Generation and Characterization of Antibodies against Opioid Receptors from Zebrafish
Abstract
:1. Introduction
2. Results
2.1. Alignment of Opioid Receptor Sequences
2.2. Generation of Specific Zebrafish Opioid Receptor Antibodies
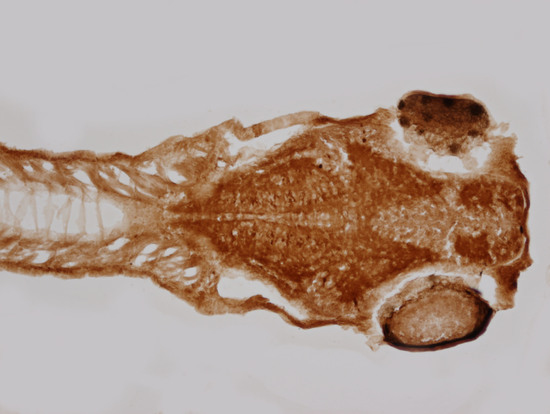
2.3. The Generated Antibodies Recognize Zebrafish Opioid Receptors
3. Discussion
4. Materials and Methods
4.1. Plasmids
4.2. DNA Transfections
4.3. Western Blot Analysis and Immunoprecipitation
4.4. Recombinant Protein Production
4.5. Immunization of Rabbits
4.6. Purification of Antibodies
4.7. Immunohistochemistry on Zebrafish Embryos
4.8. Immunohistochemistry on Zebrafish Sections
4.9. Morpholino Microinjection
4.10. mRNA Probes and Whole-Mount in Situ Hybridization
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Corbett, A.D.; Henderson, G.; McKnight, A.T.; Paterson, S.J. 75 years of opioid research: The exciting but vain quest for the holy grail. Br. J. Pharmacol. 2006, 147, S153–S162. [Google Scholar] [CrossRef] [PubMed]
- Waldhoer, M.; Bartlett, S.E.; Whistler, J.L. Opioid receptors. Annu. Rev. Biochem. 2004, 73, 953–990. [Google Scholar] [CrossRef] [PubMed]
- Loh, H.H.; Liu, H.C.; Cavalli, A.; Yang, W.; Chen, Y.F.; Wei, L.N. Mu opioid receptor knockout in mice: Effects on ligand-induced analgesia and morphine lethality. Brain Res. Mol. Brain Res. 1998, 54, 321–326. [Google Scholar] [CrossRef]
- Matthes, H.W.; Maldonado, R.; Simonin, F.; Valverde, O.; Slowe, S.; Kitchen, I.; Befort, K.; Dierich, A.; Le Meur, M.; Dolle, P.; et al. Loss of morphine-induced analgesia, reward effect and withdrawal symptoms in mice lacking the mu-opioid-receptor gene. Nature 1996, 383, 819–823. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Zhang, Y.; Kalyuzhny, A.E.; Pan, Z.Z. Emergence of functional delta-opioid receptors induced by long-term treatment with morphine. Mol. Pharmacol. 2006, 69, 1137–1145. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; King, M.A.; Schuller, A.G.; Nitsche, J.F.; Reidl, M.; Elde, R.P.; Unterwald, E.; Pasternak, G.W.; Pintar, J.E. Retention of supraspinal delta-like analgesia and loss of morphine tolerance in delta opioid receptor knockout mice. Neuron 1999, 24, 243–252. [Google Scholar] [CrossRef]
- Rudd, R.A.; Seth, P.; David, F.; Scholl, L. Increases in drug and opioid-involved overdose deaths—United States, 2010–2015. Morb. Mortal. Wkly. Rep. 2016, 65, 1445–1452. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Pan, Z.Z. Synaptic mechanism for functional synergism between delta- and mu-opioid receptors. J. Neurosci. 2010, 30, 4735–4745. [Google Scholar] [CrossRef] [PubMed]
- Santoriello, C.; Anelli, V.; Alghisi, E.; Mione, M. Highly penetrant melanoma in a zebrafish model is independent of erbb3b signaling. Pigment Cell Melanoma Res. 2012, 25, 287–289. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, F.A.; Rodriguez-Martin, I.; Gonzalez-Nunez, V.; de Velasco, E.M.; Gonzalez Sarmiento, R.; Rodriguez, R.E. New kappa opioid receptor from zebrafish Danio rerio. Neurosci. Lett. 2006, 405, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Barrallo, A.; Gonzalez-Sarmiento, R.; Alvar, F.; Rodriguez, R.E. Zfor2, a new opioid receptor-like gene from the teleost zebrafish (Danio rerio). Brain Res. Mol. Brain Res. 2000, 84, 1–6. [Google Scholar] [CrossRef]
