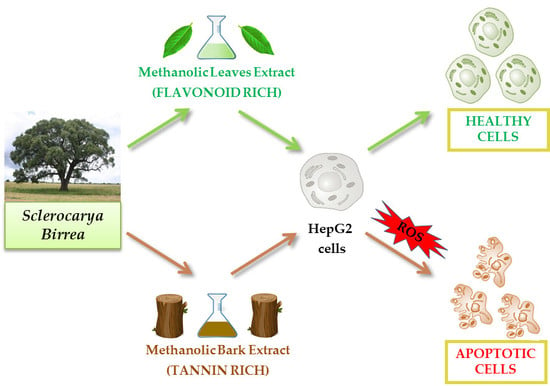
A Comparative Study on Phytochemical Profiles and Biological Activities of Sclerocarya birrea (A.Rich.) Hochst Leaf and Bark Extracts
Abstract
:1. Introduction
2. Results
2.1. Total Polyphenol (TPC), Flavonoid (TFC), and Tannin Content (TTC) Evaluation
2.2. Antioxidant Activity
2.3. Cytotoxic Effect of Bark and Leaf Methanol Extracts
2.4. Cell Morphology Analysis
2.5. Evaluation of Apoptosis in HepG2 Cells Treated with MLE and MBE
2.6. Effects of MLE and MBE on Both ROS Production and Mitochondrial Membrane Potential (ΔΨm)
2.7. Apoptosis Analysis by Western Blotting
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Plant Extracts
4.3. Total Content of Polyphenols, Flavonoids, and Tannins
4.4. In Vitro Antioxidant Activity
4.4.1. ABTS Test
4.4.2. BCB Assay
4.5. Nitric Oxide (NO) Scavenging Activity
4.6. Superoxide Anion (O2−•) Scavenging Activity
4.7. Cell Culture and Treatment with Extracts
4.8. Cytotoxicity Analysis
4.9. Cell Imaging
4.10. Apoptosis Assay
4.11. Reactive Oxygen Species (ROS) Measurements
4.12. Mitochondrial Membrane Potential (MMP) Evaluation
4.13. Western Blot Analysis
4.14. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cragg, G.M.; Newman, D.J. Natural products: A continuing source of novel drug leads. Biochim. Biophys. Acta 2013, 1830, 3670–3695. [Google Scholar] [CrossRef] [PubMed]
- Bisio, A.; de Mieri, M.; Milella, L.; Schito, A.M.; Parricchi, A.; Russo, D.; Alfei, S.; Lapillo, M.; Tuccinardi, T.; Hamburger, M. Antibacterial and Hypoglycemic Diterpenoids from Salvia chamaedryoides. J. Nat. Prod. 2017, 80, 503–514. [Google Scholar] [CrossRef] [PubMed]
- Milella, L.; Milazzo, S.; de Leo, M.; Vera Saltos, M.B.; Faraone, I.; Tuccinardi, T.; Lapillo, M.; de Tommasi, N.; Braca, A. α-Glucosidase and α-Amylase Inhibitors from Arcytophyllum thymifolium. J. Nat. Prod. 2016, 79, 2104–2112. [Google Scholar] [CrossRef] [PubMed]
- Gerbino, A.; Schena, G.; Milano, S.; Milella, L.; Barbosa, A.F.; Armentano, F.; Procino, G.; Svelto, M.; Carmosino, M. Spilanthol from Acmella oleracea lowers the intracellular levels of cAMP impairing NKCC2 phosphorylation and water channel AQP2 membrane expression in mouse kidney. PLoS ONE 2016, 11, e0156021. [Google Scholar] [CrossRef] [PubMed]
- Ntie-Kang, F.; Njume, L.E.; Malange, Y.I.; Günther, S.; Sippl, W.; Yong, J.N. The chemistry and biological activities of natural products from northern african plant families: From taccaceae to Zygophyllaceae. Nat. Prod. Bioprospect. 2016, 6, 63–96. [Google Scholar] [CrossRef] [PubMed]
- Mileo, A.M.; Miccadei, S. Polyphenols as modulator of oxidative stress in cancer disease: New therapeutic strategies. Oxid. Med. Cell. Longev. 2016, 2016. [Google Scholar] [CrossRef] [PubMed]
- Malongane, F.; McGaw, L.; Mudau, F. The synergistic potential of various teas, herbs and therapeutic drugs in health improvement: A review. J. Sci. Food Agric. 2017, 97, 4679–4689. [Google Scholar] [CrossRef] [PubMed]
- León-González, A.J.; Auger, C.; Schini-Kerth, V.B. Pro-oxidant activity of polyphenols and its implication on cancer chemoprevention and chemotherapy. Biochem. Pharmacol. 2015, 98, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Braca, A.; Politi, M.; Sanogo, R.; Sanou, H.; Morelli, I.; Pizza, C.; de Tommasi, N. Chemical composition and antioxidant activity of phenolic compounds from wild and cultivated Sclerocarya birrea (Anacardiaceae) leaves. J. Agric. Food Chem. 2003, 51, 6689–6695. [Google Scholar] [CrossRef] [PubMed]
