Isorhamnetin and Quercetin Derivatives as Anti-Acetylcholinesterase Principles of Marigold (Calendula officinalis) Flowers and Preparations
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemical Composition and Anti-Acetylcholinesterase Potential of 23 Varieties of C. officinalis Flowers
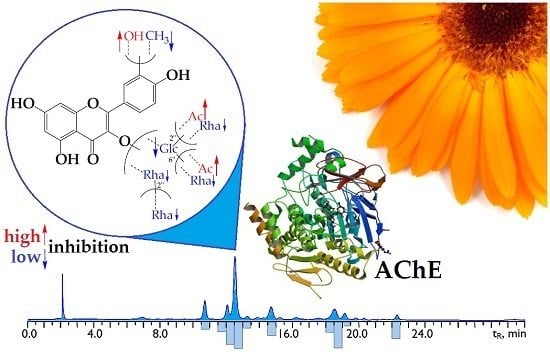
2.2. Flavonoid Profile of C. officinalis Flowers’ Extract and High-Performance Liquid Chromatography (HPLC) Activity-Based Profiling of Acetylcholinesterase Inhibitors
2.3. Acetylcholinesterase Inhibitory Activity of C. officinalis Flavonoids
2.4. Flavonoid Content in Marigold Flower Products
3. Materials and Methods
3.1. Chemicals
3.2. Plant Material
3.3. Sample Preparation for the Extraction of Total Phytochemicals and Anti-acetylcolinesterase Acitivity Determination
3.4. Chemical Composition Analytical Methods
3.5. Anti-Acetylcholinesterase Activity Microplate Assay
3.6. Microcolumn Reversed-Phase High-Performance Liquid Chromatography with Ultraviolet Detection (MC-RP-HPLC-UV) Conditions
3.7. Microcolumn Reversed-Phase High-Performance Liquid Chromatography with Electrospray Ionization Mass Spectrometry Detection (MC-RP-HPLC-ESI-MS) Conditions
3.8. HPLC Activity-Based Profiling
3.9. Statistical Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Brühlmann, C.; Marston, A.; Hostettmann, K.; Carrupt, P.A.; Testa, B. Screening of non-alkaloidal natural compounds as acetylcholinesterase inhibitors. Chem. Biodivers. 2004, 1, 819–829. [Google Scholar] [CrossRef] [PubMed]
- Ni, R.; Marutle, A.; Nordberg, A. Modulation of α7 nicotinic acetylcholine receptor and fibrillary amyloid-β interactions in Alzheimer’s disease brain. J. Alzheimers Dis. 2013, 33, 841–851. [Google Scholar] [CrossRef] [PubMed]
- Moss, D.E.; Perez, R.G.; Kobayashi, H. Cholinesterase inhibitor therapy in Alzheimer’s: The limits and tolerability of irreversible CNS-selective acetylcholinesterase inhibition in primates. J. Alzheimers Dis. 2017, 55, 1285–1294. [Google Scholar] [CrossRef] [PubMed]
- Nelson, P.T.; Kryscio, R.G.; Abner, E.L.; Schmitt, F.A.; Jicha, G.A.; Mendiondo, M.S.; Cooper, G.; Smith, C.B.; Markesbery, W.R. Acetylcholinesterase inhibitor treatment is associated with relatively slow cognitive decline in patients with Alzheimer’s disease and AD + DLB. J. Alzheimers Dis. 2009, 16, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Filho, J.M.B.; Medeiros, K.C.P.; Diniz, M.F.F.M.; Batista, L.M.; Athayde-Filho, P.F.; Silva, M.S.; da-Cunha, E.V.L.; Almeida, J.R.G.S.; Quintans-Júnior, L.J. Natural products inhibitors of the enzyme acetylcholinesterase. Rev. Bras. Farmacogn. 2006, 16, 258–285. [Google Scholar] [CrossRef]
- Ercetin, T.; Senol, F.S.; Orhan, I.E.; Toker, G. Comparative assessment of antioxidant and cholinesterase inhibitory properties of the marigold extracts from Calendula arvensis L. and Calendula officinalis L. Ind. Crops Prod. 2012, 36, 203–208. [Google Scholar] [CrossRef]
