Extracorporeal Shock Wave Therapy Alters the Expression of Fibrosis-Related Molecules in Fibroblast Derived from Human Hypertrophic Scar
Abstract
:1. Introduction
2. Results
2.1. Characterization of HTSFs
2.2. Effects of ESWT on HTSF Viability
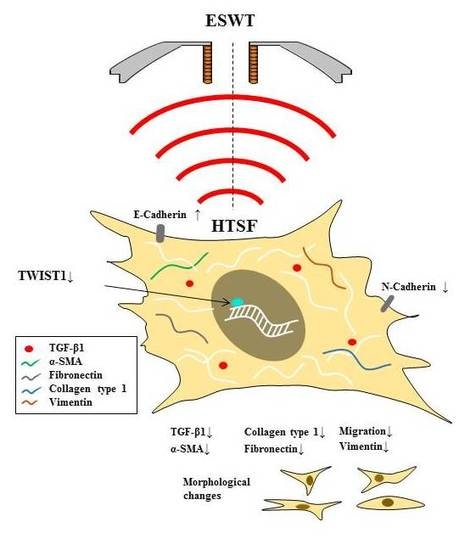
2.3. Effects of ESWT on TGF-β1, α-SMA and Vimentin Expression in HTSFs
2.4. Effects of ESWT on Expression of ECM-Related Proteins in HTSFs
2.5. Effects of ESWT on Expression of N- and E-Cadherin in HTSFs
2.6. Effects of ESWT on Transcription Factor Expression in HTSFs
2.7. Effects of ESWT on HTSF Migration
3. Discussion
4. Materials and Methods
4.1. Primary Cell Culture
4.2. ESWT
4.3. Cell Viability Assay
4.4. HTSF Migration Assay
4.5. qRT-PCR
4.6. Western Blotting
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gauglitz, G.G.; Korting, H.C.; Pavicic, T.; Ruzicka, T.; Jeschke, M.G. Hypertrophic scarring and keloids: Pathomechanisms and current and emerging treatment strategies. Mol. Med. 2011, 17, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Aarabi, S.; Longaker, M.T.; Gurtner, G.C. Hypertrophic scar formation following burns and trauma: New approaches to treatment. PLoS Med. 2007, 4, e234. [Google Scholar] [CrossRef] [PubMed]
- Armour, A.; Scott, P.G.; Tredget, E.E. Cellular and molecular pathology of HTS: Basis for treatment. Wound Repair Regen. 2007, 15 (Suppl. 1), S6–S17. [Google Scholar] [CrossRef] [PubMed]
- Wynn, T.A. Fibrotic disease and the T(H)1/T(H)2 paradigm. Nat. Rev. Immunol. 2004, 4, 583–594. [Google Scholar] [CrossRef] [PubMed]
- Tredget, E.; Shankowsky, H.; Pannu, R.; Nedelec, B.; Iwashina, T.; Ghahary, A.; Taerum, T.; Scott, P. Transforming growth factor-β in thermally injured patients with hypertrophic scars: Effects of interferon α2b. Plast. Reconstr. Surg. 1998, 102, 1317–1328. [Google Scholar] [CrossRef] [PubMed]
- Schmid, P.; Itin, P.; Cherry, G.; Bi, C.; Cox, D.A. Enhanced expression of transforming growth factor-β type I and type II receptors in wound granulation tissue and hypertrophic scar. Am. J. Pathol. 1998, 152, 485–493. [Google Scholar] [PubMed]
- Yan, C.; Grimm, W.A.; Garner, W.L.; Qin, L.; Travis, T.; Tan, N.; Han, Y.P. Epithelial to mesenchymal transition in human skin wound healing is induced by tumor necrosis factor-α through bone morphogenic protein-2. Am. J. Pathol. 2010, 176, 2247–2258. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Ghahary, A.; Shen, Q.; Scott, P.G.; Roy, K.; Tredget, E.E. Hypertrophic scar tissues and fibroblasts produce more transforming growth factor-β1 mRNA and protein than normal skin and cells. Wound Repair. Regen. 2000, 8, 128–137. [Google Scholar] [CrossRef] [PubMed]
- Nedelec, B.; Shankowsky, H.; Scott, P.G.; Ghahary, A.; Tredget, E.E. Myofibroblasts and apoptosis in human hypertrophic scars: The effect of interferon-α2b. Surgery 2001, 130, 798–808. [Google Scholar] [CrossRef] [PubMed]
- Garner, W.L.; Karmiol, S.; Rodriguez, J.L.; Smith, D.J., Jr.; Phan, S.H. Phenotypic differences in cytokine responsiveness of hypertrophic scar versus normal dermal fibroblasts. J. Investig. Dermatol. 1993, 101, 875–879. [Google Scholar] [CrossRef] [PubMed]
