vivo
3.6. Histology
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Falvo, E.; Tremante, E.; Fraioli, R.; Leonetti, C.; Zamparelli, C.; Boffi, A.; Morea, V.; Ceci, P.; Giacomini, P. Antibody-drug conjugates: Targeting melanoma with cisplatin encapsulated in protein-cage nanoparticles based on human ferritin. Nanoscale 2013, 5, 12278–12285. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.Y.; Wei, W.; Huang, S.J.; Lin, S.S.; Zhang, X.; Zhang, C.M.; Du, Y.G.; Ma, G.H.; Li, M.; Mann, S.; et al. Bio-inspired protein-gold nanoconstruct with core-void-shell structure: Beyond a chemo drug carrier. J. Mater. Chem. B 2013, 1, 3136–3143. [Google Scholar] [CrossRef]
- Liang, M.; Fan, K.; Zhou, M.; Duan, D.; Zheng, J.; Yang, D.; Feng, J.; Yan, X. H-ferritin-nanocaged doxorubicin nanoparticles specifically target and kill tumors with a single-dose injection. Proc. Natl. Acad. Sci. USA 2014, 111, 14900–14905. [Google Scholar] [CrossRef] [PubMed]
- Falvo, E.; Tremante, E.; Arcovito, A.; Papi, M.; Elad, N.; Boffi, A.; Morea, V.; Conti, G.; Toffoli, G.; Fracasso, G.; et al. Improved Doxorubicin Encapsulation and Pharmacokinetics of Ferritin-Fusion Protein Nanocarriers Bearing Proline, Serine, and Alanine Elements. Biomacromolecules 2015, 17, 514. [Google Scholar] [CrossRef] [PubMed]
- Kilic, M.A.; Ozlu, E.; Calis, S. A Novel Protein-Based Anticancer Drug Encapsulating Nanosphere: Apoferritin-Doxorubicin Complex. J. Biomed. Nanotechnol. 2012, 8, 508–514. [Google Scholar] [CrossRef] [PubMed]
- Zhen, Z.; Tang, W.; Chen, H.; Lin, X.; Todd, T.; Wang, G.; Cowger, T.; Chen, X.; Xie, J. RGD-Modified Apoferritin Nanoparticles for Efficient Drug Delivery to Tumors. ACS Nano 2013, 7, 4830–4837. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.B.; Li, L.; di Penta, A.; Carmona, U.; Yang, F.; Schops, R.; Brandsch, M.; Zugaza, J.L.; Knez, M. H-Chain Ferritin: A Natural Nuclei Targeting and Bioactive Delivery Nanovector. Adv. Healthc. Mater. 2015, 4, 1305–1310. [Google Scholar] [CrossRef] [PubMed]
- Blazkova, I.; Nguyen, H.V.; Dostalova, S.; Kopel, P.; Stanisavljevic, M.; Vaculovicova, M.; Stiborova, M.; Eckschlager, T.; Kizek, R.; Adam, V. Apoferritin modified magnetic particles as Doxorubicin carriers for anticancer drug delivery. Int. J. Mol. Sci. 2013, 14, 13391–13402. [Google Scholar] [CrossRef] [PubMed]
- Vannucci, L.; Falvo, E.; Fornara, M.; di Micco, P.; Benada, O.; Krizan, J.; Svoboda, J.; Hulikova-Capkova, K.; Morea, V.; Boffi, A.; et al. Selective targeting of melanoma by PEG-masked protein-based multifunctional nanoparticles. Int. J. Nanomed. 2012, 7, 1489–1509. [Google Scholar]
- Heger, Z.; Skalickova, S.; Zitka, O.; Adam, V.; Kizek, R. Apoferritin applications in nanomedicine. Nanomedicine 2014, 9, 2233–2245. [Google Scholar] [CrossRef] [PubMed]
- Truffi, M.; Fiandra, L.; Sorrentino, L.; Monieri, M.; Corsi, F.; Mazzucchelli, S. Ferritin nanocages: A biological platform for drug delivery, imaging and theranostics in cancer. Pharmacol. Res. 2016, 107, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Bode, S.A.; Minten, I.J.; Nolte, R.J.; Cornelissen, J.J. Reactions inside nanoscale protein cages. Nanoscale 2011, 3, 2376–2389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bellapadrona, G.; Sinkar, S.; Sabanay, H.; Liljeström, V.; Kostiainen, M.; Elbaum, M. Supramolecular assembly and coalescence of ferritin cages driven by designed protein-protein interactions. Biomacromolecules 2015, 16, 2006–2011. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.J.; Lee, N.K.; Kim, I.S. Bioengineered protein-based nanocage for drug delivery. Adv. Drug Deliv. Rev. 2016, 106, 157–171. [Google Scholar] [CrossRef] [PubMed]
- Schoonen, L.; van Hest, J.C.M. Functionalization of protein-based nanocages for drug delivery applications. Nanoscale 2014, 6, 7124–7141. [Google Scholar] [CrossRef] [PubMed]