- Hamza, O.J.; van den Bout-van, C.J.; Matee, M.I.; Moshi, M.J.; Mikx, F.H.; Selemani, H.O.; Mbwambo, Z.H.; van der Ven, A.J.; Verweij, P.E. Antifungal activity of some Tanzanian plants used traditionally for the treatment of fungal infections. J. Ethnopharmacol. 2006, 108, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Mariod, A.A.; Matthäus, B.; Hussein, I.H. Antioxidant properties of methanolic extracts from different parts of Sclerocarya birrea. Int. J. Food Sci. Technol. 2008, 43, 921–926. [Google Scholar] [CrossRef]
- Armentano, M.F.; Bisaccia, F.; Miglionico, R.; Russo, D.; Nolfi, N.; Carmosino, M.; Andrade, P.B.; Valentão, P.; Diop, M.S.; Milella, L. Antioxidant and proapoptotic activities of Sclerocarya birrea [(A. Rich.) Hochst.] methanolic root extract on the hepatocellular carcinoma cell line HepG2. BioMed Res. Int. 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Tanih, N.F.; Ndip, R.N. The acetone extract of Sclerocarya birrea (Anacardiaceae) possesses antiproliferative and apoptotic potential against human breast cancer cell lines (MCF-7). Sci. World J. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Russo, D.; Kenny, O.; Smyth, T.J.; Milella, L.; Hossain, M.B.; Diop, M.S.; Rai, D.K.; Brunton, N.P. Profiling of phytochemicals in tissues from Sclerocarya birrea by HPLC-MS and their link with antioxidant activity. ISRN Chromatogr. 2013, 2013. [Google Scholar] [CrossRef]
- Schulze-Kaysers, N.; Feuereisen, M.; Schieber, A. Phenolic compounds in edible species of the Anacardiaceae family—A review. RSC Adv. 2015, 5, 73301–73314. [Google Scholar] [CrossRef]
- Jiménez-Sánchez, C.; Lozano-Sánchez, J.; Gabaldón-Hernández, J.A.; Segura-Carretero, A.; Fernández-Gutiérrez, A. RP-HPLC-ESI-QTOF/MS 2 based strategy for the comprehensive metabolite profiling of Sclerocarya birrea (marula) bark. Ind. Crops Prod. 2015, 71, 214–234. [Google Scholar] [CrossRef]
- Milella, L. Antioxidant Activity of Different Extracts from Marula; Department of Science, University of Basilicata: Potenza, Italy, 2017. [Google Scholar]
- Russo, D.; Valentão, P.; Andrade, P.B.; Fernandez, E.C.; Milella, L. Evaluation of antioxidant, antidiabetic and anticholinesterase activities of Smallanthus sonchifolius landraces and correlation with their phytochemical profiles. Int. J. Mol. Sci. 2015, 16, 17696–17718. [Google Scholar] [CrossRef] [PubMed]
- Hilou, A.; Bougma, A.; Dicko, M.H. Phytochemistry and Agro-Industrial Potential of Native Oilseeds from West Africa: African Grape (Lannea microcarpa), Marula (Sclerocarya birrea), and Butter Tree (Pentadesma butyracea). Agriculture 2017, 7, 24. [Google Scholar] [CrossRef]
- Ugartondo, V.; Mitjans, M.; Touriño, S.; Torres, J.L.; Vinardell, M.P. Comparative antioxidant and cytotoxic effect of procyanidin fractions from grape and pine. Chem. Res. Toxicol. 2007, 20, 1543–1548. [Google Scholar] [CrossRef] [PubMed]
- Rue, E.A.; Rush, M.D.; van Breemen, R.B. Procyanidins: A comprehensive review encompassing structure elucidation via mass spectrometry. Phytochem. Rev. 2017, 1–16. [Google Scholar] [CrossRef]