- Shivasharan, B.D.; Nagakannan, P.; Thippeswamy, B.S.; Veerapur, V.P. Protective effect of Calendula officinalis L. flowers against monosodium glutamate induced oxidative stress and excitotoxic brain damage in rats. Ind. J. Clin. Biochem. 2013, 28, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, S.; Mahmoodi, M.; Farahmandlou, N. Antinociceptive properties of hydro-alcoholic extract of Calendula officinalis in rats. Basic Clin. Neurosci. 2012, 3, 45–48. [Google Scholar]
- Bashir, S.; Janbaz, K.H.; Jabeen, Q.; Gilani, A.H. Studies on spasmogenic and spasmolytic activities of Calendula officinalis flowers. Phytother. Res. 2006, 20, 906–910. [Google Scholar] [CrossRef] [PubMed]
- Hechtman, L. Clinical Naturopathic Medicine; Elsevier Science: Chatswood, Australia, 2012; pp. 301–337. [Google Scholar]
- Lagarto, A.; Bueno, V.; Guerra, I.; Valdes, O.; Vega, Y.; Torres, L. Acute and subchronic oral toxicities of Calendula officinalis extract in Wistar rats. Exp. Toxicol. Pathol. 2011, 63, 387–391. [Google Scholar] [CrossRef] [PubMed]
- Gazim, Z.C.; Rezende, C.M.; Fraga, S.R.; Svidzinski, T.I.E.; Cortez, D.A.G. Antifungal activity of the essential oil from Calendula officinalis L. (asteraceae) growing in Brazil. Braz. J. Microbiol. 2008, 39, 61–63. [Google Scholar] [CrossRef] [PubMed]
- Khalid, K.A.; da Silva, J.A.T. Yield, essential oil and pigment content of Calendula officinalis L. flower heads cultivated under salt stress conditions. Sci. Hort. 2010, 126, 297–305. [Google Scholar] [CrossRef]
- Okoh, O.O.; Sadimenko, A.A.; Afolayan, A.J. The effects of age on the yield and composition of the essential oils of Calendula officinalis. J. Appl. Sci. 2007, 7, 3806–3810. [Google Scholar] [CrossRef]
- Piccaglia, R.; Marotti, M.; Chiavari, G.; Gandini, N. Effects of harvesting date and climate on the flavonoid and carotenoid contents of marigold (Calendula officinalis L.). Flavor Fragr. J. 1997, 12, 85–90. [Google Scholar] [CrossRef]
- Pintea, A.; Bele, C.; Andrei, S.; Socaciu, C. HPLC analysis of carotenoids in four varieties of Calendula officinalis L. flowers. Acta Biol. Szeged. 2003, 47, 37–40. [Google Scholar]
- Kishimoto, S.; Sumitomo, K.; Yagi, M.; Nakayama, M.; Ohmiya, A. Three routes to orange petal color via carotenoids components in 9 compositae species. J. Jpn. Soc. Hort. Sci. 2007, 76, 250–257. [Google Scholar] [CrossRef]
- Raal, A.; Kirsipuu, K.; Must, R.; Tenno, S. Content of total carotenoids in Calendula officinalis L. from different countries cultivated in Estonia. Nat. Prod. Commun. 2009, 4, 35–38. [Google Scholar] [PubMed]
- Jäger, S.; Trojan, H.; Kopp, T.; Laszczyk, M.N.; Scheffler, A. Pentacyclic triterpene distribution in various plants, rich sources for a new group of multi-potent plant extracts. Molecules 2009, 14, 2016–2031. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, R. Studies of selected plant raw materials as alternative sources of triterpenes of oleanolic and ursolic acid types. J. Agric. Food Chem. 2007, 55, 656–662. [Google Scholar] [CrossRef] [PubMed]
- Neukirch, H.; D’Ambrosio, M.; Dalla, V.J.; Guerriero, A. Simultaneous quantitative determination of eight triterpenoid monoesters from flowers of 10 varieties of Calendula officinalis L. and characterization of a new triterpenoid monoester. Phytochem. Anal. 2004, 15, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Raal, A.; Kirsipuu, K. Total flavonoid content in varieties of Calendula officinalis L. originating from different countries and cultivated in Estonia. Nat. Prod. Res. 2011, 25, 658–662. [Google Scholar] [CrossRef] [PubMed]