- Pinal-Seoane, N.; Martin, I.R.; Gonzalez-Nunez, V.; de Velasco, E.M.; Alvarez, F.A.; Sarmiento, R.G.; Rodriguez, R.E. Characterization of a new duplicate delta-opioid receptor from zebrafish. J. Mol. Endocrinol. 2006, 37, 391–403. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, R.E.; Barrallo, A.; Garcia-Malvar, F.; McFadyen, I.J.; Gonzalez-Sarmiento, R.; Traynor, J.R. Characterization of zfor1, a putative delta-opioid receptor from the teleost zebrafish (Danio rerio). Neurosci. Lett. 2000, 288, 207–210. [Google Scholar] [CrossRef]
- Porteros, A.; Garcia-Isidoro, M.; Barrallo, A.; Gonzalez-Sarmiento, R.; Rodriguez, R.E. Expression of zfor1, a delta-opioid receptor, in the central nervous system of the zebrafish (Danio rerio). J. Comp. Neurol. 1999, 412, 429–438. [Google Scholar] [CrossRef]
- Gonzalez-Nunez, V.; Jimenez Gonzalez, A.; Barreto-Valer, K.; Rodriguez, R.E. In vivo regulation of the mu opioid receptor: Role of the endogenous opioid agents. Mol. Med. 2013, 19, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Matrone, G.; Taylor, J.M.; Wilson, K.S.; Baily, J.; Love, G.D.; Girkin, J.M.; Mullins, J.J.; Tucker, C.S.; Denvir, M.A. Laser-targeted ablation of the zebrafish embryonic ventricle: A novel model of cardiac injury and repair. Int. J. Cardiol. 2013, 168, 3913–3919. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McLawhon, R.W.; Cermak, D.; Ellory, J.C.; Dawson, G. Glycosylation-dependent regulation of opiate (enkephalin) receptors in neurotumor cells. J. Neurochem. 1983, 41, 1286–1296. [Google Scholar] [CrossRef] [PubMed]
- Garzon, J.; Juarros, J.L.; Castro, M.A.; Sanchez-Blazquez, P. Antibodies to the cloned mu-opioid receptor detect various molecular weight forms in areas of mouse brain. Mol. Pharmacol. 1995, 47, 738–744. [Google Scholar] [PubMed]
- Arevalo, J.C.; Waite, J.; Rajagopal, R.; Beyna, M.; Chen, Z.Y.; Lee, F.S.; Chao, M.V. Cell survival through trk neurotrophin receptors is differentially regulated by ubiquitination. Neuron 2006, 50, 549–559. [Google Scholar] [CrossRef] [PubMed]
- Cachat, J.; Canavello, P.; Elegante, M.; Bartels, B.; Hart, P.; Bergner, C.; Egan, R.; Duncan, A.; Tien, D.; Chung, A.; et al. Modeling withdrawal syndrome in zebrafish. Behav. Brain Res. 2010, 208, 371–376. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, C.; Hsu, C.H.; Wen, Z.H.; Lin, C.S.; Agoramoorthy, G. Zebrafish: A complete animal model for in vivo drug discovery and development. Curr. Drug Metab. 2009, 10, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Guo, S. Using zebrafish to assess the impact of drugs on neural development and function. Expert Opin. Drug Discov. 2009, 4, 715–726. [Google Scholar] [CrossRef] [PubMed]
- Kari, G.; Rodeck, U.; Dicker, A.P. Zebrafish: An emerging model system for human disease and drug discovery. Clin. Pharmacol. Ther. 2007, 82, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Ninkovic, J.; Bally-Cuif, L. The zebrafish as a model system for assessing the reinforcing properties of drugs of abuse. Methods 2006, 39, 262–274. [Google Scholar] [CrossRef] [PubMed]
- Stewart, A.; Wong, K.; Cachat, J.; Gaikwad, S.; Kyzar, E.; Wu, N.; Hart, P.; Piet, V.; Utterback, E.; Elegante, M.; et al. Zebrafish models to study drug abuse-related phenotypes. Rev. Neurosci. 2011, 22, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Lohi, O.; Parikka, M.; Ramet, M. The zebrafish as a model for paediatric diseases. Acta Paediatr. 2013, 102, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Nasiraei-Moghadam, S.; Kazeminezhad, B.; Dargahi, L.; Ahmadiani, A. Maternal oral consumption of morphine increases bax/bcl-2 ratio and caspase 3 activity during early neural system development in rat embryos. J. Mol. Neurosci. 