- Dekdouk, N.; Malafronte, N.; Russo, D.; Faraone, I.; de Tommasi, N.; Ameddah, S.; Severino, L.; Milella, L. Phenolic compounds from Olea europaea L. possess antioxidant activity and inhibit carbohydrate metabolizing enzymes in vitro. Evid.-Based Complement. Altern. Med. 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Ashkenazi, A. Targeting the extrinsic apoptotic pathway in cancer: Lessons learned and future directions. J. Clin. Investig. 2015, 125. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.; Watari, H.; AbuAlmaaty, A.; Ohba, Y.; Sakuragi, N. Apoptosis and molecular targeting therapy in cancer. BioMed Res. Int. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Somasagara, R.R.; Hegde, M.; Nishana, M.; Tadi, S.K.; Srivastava, M.; Choudhary, B.; Raghavan, S.C. Quercetin, a natural flavonoid interacts with DNA, arrests cell cycle and causes tumor regression by activating mitochondrial pathway of apoptosis. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Ha, S.H.; Jin, F.; Kwak, C.H.; Abekura, F.; Park, J.Y.; Park, N.G.; Chang, Y.C.; Lee, Y.C.; Chung, T.W.; Ha, K.T. Jellyfish extract induces apoptotic cell death through the p38 pathway and cell cycle arrest in chronic myelogenous leukemia K562 cells. PeerJ 2017, 5, e2895. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, T.; Song, Y.; He, C.; Wang, D.; Morita, K.; Tsukada, J.; Kanazawa, T.; Yoshida, Y. Relationship between triterpenoid anticancer drug resistance, autophagy, and caspase-1 in adult T-cell leukemia. PeerJ 2016, 4, e2026. [Google Scholar] [CrossRef] [PubMed]
- Zielińska-Przyjemska, M.; Kaczmarek, M.; Krajka-Kuźniak, V.; Łuczak, M.; Baer-Dubowska, W. The effect of resveratrol, its naturally occurring derivatives and tannic acid on the induction of cell cycle arrest and apoptosis in rat C6 and human T98G glioma cell lines. Toxicol. In Vitro 2017, 43, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Giorgio, V.; Guo, L.; Bassot, C.; Petronilli, V.; Bernardi, P. Calcium and regulation of the mitochondrial permeability transition. Cell Calcium 2017. [Google Scholar] [CrossRef] [PubMed]
- Edlich, F.; Martinou, J.C. Bcl-2 Protein Interplay on the Outer Mitochondrial Membrane. In Mitochondria and Cell Death; Cell Death in Biology and Diseases Series; Hockenbery, D., Ed.; Humana Press: New York, NY, USA, 2013; pp. 69–83. ISBN 978-1-4939-3610-6. [Google Scholar]
- Vizetto-Duarte, C.; Custódio, L.; Gangadhar, K.N.; Lago, J.H.G.; Dias, C.; Matos, A.M.; Neng, N.; Nogueira, J.M.F.; Barreira, L.; Albericio, F. Isololiolide, a carotenoid metabolite isolated from the brown alga Cystoseira tamariscifolia, is cytotoxic and able to induce apoptosis in hepatocarcinoma cells through caspase-3 activation, decreased Bcl-2 levels, increased p53 expression and PARP cleavage. Phytomedicine 2016, 23, 550–557. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Pandey, A.K. Chemistry and biological activities of flavonoids: An overview. Sci. World J. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Massini, L.; Rico, D.; Martin-Díana, A.; Barry-Ryan, C. Study of antioxidant properties of fractionated apple peel phenolics using a multiple-assay approach. Int. Food Res. J. 2016, 23, 1996–2005. [Google Scholar]
- Chen, J.; Teng, J.; Ma, L.; Tong, H.; Ren, B.; Wang, L.; Li, W. Flavonoids isolated from the flowers of Limonium bicolor and their in vitro antitumor evaluation. Pharmacogn. Mag. 2017, 13, 222–225. [Google Scholar] [CrossRef] [PubMed]
- Karas, D.; Ulrichová, J.; Valentová, K. Galloylation of polyphenols alters their biological activity. Food Chem. Toxicol. 2017, 105, 223–240. [Google Scholar] [CrossRef] [PubMed]
- Avelar, M.M.; Gouvêa, C.M. Procyanidin B2 cytotoxicity to MCF-7 human breast adenocarcinoma cells. Indian J. Pharm. Sci. 2012, 74. [Google Scholar] [CrossRef]