- Fan, K.; Cao, C.; Pan, Y.; Lu, D.; Yang, D.; Feng, J.; Song, L.; Liang, M.; Yan, X. Magnetoferritin nanoparticles for targeting and visualizing tumor tissues. Nat. Nanotechnol. 2012, 7, 459–464. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Fang, C.J.; Ryan, J.C.; Niemi, E.C.; Lebron, J.A.; Bjorkman, P.J.; Arase, H.; Torti, F.M.; Torti, S.V.; Nakamura, M.C.; Seaman, W.E. Binding and uptake of H-ferritin are mediated by human transferrin receptor-1. Proc. Natl. Acad. Sci. USA 2010, 107, 3505–3510. [Google Scholar] [CrossRef] [PubMed]
- Fracasso, G.; Falvo, E.; Colotti, G.; Fazi, F.; Ingegnere, T.; Amalfitano, A.; Doglietto, G.B.; Alfieri, S.; Boffi, A.; Morea, V.; et al. Selective delivery of doxorubicin by novel stimuli-sensitive nano-ferritins overcomes tumor refractoriness. J. Control. Release 2016, 239, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2017. CA Cancer J. Clin. 2017, 67, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Adelstein, D.J.; Saxton, J.P.; Rybicki, L.A.; Esclamado, R.M.; Wood, B.G.; Strome, M.; Lavertu, P.; Lorenz, R.R.; Carroll, M.A. Multiagent concurrent chemoradiotherapy for locoregionally advanced squamous cell head and neck cancer: Mature results from a single institution. J. Clin. Oncol. 2006, 24, 1064–1071. [Google Scholar] [CrossRef] [PubMed]
- Kogashiwa, Y.; Yamauchi, K.; Nagafuji, H.; Matsuda, T.; Tsubosaka, T.; Tsutsumi, T.; Karaho, T.; Kohno, N. Concurrent chemoradiotherapy for organ function preservation in advanced patients with hypopharyngeal and laryngeal cancer. Oncol. Rep. 2009, 22, 1163–1167. [Google Scholar] [PubMed]
- Bellini, M.; Mazzucchelli, S.; Galbiati, E.; Sommaruga, S.; Fiandra, L.; Truffi, M.; Rizzuto, M.A.; Colombo, M.; Tortora, P.; Corsi, F.; et al. Protein nanocages for self-triggered nuclear delivery of DNA-targeted chemotherapeutics in Cancer Cells. J. Control. Release 2014, 196, 184–196. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.C.; Gillies, E.R.; Fox, M.E.; Guillaudeu, S.J.; Frechet, J.M.J.; Dy, E.E.; Szoka, F.C. A single dose of doxorubicin-functionalized bow-tie dendrimer cures mice bearing C-26 colon carcinomas. Proc. Natl. Acad. Sci. USA 2006, 103, 16649–16654. [Google Scholar] [CrossRef] [PubMed]
- Mazzucchelli, S.; Bellini, M.; Fiandra, L.; Truffi, M.; Maria, A.; Sorrentino, L.; Longhi, E.; Nebuloni, M.; Prosperi, D.; Corsi, F. Nanometronomic treatment of 4T1 breast cancer with nanocaged doxorubicin prevents drug resistance and circumvents cardiotoxicity. Oncotarget 2017, 8, 8383–8396. [Google Scholar] [CrossRef] [PubMed]





© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Damiani, V.; Falvo, E.; Fracasso, G.; Federici, L.; Pitea, M.; De Laurenzi, V.; Sala, G.; Ceci, P. Therapeutic Efficacy of the Novel Stimuli-Sensitive Nano-Ferritins Containing Doxorubicin in a Head and Neck Cancer Model. Int. J. Mol. Sci. 2017, 18, 1555. https://doi.org/10.3390/ijms18071555
Damiani V, Falvo E, Fracasso G, Federici L, Pitea M, De Laurenzi V, Sala G, Ceci P. Therapeutic Efficacy of the Novel Stimuli-Sensitive Nano-Ferritins Containing Doxorubicin in a Head and Neck Cancer Model. International Journal of Molecular Sciences. 2017; 18(7):1555. https://doi.org/10.3390/ijms18071555
Chicago/Turabian StyleDamiani, Verena, Elisabetta Falvo, Giulio Fracasso, Luca Federici, Martina Pitea, Vincenzo De Laurenzi, Gianluca Sala, and Pierpaolo Ceci. 2017. "Therapeutic Efficacy of the Novel Stimuli-Sensitive Nano-Ferritins Containing Doxorubicin in a Head and Neck Cancer Model" International Journal of Molecular Sciences 18, no. 7: 1555. https://doi.org/10.3390/ijms18071555
APA StyleDamiani, V., Falvo, E., Fracasso, G., Federici, L., Pitea, M., De Laurenzi, V., Sala, G., & Ceci, P. (2017). Therapeutic Efficacy of the Novel Stimuli-Sensitive Nano-Ferritins Containing Doxorubicin in a Head and Neck Cancer Model. International Journal of Molecular Sciences, 18(7), 1555. https://doi.org/10.3390/ijms18071555