- Masterova, I.; Grancalova, Z.; Uhrinova, S.; Suchy, V.; Ubik, K.; Nagy, M. Flavonoids in flowers of Calendula officinalis L. Сhem. Pap. 1991, 45, 105–108. [Google Scholar]
- Borella, J.C.; de Carvalho, D.M.A. Quality comparison of extracts from Calendula officinalis L. (Asteraceae) sold from pharmacies in Ribeirão Preto, SP. Rev. Bras. Farm. 2011, 92, 13–18. [Google Scholar]
- Russo, D.; Valentão, P.; Andrade, P.B.; Fernandez, E.C.; Milella, L. Evaluation of antioxidant, antidiabetic and anticholinesterase activities of Smallanthus sonchifolius landraces and correlation with their phytochemical profiles. Int. J. Mol. Sci. 2015, 16, 17696–17718. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Sankarapandian, K.; Cheng, Y.; Woo, S.O.; Kwon, H.W.; Perumalsamy, H.; Ahn, Y. Relationship between total phenolic contents and biological properties of propolis from 20 different regions in South Korea. BMC Complement. Altern. Med. 2016, 16, 65. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.H.A.; Fry, J.R.; Bakar, M.F.A. Phytochemicals content, antioxidant activity and acetylcholinesterase inhibition properties of indigenous Garcinia parvifolia fruit. BioMed Res. Int. 2013, 2013. [Google Scholar] [CrossRef]
- Olennikov, D.N.; Kashchenko, N.I. New isorhamnetin glycosides and other phenolic compounds from Calendula officinalis. Chem. Nat. Comp. 2013, 49, 833–839. [Google Scholar] [CrossRef]
- Komissarenko, N.F.; Chernobai, V.T.; Derkach, A.T. Flavonoids of inflorescences of Calendula officinalis. Chem. Nat. Compd. 1988, 24, 675–680. [Google Scholar] [CrossRef]
- Vidal-Ollivier, E.; Elias, R.; Faure, F.; Babadjamian, A.; Crespin, F.; Balansard, G.; Boudon, G. Flavonol glycosides from Calendula officinalis flowers. Planta Med. 1989, 55, 73–74. [Google Scholar] [CrossRef] [PubMed]
- Dittmann, K.; Riese, U.; Hamburger, M. HPLC-based bioactivity profiling of plant extracts: A kinetic assay for the identification of monoamine oxidase-A inhibitors using human recombinant monoamine oxidase-A. Phytochemistry 2004, 65, 2885–2891. [Google Scholar] [CrossRef] [PubMed]
- Julianti, T.; Mieri, M.D.; Zimmermann, S.; Ebrahimi, S.N.; Kaiser, M.; Neuburger, M.; Raith, M.; Brun, R.; Hamburger, M. HPLC-based activity profiling for antiplasmodial compounds in the traditional Indonesian medicinal plant Carica papaya L. J. Ethnopharmacol. 2014, 155, 426–434. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Baburin, I.; Plitzko, I.; Hering, S.; Hamburger, M. HPLC-based activity profiling for GABAa receptor modulators from the traditional Chinese herbal drug Kushen (Sophora flavescens root). Mol. Divers. 2011, 15, 361–372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olennikov, D.N.; Kashchenko, N.I. 1,5-Di-O-isoferuloylquinic acid and other phenolic compounds from pollen of Calendula officinalis. Chem. Nat. Compd. 2014, 50, 589–593. [Google Scholar] [CrossRef]