- Hah, Y.S.; Kim, J.G.; Cho, H.Y.; Park, J.S.; Heo, E.P.; Yoon, T.J. Procyanidins from Vitis vinifera seeds induce apoptotic and autophagic cell death via generation of reactive oxygen species in squamous cell carcinoma cells. Oncol. Lett. 2017, 14, 1925–1932. [Google Scholar] [CrossRef] [PubMed]
- Taparia, S.S.; Khanna, A. Procyanidin-rich extract of natural cocoa powder causes ROS-mediated caspase-3 dependent apoptosis and reduction of pro-MMP-2 in epithelial ovarian carcinoma cell lines. Biomed. Pharmacother. 2016, 83, 130–140. [Google Scholar] [CrossRef] [PubMed]
- Actis-Goretta, L.; Romanczyk, L.J.; Rodriguez, C.A.; Kwik-Uribe, C.; Keen, C.L. Cytotoxic effects of digalloyl dimer procyanidins in human cancer cell lines. J. Nutr. Biochem. 2008, 19, 797–808. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Xu, H.; Luo, X.; Zhang, H.; He, Y.; Sun, G.; Sun, X. Procyanidins from Nelumbo nucifera Gaertn. Seedpod induce autophagy mediated by reactive oxygen species generation in human hepatoma G2 cells. Biomed. Pharmacother. 2016, 79, 135–152. [Google Scholar] [CrossRef] [PubMed]
- Mezrag, A.; Malafronte, N.; Bouheroum, M.; Travaglino, C.; Russo, D.; Milella, L.; Severino, L.; de Tommasi, N.; Braca, A.; Dal Piaz, F. Phytochemical and antioxidant activity studies on Ononis angustissima L. aerial parts: Isolation of two new flavonoids. Nat. Prod. Res. 2017, 31, 507–514. [Google Scholar] [CrossRef] [PubMed]








| TPC (mg GAE/g) | TTC (mg TAE/g) | TFC (mg QE/g) | |
|---|---|---|---|
| HLE | 30.2 ± 1.3 | 102.4 ± 3.1 | 8.3 ± 1.2 |
| CLE | 41.7 ± 0.6 | 175.8 ± 5.5 | 17.7 ± 2.0 |
| CMLE | 49.9 ± 3.7 | 74.2 ± 5.5 | 31.1 ± 4.1 |
| MLE | 62.6 ± 0.8 | 90.2 ± 4.7 | 132.7 ± 10.4 |
| HBE | 79.9 ± 0.7 | 196.1 ± 12.5 | 4.7 ± 0.5 |
| CBE | 31.9 ± 1.6 | 211.2 ± 14.8 | 9.5 ± 1.2 |
| CMBE | 31.3 ± 0.2 | 158.7 ± 1.6 | 15.6 ± 2.1 |
| MBE | 241.3 ± 8.5 | 949.5 ± 29.7 | 57.7 ± 3.5 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Russo, D.; Miglionico, R.; Carmosino, M.; Bisaccia, F.; Andrade, P.B.; Valentão, P.; Milella, L.; Armentano, M.F. A Comparative Study on Phytochemical Profiles and Biological Activities of Sclerocarya birrea (A.Rich.) Hochst Leaf and Bark Extracts. Int. J. Mol. Sci. 2018, 19, 186. https://doi.org/10.3390/ijms19010186
Russo D, Miglionico R, Carmosino M, Bisaccia F, Andrade PB, Valentão P, Milella L, Armentano MF. A Comparative Study on Phytochemical Profiles and Biological Activities of Sclerocarya birrea (A.Rich.) Hochst Leaf and Bark Extracts. International Journal of Molecular Sciences. 2018; 19(1):186. https://doi.org/10.3390/ijms19010186
Chicago/Turabian StyleRusso, Daniela, Rocchina Miglionico, Monica Carmosino, Faustino Bisaccia, Paula B. Andrade, Patrícia Valentão, Luigi Milella, and Maria Francesca Armentano. 2018. "A Comparative Study on Phytochemical Profiles and Biological Activities of Sclerocarya birrea (A.Rich.) Hochst Leaf and Bark Extracts" International Journal of Molecular Sciences 19, no. 1: 186. https://doi.org/10.3390/ijms19010186
APA StyleRusso, D., Miglionico, R., Carmosino, M., Bisaccia, F., Andrade, P. B., Valentão, P., Milella, L., & Armentano, M. F. (2018). A Comparative Study on Phytochemical Profiles and Biological Activities of Sclerocarya birrea (A.Rich.) Hochst Leaf and Bark Extracts. International Journal of Molecular Sciences, 19(1), 186. https://doi.org/10.3390/ijms19010186