2010, 41, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Nasiraei-Moghadam, S.; Sahraei, H.; Bahadoran, H.; Sadooghi, M.; Salimi, S.H.; Kaka, G.R.; Imani, H.; Mahdavi-Nasab, H.; Dashtnavard, H. Effects of maternal oral morphine consumption on neural tube development in wistar rats. Brain Res. Dev. Brain Res. 2005, 159, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Malafoglia, V.; Colasanti, M.; Raffaeli, W.; Balciunas, D.; Giordano, A.; Bellipanni, G. Extreme thermal noxious stimuli induce pain responses in zebrafish larvae. J. Cell. Physiol. 2014, 229, 300–308. [Google Scholar] [CrossRef] [PubMed]
- Stevens, C.W. The evolution of vertebrate opioid receptors. Front. Biosci. 2009, 14, 1247–1269. [Google Scholar] [CrossRef]
- Sambrook, J.; Maniatis, T.; Fritsch, E.F. Molecular Cloning: A Laboratory Manual; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 1993. [Google Scholar]
- Yu, T.; Calvo, L.; Anta, B.; Lopez-Benito, S.; Southon, E.; Chao, M.V.; Tessarollo, L.; Arevalo, J.C. Regulation of trafficking of activated trka is critical for ngf-mediated functions. Traffic 2011, 12, 521–534. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Bellido, R.; Barreto-Valer, K.; Sanchez-Simon, F.M.; Rodriguez, R.E. Cocaine modulates the expression of opioid receptors and mir-let-7d in zebrafish embryos. PLoS ONE 2012, 7, e50885. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Simon, F.M.; Zhang, X.X.; Loh, H.H.; Law, P.Y.; Rodriguez, R.E. morphine regulates dopaminergic neuron differentiation via miR-133b. Mol. Pharmacol. 2010, 78, 935–942. [Google Scholar] [CrossRef] [PubMed]
- Nasevicius, A.; Ekker, S.C. Effective targeted gene ‘knockdown’ in zebrafish. Nat. Genet. 2000, 26, 216–220. [Google Scholar] [CrossRef] [PubMed]
- Calvo, L.; Anta, B.; López-Benito, S.; Martín-Rodriguez, C.; Lee, F.S.; Pérez, P.; Martín-Zanca, D.; Arévalo, J.C. Bex3 Dimerization Regulates NGF-Dependent Neuronal Survival and Differentiation by Enhancing trkA Gene Transcription. J. Neurosci. 2015, 35, 7190–7202. [Google Scholar] [CrossRef] [PubMed]





© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arévalo, J.C.; Hernández-Jiménez, E.; Jiménez-González, A.; Torres-Valle, M.; Iwasaki, R.S.; López-Bellido, R.; Vicente-García, C.; Rodríguez, R.E. Generation and Characterization of Antibodies against Opioid Receptors from Zebrafish. Int. J. Mol. Sci. 2018, 19, 14. https://doi.org/10.3390/ijms19010014
Arévalo JC, Hernández-Jiménez E, Jiménez-González A, Torres-Valle M, Iwasaki RS, López-Bellido R, Vicente-García C, Rodríguez RE. Generation and Characterization of Antibodies against Opioid Receptors from Zebrafish. International Journal of Molecular Sciences. 2018; 19(1):14. https://doi.org/10.3390/ijms19010014
Chicago/Turabian StyleArévalo, Juan C., Enrique Hernández-Jiménez, Ada Jiménez-González, María Torres-Valle, Roman S. Iwasaki, Roger López-Bellido, Cristina Vicente-García, and Raquel E. Rodríguez. 2018. "Generation and Characterization of Antibodies against Opioid Receptors from Zebrafish" International Journal of Molecular Sciences 19, no. 1: 14. https://doi.org/10.3390/ijms19010014
APA StyleArévalo, J. C., Hernández-Jiménez, E., Jiménez-González, A., Torres-Valle, M., Iwasaki, R. S., López-Bellido, R., Vicente-García, C., & Rodríguez, R. E. (2018). Generation and Characterization of Antibodies against Opioid Receptors from Zebrafish. International Journal of Molecular Sciences, 19(1), 14. https://doi.org/10.3390/ijms19010014