- Olennikov, D.N.; Kashchenko, N.I. Calendosides I-IV, new quercetin and isorhamnetin rhamnoglucosides from Calendula officinalis. Chem. Nat. Compd. 2014, 50, 633–637. [Google Scholar] [CrossRef]
- Olennikov, D.N.; Kashchenko, N.I. Componential profile and amylase inhibiting activity of phenolic compounds from Calendula officinalis L. leaves. Sci. World J. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Di Giovanni, S.; Borloz, A.; Urbain, A.; Marston, A.; Hostettmann, K.; Carrupt, P.-A.; Reist, M. In vitro screening assays to identify natural or synthetic acetylcholinesterase inhibitors: Thin layer chromatography versus microplate methods. Eur. J. Pharm. Sci. 2008, 33, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Park, B.S.; Lee, K.G.; Choi, C.Y.; Jang, S.S.; Kim, Y.L.; Lee, S.E. Effect of naturally occurring compounds on fibril formation and oxidative stress of beta amyloid. J. Agric. Food Chem. 2005, 53, 8537–8541. [Google Scholar] [CrossRef] [PubMed]
- Pietta, P.G. Flavonoids as antioxidants. J. Nat. Prod. 2000, 63, 1035–1042. [Google Scholar] [CrossRef] [PubMed]
- Remya, C.; Dileep, K.V.; Tintu, I.; Variyar, E.J.; Sadasivan, C. Design of potent inhibitors of acetylcholinesterase using morin as the starting compound. Front. Life Sci. 2012, 6, 107–117. [Google Scholar] [CrossRef]
- Balkis, A.; Tran, K.; Lee, Y.Z.; Ng, K. Screening flavonoids for inhibition of acetylcholinesterase identified baicalein as the most potent inhibitor. J. Agric. Sci. 2015, 7, 26–35. [Google Scholar] [CrossRef]
- Jung, J.; Park, M. Acetylcholinesterase inhibition by flavonoids from Agrimonia pilosa. Molecules 2007, 12, 2130–2139. [Google Scholar] [CrossRef] [PubMed]
- Orhan, I.; Kartal, M.; Tosun, F.; Sener, B. Screening of various phenolic acids and flavonoid derivatives for their anticholinesterase potential. Z. Naturforsch. 2007, 62, 829–832. [Google Scholar] [CrossRef]
- Katalinic, M.; Rusak, G.; Barovic, J.D.; Šinko, G.; Jelic, D.; Antolovic, R.; Kovarik, Z. Structural aspects of flavonoids as inhibitors of human butyrylcholinesterase. Eur. J. Med. Chem. 2010, 45, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Mills, S.; Bone, K. The Essential Guide to Herbal Safety; Elsevier: St. Louis, MO, USA, 2005; pp. 309–312. [Google Scholar]
- Olennikov, D.N.; Kashchenko, N.I.; Chirikova, N.K. Meadowsweet teas as new functional beverages: Comparative analysis of nutrients, phytochemicals and biological effects of four Filipendula species. Molecules 2017, 22, 16. [Google Scholar] [CrossRef] [PubMed]
- Bezbradica, D.; Milic-Ascrabic, J.; Petrobic, S.D.; Siler-Marinkovic, S. An investigation of influence of solvent on the degradation kinetics of carotenoids in oil extracts of Calendula officinalis. J. Serb. Chem. Soc. 2005, 70, 115–124. [Google Scholar] [CrossRef]
- Wójciak-Kosior, M. Separation and determination of closely related triterpenic acids by high performance thin-layer chromatography after iodine derivatization. J. Pharm. Biomed. Anal. 2007, 45, 337–340. [Google Scholar] [CrossRef] [PubMed]
- Chirikova, N.K.; Olennikov, D.N.; Tankhaeva, L.M. Quantitative determination of flavonoid content in the aerial part of Baical scullcap (Scutellaria baicalensis Georgi). Russ. J. Bioorg. Chem. 2010, 36, 915–922. [Google Scholar] [CrossRef]