- Honardoust, D.; Ding, J.; Varkey, M.; Shankowsky, H.A.; Tredget, E.E. Deep dermal fibroblasts refractory to migration and decorin-induced apoptosis contribute to hypertrophic scarring. J. Burn Care Res. 2012, 33, 668–677. [Google Scholar] [CrossRef] [PubMed]
- Notarnicola, A.; Moretti, B. The biological effects of extracorporeal shock wave therapy (ESWT) on tendon tissue. Muscles Ligaments Tendons J. 2012, 2, 33–37. [Google Scholar] [PubMed]
- Wang, C.J. Extracorporeal shockwave therapy in musculoskeletal disorders. J. Orthop. Surg. Res. 2012, 20, 11. [Google Scholar] [CrossRef] [PubMed]
- Mariotto, S.; Cavalieri, E.; Amelio, E.; Ciampa, A.R.; de Prati, A.C.; Marlinghaus, E.; Russo, S.; Suzuki, H. Extracorporeal shock waves: From lithotripsy to anti-inflammatory action by NO production. Nitric Oxide 2005, 12, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Zins, S.R.; Amare, M.F.; Tadaki, D.K.; Elster, E.A.; Davis, T.A. Comparative analysis of angiogenic gene expression in normal and impaired wound healing in diabetic mice: Effects of extracorporeal shock wave therapy. Angiogenesis 2010, 13, 293–304. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.J.; Huang, H.Y.; Pai, C.H. Shock wave enhances neovascularization at the tendon-bone junction. J. Foot Ankle Surg. 2002, 41, 16–22. [Google Scholar] [CrossRef]
- Wang, C.J.; Yang, K.D.; Wang, F.S.; Huang, C.C.; Yang, L.J. Shock wave induces neovascularization at the tendon-bone junction. A study in rabbits. J. Orthop. Res. 2003, 21, 984–989. [Google Scholar] [CrossRef]
- Schaden, W.; Thiele, R.; Kolpl, C.; Pusch, M.; Nissan, A.; Attinger, C.E.; Maniscalco-Theberge, M.E.; Peoples, G.E.; Elster, E.A.; Stojadinovic, A. Shock wave therapy for acute and chronic soft tissue wounds: A feasibility study. J. Surg. Res. 2007, 143, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Fioramonti, P.; Cigna, E.; Onesti, M.G.; Fino, P.; Fallico, N.; Scuderi, N. Extracorporeal shock wave therapy for the management of burn scars. Dermatol. Surg. 2012, 38, 778–782. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.S.; Joo, S.Y.; Cui, H.; Cho, S.R.; Yim, H.; Seo, C.H. Effect of extracorporeal shock wave therapy on scar pain in burn patients: A prospective, randomized, single-blind, placebo-controlled study. Medicine 2016, 95, e4575. [Google Scholar] [CrossRef] [PubMed]
- Joo, S.Y.; Cho, Y.S.; Seo, C.H. The clinical utility of extracorporeal shock wave therapy for burn pruritus: A prospective, randomized, single-blind study. Burns 2017. [Google Scholar] [CrossRef] [PubMed]
- Je, Y.J.; Choi, D.K.; Sohn, K.C.; Kim, H.R.; Im, M.; Lee, Y.; Lee, J.H.; Kim, C.D.; Seo, Y.J. Inhibitory role of Id1 on TGF-β-induced collagen expression in human dermal fibroblasts. Biochem. Biophys. Res. Commun. 2014, 444, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Velikoff, M.; Agarwal, M.; Disayabutr, S.; Wolters, P.J.; Kim, K.K. Overexpression of inhibitor of DNA-binding 2 attenuates pulmonary fibrosis through regulation of c-Abl and Twist. Am. J. Pathol. 2015, 185, 1001–1011. [Google Scholar] [CrossRef] [PubMed]
- Barriere, G.; Fici, P.; Gallerani, G.; Fabbri, F.; Rigaud, M. Epithelial Mesenchymal Transition: A double-edged sword. Clin. Transl. Med. 2015, 14, 4. [Google Scholar] [CrossRef] [PubMed]