- Galvez, M.; Martin-Cordero, C.; Houghton, P.J.; Ayuso, M.J. Antioxidant activity of methanol extracts obtained from Plantago species. J. Agric. Food Chem. 2005, 53, 1927–1933. [Google Scholar] [CrossRef] [PubMed]
- Olennikov, D.N.; Tankhaeva, L.M.; Samuelsen, A.B. Quantitative analysis of polysaccharides from Plantago major leaves using the Dreywood method. Chem. Nat. Compd. 2006, 42, 265–268. [Google Scholar] [CrossRef]




| Variety | Essential Oil | Carotenoids | Triterpenes | Flavonoids | Phenylpropanoids | Polysaccharides | AChA, IC50, μg/mL |
|---|---|---|---|---|---|---|---|
| Amber Bay | 1.12 ± 0.04 | 4.12 ± 0.10 | 25.17 ± 0.48 | 18.53 ± 0.42 | 19.45 ± 0.33 | 30.27 ± 0.48 | 133.9 ± 4.3 |
| Big Orange | 2.65 ± 0.12 | 6.56 ± 0.17 | 39.11 ± 0.82 | 26.79 ± 0.56 | 18.54 ± 0.35 | 35.62 ± 0.64 | 123.7 ± 3.8 |
| Cardinal | 0.32 ± 0.01 | 8.29 ± 0.19 | 27.63 ± 0.50 | 12.65 ± 0.29 | 25.09 ± 0.40 | 25.11 ± 0.50 | 197.2 ± 6.5 |
| Egypt Sun | 1.82 ± 0.09 | 5.47 ± 0.12 | 65.70 ± 1.31 | 19.24 ± 0.38 | 7.30 ± 0.14 | 39.16 ± 0.67 | 123.8 ± 4.2 |
| Fiesta | 1.43 ± 0.05 | 10.35 ± 0.27 | 37.16 ± 0.71 | 16.35 ± 0.38 | 15.17 ± 0.27 | 42.54 ± 0.68 | 152.4 ± 5.2 |
| Flame Dancer | 2.93 ± 0.12 | 7.59 ± 0.20 | 10.28 ± 0.19 | 10.52 ± 0.24 | 22.63 ± 0.41 | 11.97 ± 0.22 | 187.2 ± 6.2 |
| Gavrish | 2.20 ± 0.10 | 5.22 ± 0.12 | 12.04 ± 0.24 | 29.37 ± 0.67 | 20.01 ± 0.36 | 28.14 ± 0.59 | 104.2 ± 3.2 |
| Geisha Girl | 2.55 ± 0.12 | 6.97 ± 0.16 | 20.33 ± 0.43 | 20.11 ± 0.44 | 24.16 ± 0.56 | 22.67 ± 0.43 | 137.3 ± 4.5 |
| Gitana Orange | 0.93 ± 0.04 | 6.31 ± 0.15 | 20.16 ± 0.44 | 32.54 ± 0.75 | 4.93 ± 0.09 | 19.27 ± 0.39 | 125.2 ± 4.3 |
| Golden Imperator | 1.12 ± 0.05 | 4.09 ± 0.11 | 42.82 ± 0.86 | 26.68 ± 0.62 | 33.47 ± 0.60 | 17.06 ± 0.34 | 153.9 ± 5.5 |
| Golden Prince | 2.43 ± 0.12 | 3.31 ± 0.09 | 14.15 ± 0.25 | 25.14 ± 0.58 | 6.07 ± 0.12 | 23.69 ± 0.50 | 161.9 ± 5.5 |
| Green Heart Orange | 0.89 ± 0.04 | 8.61 ± 0.22 | 15.80 ± 0.25 | 46.87 ± 1.03 | 30.24 ± 0.51 | 39.02 ± 0.70 | 63.5 ± 2.1 |
| Honey Cardinal | 1.03 ± 0.04 | 7.22 ± 0.15 | 30.70 ± 0.52 | 37.18 ± 0.78 | 26.12 ± 0.52 | 44.15 ± 0.79 | 105.2 ± 3.6 |
| Indian Prince | 1.47 ± 0.07 | 5.14 ± 0.11 | 37.93 ± 0.72 | 17.25 ± 0.36 | 18.69 ± 0.39 | 25.10 ± 0.40 | 169.5 ± 6.1 |
| Jiga-Jiga | 3.04 ± 0.15 | 5.22 ± 0.15 | 16.72 ± 0.27 | 11.67 ± 0.23 | 8.53 ± 0.14 | 27.82 ± 0.45 | 223.9 ± 7.6 |
| Lemon Juice | 2.47 ± 0.10 | 5.67 ± 0.14 | 53.74 ± 1.07 | 21.38 ± 0.49 | 25.84 ± 0.52 | 19.82 ± 0.36 | 150.1 ± 5.4 |
| Orange Balls | 1.45 ± 0.06 | 7.14 ± 0.17 | 25.37 ± 0.51 | 19.53 ± 0.43 | 11.47 ± 0.21 | 16.37 ± 0.31 | 179.5 ± 5.7 |
| Orange King | 1.39 ± 0.05 | 8.26 ± 0.22 | 53.41 ± 1.07 | 16.38 ± 0.33 | 16.72 ± 0.35 | 38.25 ± 0.77 | 202.4 ± 7.3 |
| Radio | 1.81 ± 0.09 | 5.98 ± 0.14 | 61.37 ± 1.29 | 18.42 ± 0.40 | 10.83 ± 0.22 | 22.16 ± 0.42 | 133.6 ± 4.5 |
| Red Black Centered | 2.12 ± 0.11 | 6.77 ± 0.15 | 11.39 ± 0.23 | 16.34 ± 0.32 | 8.42 ± 0.17 | 20.38 ± 0.37 | 175.3 ± 6.3 |
| Rose Surprise | 0.73 ± 0.03 | 11.39 ± 0.29 | 33.44 ± 0.54 | 19.63 ± 0.39 | 29.06 ± 0.64 | 11.09 ± 0.22 | 122.2 ± 4.5 |
| Touch of Red | 1.64 ± 0.07 | 2.63 ± 0.06 | 35.25 ± 0.60 | 28.16 ± 0.54 | 28.24 ± 0.48 | 15.23 ± 0.30 | 105.6 ± 3.3 |
| Tutti-Frutti | 1.73 ± 0.08 | 9.23 ± 0.23 | 28.16 ± 0.45 | 18.37 ± 0.42 | 18.28 ± 0.29 | 18.09 ± 0.36 | 169.8 ± 5.8 |
| Compound | tR, min | UV, λmax, nm | ESI-MS, m/z | Content, mg/g DW ± SD 1 |
|---|---|---|---|---|
| Phenylpropanoids | ||||
| 3-O-Caffeoylquinic acid | 6.83 | 324 | 353 [M − H]−, 191, 179, 135 | 3.32 ± 0.08 |
| Caffeic acid | 7.90 | 325 | 179 [M − H]−, 135 | 0.92 ± 0.02 |
| 3,5-Di-O-caffeoylquinic acid | 15.31 | 333 | 515 [M − H]−, 353, 191, 179, 135 | 1.16 ± 0.03 |
| 1,5-Di-O-caffeoylquinic acid | 17.52 | 332 | 515 [M − H]−, 353, 191, 179 | 3.03 ± 0.07 |
| 4,5-Di-O-caffeoylquinic acid | 20.37 | 331 | 515 [M − H]−, 353, 179 | 1.02 ± 0.02 |
| Flavonoids. Quercetin derivatives | ||||
| Manghaslin | 10.63 | 255, 356 | 757 [M + H]+, 611, 465, 303 | 12.62 ± 0.32 |
| Calendoflavobioside | 12.06 | 255, 356 | 611 [M + H]+, 465, 303 | 10.12 ± 0.25 |
| Rutin | 13.18 | 255, 356 | 611 [M + H]+, 465, 303 | 2.26 ± 0.05 |
| Isoquercitrin | 13.87 | 257, 356 | 465 [M + H]+, 303 | 0.66 ± 0.01 |
| Quercetin-3-O-(2″-ramnosyl)-rhamnoside | 14.16 | 259, 353 | 595 [M + H]+, 449, 303 | 0.49 ± 0.01 |
| Quercetin-3-O-(6″-acetyl)-glucoside | 16.14 | 261, 352 | 507 [M + H]+, 303 | 0.60 ± 0.01 |
| Flavonoids. Isorhamnetin derivatives | ||||
| Typhaneoside | 10.51 | 254, 356 | 771 [M + H]+, 625, 479, 317 | 42.46 ± 1.10 |
| Calendoflavoside | 14.63 | 255, 357 | 625 [M + H]+, 479, 317 | 6.43 ± 0.16 |
| Narcissin | 18.46 | 255, 355 | 625 [M + H]+, 479, 317 | 12.92 ± 0.33 |
| Isorhamnetin-3-O-glucoside | 19.09 | 258, 361 | 479 [M + H]+, 317 | 1.79 ± 0.04 |
| Calendoflaside | 19.82 | 253, 354 | 609 [M + H]+, 463, 317 | 0.33 ± 0.01 |
| Isorhamnetin-3-O-(6″-acetyl)-glucoside | 22.21 | 253, 354 | 521 [M + H]+, 317 | 1.86 ± 0.04 |
| Total content: | ||||
| phenylpropanoids | 9.45 | |||
| quercetin derivatives | 26.75 | |||
| isorhamnetin derivatives | 65.79 | |||
| flavonoids | 92.54 |
| Carbohydrate Unit | Isorhamnetin | Quercetin |
|---|---|---|
| - | 24.18 ± 0.74 | 14.37 ± 0.34 |
| 3-O-Glcp | 89.04 ± 2.49 | 70.12 ± 1.82 |
| 3-O-(2″-Ac)-Glcp | 70.85 ± 1.84 | 48.01 ± 1.20 |
| 3-O-(6″-Ac)-Glcp | 68.22 ± 1.71 | 45.16 ± 1.12 |
| 3-O-(2″,6″-di-Ac)-Glcp | 51.26 ± 1.53 | 36.47 ± 1.02 |
| 3-O-(2″-Rhap)-Glcp | 94.27 ± 2.82 | 71.86 ± 1.94 |
| 3-O-(3″-Rhap)-Glcp | 91.16 ± 2.73 | 69.15 ± 1.84 |
| 3-O-(4″-Rhap)-Glcp | 92.07 ± 2.85 | 70.35 ± 1.90 |
| 3-O-(6″-Rhap)-Glcp | 97.32 ± 2.91 | 72.09 ± 2.04 |
| 3-O-(2″,6″-di-Rhap)-Glcp | 98.45 ± 3.04 | 94.92 ± 2.65 |
| 3-O-Rhap | 73.96 ± 2.14 | 48.80 ± 1.26 |
| 3-O-(2″-Rhap)-Rhap | 84.90 ± 2.37 | 67.91 ± 1.76 |
| Compound | 01 | 02 | 03 | 04 | 05 | 06 | 07 | 08 |
|---|---|---|---|---|---|---|---|---|
| Quercetin derivatives | ||||||||
| Manghaslin | 0.21 ± 0.00 | 0.43 ± 0.01 | 0.72 ± 0.02 | 0.27 ± 0.00 | 0.52 ± 0.01 | 0.28 ± 0.00 | 0.30 ± 0.01 | 0.33 ± 0.00 |
| Calendoflavobioside | 0.54 ± 0.01 | 0.71 ± 0.02 | 0.38 ± 0.01 | 0.35 ± 0.01 | 0.96 ± 0.02 | 0.32 ± 0.01 | 0.40 ± 0.01 | 0.38 ± 0.00 |