- Weber, C.E.; Li, N.Y.; Wai, P.Y.; Kuo, P.C. Epithelial-mesenchymal transition, TGF-β, and osteopontin in wound healing and tissue remodeling after injury. J. Burn. Care Res. 2012, 33, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, R.; Neilson, E.G. Epithelial-mesenchymal transition and its implications for fibrosis. J. Clin. Investig. 2003, 112, 1776–1784. [Google Scholar] [CrossRef] [PubMed]
- Song, R.; Bian, H.N.; Lai, W.; Chen, H.D.; Zhao, K.S. Normal skin and hypertrophic scar fibroblasts differentially regulate collagen and fibronectin expression as well as mitochondrial membrane potential in response to basic fibroblast growth factor. Braz. J. Med. Biol. Res. 2011, 44, 402–410. [Google Scholar] [CrossRef] [PubMed]
- Penn, J.W.; Grobbelaar, A.O.; Rolfe, K.J. The role of the TGF-β family in wound healing, burns and scarring: A review. Int. J. Burns Trauma 2012, 2, 18–28. [Google Scholar] [PubMed]
- Vetrano, M.; d’Alessandro, F.; Torrisi, M.R.; Ferretti, A.; Vulpiani, M.C.; Visco, V. Extracorporeal shock wave therapy promotes cell proliferation and collagen synthesis of primary cultured human tenocytes. Knee Surg. Sports Traumatol. Arthrosc. 2011, 19, 2159–2168. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, A.; Ritz, U.; Hessmann, M.H.; Alini, M.; Rommens, P.M.; Rompe, J.D. Extracorporeal shock wave-mediated changes in proliferation, differentiation, and gene expression of human osteoblasts. J. Trauma 2008, 65, 1402–1410. [Google Scholar] [CrossRef] [PubMed]
- Saggini, R.; Saggini, A.; Spagnoli, A.M.; Dodaj, I.; Cigna, E.; Maruccia, M.; Soda, G.; Bellomo, R.G.; Scuderi, N. Extracorporeal Shock Wave Therapy: An Emerging Treatment Modality for Retracting Scars of the Hands. Ultrasound Med. Biol. 2016, 42, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Arnó, A.; García, O.; Hernán, I.; Sancho, J.; Acosta, A.; Barret, J.P. Extracorporeal shock waves, a new non-surgical method to treat severe burns. Burns 2010, 36, 844–849. [Google Scholar] [CrossRef] [PubMed]
- Linge, C.; Richardson, J.; Vigor, C.; Clayton, E.; Hardas, B.; Rolfe, K. Hypertrophic scar cells fail to undergo a form of apoptosis specific to contractile collagen-the role of tissue transglutaminase. J. Investig. Dermatol. 2005, 125, 72–82. [Google Scholar] [CrossRef] [PubMed]
- Albanna, M.; Homes, J.H., IV. Skin Tissue Engineering and Regenerative Medicine, 1st ed.; Academic Press: London, UK, 2016; pp. 23–32. ISBN 9780128016541. [Google Scholar]
- Lee, C.G.; Homer, R.J.; Zhu, Z.; Lanone, S.; Wang, X.; Koteliansky, V.; Shipley, J.M.; Gotwals, P.; Noble, P.; Chen, Q.; et al. Interleukin-13 induces tissue fibrosis by selectively stimulating and activating transforming growth factor β1. J. Exp. Med. 2001, 194, 809–821. [Google Scholar] [CrossRef] [PubMed]
- Zeisberg, M.; Neilson, E.G. Biomarkers for epithelial-mesenchymal transitions. J. Clin. Investig. 2009, 119, 1429–1437. [Google Scholar] [CrossRef] [PubMed]
- Derycke, L.D.; Bracke, M.E. N-Cadherin in the spotlight of cell–cell adhesion, differentiation, embryogenesis, invasion and signalling. Int. J. Dev. Biol. 2004, 48, 463–476. [Google Scholar] [CrossRef] [PubMed]