| Rutin | 0.18 ± 0.00 | 0.23 ± 0.00 | 0.18 ± 0.00 | 0.27 ± 0.00 | 0.25 ± 0.00 | 0.06 ± 0.00 | 0.08 ± 0.00 | 0.11 ± 0.00 |
| Isoquercitrin | 0.15 ± 0.00 | 0.14 ± 0.00 | 0.05 ± 0.00 | 0.07 ± 0.00 | 0.19 ± 0.00 | 0.02 ± 0.00 | 0.02 ± 0.00 | 0.06 ± 0.00 |
| Quercetin-3-O-(2″-Rha)-Rha | 0.03 ± 0.00 | 0.03 ± 0.00 | 0.04 ± 0.00 | 0.01 ± 0.00 | 0.05 ± 0.00 | 0.03 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| Quercetin-3-O-(6″-Ac)-Glc | 0.16 ± 0.00 | 0.09 ± 0.00 | 0.04 ± 0.00 | 0.17 ± 0.00 | 0.11 ± 0.00 | 0.00 ± 0.00 | 0.04 ± 0.00 | 0.09 ± 0.00 |
| Subtotal | ||||||||
| Isorhamnetin derivatives | ||||||||
| Typhaneoside | 5.01 ± 0.11 | 5.83 ± 0.14 | 10.45 ± 0.24 | 4.17 ± 0.09 | 7.18 ± 0.16 | 4.04 ± 0.09 | 3.94 ± 0.09 | 6.03 ± 0.14 |
| Calendoflavoside | 0.90 ± 0.02 | 1.11 ± 0.02 | 1.21 ± 0.03 | 0.58 ± 0.01 | 2.14 ± 0.05 | 0.87 ± 0.02 | 0.97 ± 0.02 | 1.34 ± 0.03 |
| Narcissin | 3.49 ± 0.08 | 3.50 ± 0.08 | 4.19 ± 0.09 | 4.16 ± 0.09 | 4.29 ± 0.09 | 1.85 ± 0.04 | 1.70 ± 0.04 | 3.18 ± 0.07 |
| Isorhamnetin-3-O-Glc | 0.30 ± 0.00 | 0.35 ± 0.01 | 0.08 ± 0.00 | 0.11 ± 0.00 | 0.29 ± 0.00 | 0.18 ± 0.00 | 0.17 ± 0.00 | 0.15 ± 0.00 |
| Calendoflaside | 0.15 ± 0.00 | 0.15 ± 0.00 | 0.05 ± 0.00 | 0.04 ± 0.00 | 0.14 ± 0.00 | 0.02 ± 0.00 | 0.15 ± 0.00 | 0.04 ± 0.00 |
| Isorhamnetin-3-O-(6″-Ac)-Glc | 0.68 ± 0.01 | 0.30 ± 0.01 | 0.35 ± 0.01 | 0.57 ± 0.01 | 0.31 ± 0.01 | 0.12 ± 0.00 | 0.14 ± 0.00 | 0.40 ± 0.01 |
| Subtotal | 10.53 | 11.24 | 16.33 | 9.63 | 14.35 | 7.08 | 7.07 | 11.14 |
| Total flavonoids | 11.80 | 12.87 | 17.74 | 10.77 | 16.43 | 7.79 | 7.91 | 12.11 |
| Compound | 09 | 10 | 11 | 12 | 13 | 14 | 15 | 16 |
| Quercetin derivatives | ||||||||
| Manghaslin | 0.37 ± 0.01 | 0.56 ± 0.02 | 0.41 ± 0.01 | 0.39 ± 0.01 | 0.15 ± 0.00 | 0.42 ± 0.01 | 0.49 ± 0.01 | 0.34 ± 0.01 |
| Calendoflavobioside | 0.45 ± 0.01 | 0.51 ± 0.01 | 0.47 ± 0.01 | 0.47 ± 0.01 | 0.21 ± 0.00 | 0.24 ± 0.00 | 0.57 ± 0.01 | 0.33 ± 0.01 |
| Rutin | 0.09 ± 0.00 | 0.12 ± 0.00 | 0.11 ± 0.00 | 0.11 ± 0.00 | 0.09 ± 0.00 | 0.28 ± 0.00 | 0.79 ± 0.02 | 0.15 ± 0.00 |
| Isoquercitrin | 0.07 ± 0.00 | 0.09 ± 0.00 | 0.07 ± 0.00 | 0.06 ± 0.00 | 0.05 ± 0.00 | 0.03 ± 0.00 | 0.07 ± 0.00 | 0.07 ± 0.00 |
| Quercetin-3-O-(2″-Rha)-Rha | 0.00 ± 0.00 | 0.02 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.02 ± 0.00 | 0.00 ± 0.00 | 0.03 ± 0.00 | 0.00 ± 0.00 |
| Quercetin-3-O-(6″-Ac)-Glc | 0.10 ± 0.00 | 0.11 ± 0.00 | 0.09 ± 0.00 | 0.08 ± 0.00 | 0.09 ± 0.00 | 0.11 ± 0.00 | 0.04 ± 0.00 | 0.04 ± 0.00 |
| Subtotal | 1.08 | 1.41 | 1.15 | 1.11 | 0.61 | 1.08 | 1.99 | 0.93 |
| Isorhamnetin derivatives | ||||||||
| Typhaneoside | 5.23 ± 0.12 | 5.52 ± 0.12 | 6.24 ± 0.14 | 6.17 ± 0.14 | 3.88 ± 0.08 | 7.89 ± 0.18 | 8.89 ± 0.20 | 9.16 ± 0.21 |
| Calendoflavoside | 1.35 ± 0.03 | 1.16 ± 0.03 | 1.17 ± 0.02 | 1.20 ± 0.02 | 0.28 ± 0.00 | 0.35 ± 0.01 | 0.36 ± 0.01 | 0.29 ± 0.00 |
| Narcissin | 2.25 ± 0.05 | 2.42 ± 0.06 | 2.70 ± 0.06 | 2.90 ± 0.07 | 2.00 ± 0.05 | 4.69 ± 0.11 | 7.98 ± 0.18 | 4.82 ± 0.11 |
| Isorhamnetin-3-O-Glc | 0.14 ± 0.00 | 0.15 ± 0.00 | 0.18 ± 0.00 | 0.20 ± 0.00 | 0.15 ± 0.00 | 0.34 ± 0.01 | 0.11 ± 0.00 | 0.11 ± 0.00 |
| Calendoflaside | 0.15 ± 0.00 | 0.19 ± 0.00 | 0.12 ± 0.00 | 0.17 ± 0.00 | 0.12 ± 0.00 | 0.12 ± 0.00 | 0.09 ± 0.00 | 0.14 ± 0.00 |
| Isorhamnetin-3-O-(6″-Ac)-Glc | 0.33 ± 0.01 | 0.34 ± 0.00 | 0.35 ± 0.01 | 0.35 ± 0.01 | 0.14 ± 0.00 | 0.39 ± 0.01 | 0.43 ± 0.01 | 0.56 ± 0.02 |
| Subtotal | 9.45 | 9.78 | 10.76 | 10.99 | 6.57 | 13.78 | 17.86 | 15.08 |
| Total flavonoids | 10.53 | 11.19 | 11.91 | 12.10 | 7.18 | 14.86 | 19.85 | 16.01 |
| Compound | Infusion | Decoction | Tincture | Liquid Extract |
|---|---|---|---|---|
| Quercetin derivatives | ||||
| Manghaslin | 0.02 ± 0.00 | 0.02 ± 0.00 | 0.03 ± 0.00 | 0.14 ± 0.00 |
| Calendoflavobioside | 0.02 ± 0.00 | 0.03 ± 0.00 | 0.04 ± 0.00 | 0.21 ± 0.00 |
| Rutin | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.02 ± 0.00 | 0.07 ± 0.00 |
| Isoquercitrin | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 |
| Quercetin-3-O-(2″-Rha)-Rha | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.02 ± 0.00 |
| Quercetin-3-O-(6″-Ac)- Glc | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.03 ± 0.00 |
| Subtotal | 0.08 | 0.09 | 0.12 | 0.48 |
| Isorhamnetin derivatives | ||||
| Typhaneoside | 0.19 ± 0.01 | 0.23 ± 0.01 | 0.26 ± 0.01 | 1.24 ± 0.03 |
| Calendoflavoside | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.10 ± 0.00 |
| Narcissin | 0.14 ± 0.01 | 0.21 ± 0.01 | 0.26 ± 0.01 | 1.09 ± 0.03 |
| Isorhamnetin-3-O-Glc | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.03 ± 0.00 | 0.15 ± 0.00 |
| Calendoflaside | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.02 ± 0.00 |
| Isorhamnetin-3-O-(6″-Ac)-Glc | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.02 ± 0.00 |
| Subtotal | 0.37 | 0.48 | 0.58 | 2.62 |
| Total flavonoids | 0.45 | 0.57 | 0.70 | 3.10 |
| Daily intake of flavonoids | 112.50 2 | 142.50 2 | 3.15 3 | 9.30 4 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olennikov, D.N.; Kashchenko, N.I.; Chirikova, N.K.; Akobirshoeva, A.; Zilfikarov, I.N.; Vennos, C. Isorhamnetin and Quercetin Derivatives as Anti-Acetylcholinesterase Principles of Marigold (Calendula officinalis) Flowers and Preparations. Int. J. Mol. Sci. 2017, 18, 1685. https://doi.org/10.3390/ijms18081685
Olennikov DN, Kashchenko NI, Chirikova NK, Akobirshoeva A, Zilfikarov IN, Vennos C. Isorhamnetin and Quercetin Derivatives as Anti-Acetylcholinesterase Principles of Marigold (Calendula officinalis) Flowers and Preparations. International Journal of Molecular Sciences. 2017; 18(8):1685. https://doi.org/10.3390/ijms18081685
Chicago/Turabian StyleOlennikov, Daniil N., Nina I. Kashchenko, Nadezhda K. Chirikova, Anzurat Akobirshoeva, Ifrat N. Zilfikarov, and Cecile Vennos. 2017. "Isorhamnetin and Quercetin Derivatives as Anti-Acetylcholinesterase Principles of Marigold (Calendula officinalis) Flowers and Preparations" International Journal of Molecular Sciences 18, no. 8: 1685. https://doi.org/10.3390/ijms18081685
APA StyleOlennikov, D. N., Kashchenko, N. I., Chirikova, N. K., Akobirshoeva, A., Zilfikarov, I. N., & Vennos, C. (2017). Isorhamnetin and Quercetin Derivatives as Anti-Acetylcholinesterase Principles of Marigold (Calendula officinalis) Flowers and Preparations. International Journal of Molecular Sciences, 18(8), 1685. https://doi.org/10.3390/ijms18081685