- Bhowmick, N.A.; Ghiassi, M.; Bakin, A.; Aakre, M.; Lundquist, C.A.; Engel, M.E.; Arteaga, C.L.; Moses, H.L. Transforming growth factor-β1 mediates epithelial to mesenchymal transdifferentiation through a RhoA-dependent mechanism. Mol. Biol. Cell 2001, 12, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.; Zimmerman, N.P.; Agle, K.A.; Turner, J.R.; Kumar, S.N.; Dwinell, M.B. E-Cadherin is critical for collective sheet migration and is regulated by the chemokine CXCL12 protein during restitution. J. Biol. Chem. 2012, 287, 22227–22240. [Google Scholar] [CrossRef] [PubMed]
- Benezra, R.; Davis, R.L.; Lockshon, D.; Turner, D.L.; Weintraub, H. The protein Id: A negative regulator of helix-loop-helix DNA binding proteins. Cell 1990, 6, 49–59. [Google Scholar] [CrossRef]
- Lasorella, A.; Benezra, R.; Iavarone, A. The ID proteins: Master regulators of cancer stem cells and tumour aggressiveness. Nat. Rev. Cancer 2014, 14, 77–91. [Google Scholar] [CrossRef] [PubMed]
- Hecker, L.; Jagirdar, R.; Jin, T.; Thannickal, V.J. Reversible differentiation of myofibroblasts by MyoD. Exp. Cell Res. 2011, 317, 1914–1921. [Google Scholar] [CrossRef] [PubMed]
- Garamszegi, N.; Garamszegi, S.P.; Samavarchi-Tehrani, P.; Walford, E.; Schneiderbauer, M.M.; Wrana, J.L.; Scully, S.P. Extracellular matrix-induced transforming growth factor-β receptor signaling dynamics. Oncogene 2010, 29, 2368–2380. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.S.; Joo, S.Y.; Lee, D.H.; Yu, J.H.; Jeong, J.H.; Kim, J.B.; Seo, C.H. Low temperature plasma induces angiogenic growth factor via up-regulating hypoxia-inducible factor 1α in human dermal fibroblasts. Arch. Biochem. Biophys. 2017, 630, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−∆∆Ct Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]







© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, H.S.; Hong, A.R.; Kim, J.-B.; Yu, J.H.; Cho, Y.S.; Joo, S.Y.; Seo, C.H. Extracorporeal Shock Wave Therapy Alters the Expression of Fibrosis-Related Molecules in Fibroblast Derived from Human Hypertrophic Scar. Int. J. Mol. Sci. 2018, 19, 124. https://doi.org/10.3390/ijms19010124
Cui HS, Hong AR, Kim J-B, Yu JH, Cho YS, Joo SY, Seo CH. Extracorporeal Shock Wave Therapy Alters the Expression of Fibrosis-Related Molecules in Fibroblast Derived from Human Hypertrophic Scar. International Journal of Molecular Sciences. 2018; 19(1):124. https://doi.org/10.3390/ijms19010124
Chicago/Turabian StyleCui, Hui Song, A Ram Hong, June-Bum Kim, Joo Hyang Yu, Yoon Soo Cho, So Young Joo, and Cheong Hoon Seo. 2018. "Extracorporeal Shock Wave Therapy Alters the Expression of Fibrosis-Related Molecules in Fibroblast Derived from Human Hypertrophic Scar" International Journal of Molecular Sciences 19, no. 1: 124. https://doi.org/10.3390/ijms19010124
APA StyleCui, H. S., Hong, A. R., Kim, J. -B., Yu, J. H., Cho, Y. S., Joo, S. Y., & Seo, C. H. (2018). Extracorporeal Shock Wave Therapy Alters the Expression of Fibrosis-Related Molecules in Fibroblast Derived from Human Hypertrophic Scar. International Journal of Molecular Sciences, 19(1), 124. https://doi.org/10.3390/ijms19010124