3,3′-Diindolylmethane Suppressed Cyprodinil-Induced Epithelial-Mesenchymal Transition and Metastatic-Related Behaviors of Human Endometrial Ishikawa Cells via an Estrogen Receptor-Dependent Pathway
Abstract
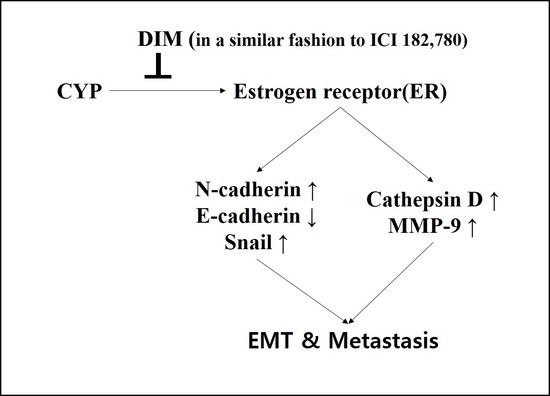
:1. Introduction
2. Results
2.1. Effects of CYP Exposure on Cell Viability of Ishikawa Endometrial Cancer Cells
2.2. Morphological Changes in Ishikawa Cells in Response to Treatment with E2 and CYP in the Presence or Absence of ICI or DIM
2.3. Effects of CYP and DIM on the Expression of EMT Related Genes
2.4. Suppression of DIM on CYP-Induced Ishikawa Endometrial Cancer Cell Migration
2.5. Suppression of DIM on CYP-Induced Ishikawa Endometrial Cancer Cell Invasion
2.6. Effects of CYP and DIM on the Expression of Metastasis Related Genes
3. Discussion
4. Materials and Methods
4.1. Reagents and Chemicals
4.2. Cell Culture and Media
4.3. Cell Viability Assay
4.4. Protein Extraction and Western Blot Assay
4.5. Effects of E2, CYP, or DIM on Ishikawa Cells Morphology
4.6. Scratch-Wound Healing Assay
4.7. Data Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Dutta, S.; Kharkar, P.S.; Sahu, N.U.; Khanna, A. Molecular docking prediction and in vitro studies elucidate anti-cancer activity of phytoestrogens. Life Sci. 2017, 185, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Murkies, A.L.; Wilcox, G.; Davis, S.R. Clinical review 92: Phytoestrogens. J. Clin. Endocrinol. Metab. 1998, 83, 297–303. [Google Scholar] [PubMed]
- Lee, G.A.; Hwang, K.A.; Choi, K.C. Roles of Dietary Phytoestrogens on the Regulation of Epithelial-Mesenchymal Transition in Diverse Cancer Metastasis. Toxins 2016, 8, 162. [Google Scholar] [CrossRef] [PubMed]
- Hwang, K.A.; Choi, K.C. Anticarcinogenic Effects of Dietary Phytoestrogens and Their Chemopreventive Mechanisms. Nutr. Cancer 2015, 67, 796–803. [Google Scholar] [CrossRef] [PubMed]
- Qadir, M.I.; Cheema, B.N. Phytoestrogens and Related Food Components in the Prevention of Cancer. Crit. Rev. Eukaryot. Gene Expr. 2017, 27, 99–112. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.; Sepkovic, D.; Bradlow, H.L.; Auborn, K.J. 3,3′-Diindolylmethane and genistein decrease the adverse effects of estrogen in LNCaP and PC-3 prostate cancer cells. J. Nutr. 2008, 138, 2379–2385. [Google Scholar] [CrossRef] [PubMed]
- Thomson, C.A.; Ho, E.; Strom, M.B. Chemopreventive properties of 3,3′-diindolylmethane in breast cancer: Evidence from experimental and human studies. Nutr. Rev. 2016, 74, 432–443. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Gao, H.; Gui, S.; Bai, G.; Lu, R.; Wang, F.; Zhang, Y. Effects of the estrogen receptor antagonist fulvestrant on F344 rat prolactinoma models. J. Neurooncol. 2014, 116, 523–531. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.A.; Hwang, K.A.; Choi, K.C. Inhibitory effects of 3,3′-diindolylmethane on epithelial-mesenchymal transition induced by endocrine disrupting chemicals in cellular and xenograft mouse models of breast cancer. Food Chem. Toxicol. 2017, 109 Pt 1, 284–295. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.A.; Choi, K.C.; Hwang, K.A. Kaempferol, a phytoestrogen, suppressed triclosan-induced epithelial-mesenchymal transition and metastatic-related behaviors of MCF-7 breast cancer cells. Environ. Toxicol. Pharmacol. 2017, 49, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, T.; Chun, S.Y.; Lee, K.S.; Kim, S.; Nam, K.S. The anti-metastatic effects of the phytoestrogen arctigenin on human breast cancer cell lines regardless of the status of ER expression. Int. J. Oncol. 2017, 50, 727–735. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Hwang, K.A.; Hyun, S.H.; Nam, K.H.; Lee, C.K.; Choi, K.C. Bisphenol A and nonylphenol have the potential to stimulate the migration of ovarian cancer cells by inducing epithelial-mesenchymal transition via an estrogen receptor dependent pathway. Chem. Res. Toxicol. 2015, 28, 662–671. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Yang, D.; Zong, H.; Zhu, L.; Wang, L.; Wang, X.; Zhu, X.; Song, X.; Wang, J. Growth-induced stress enhances epithelial-mesenchymal transition induced by IL-6 in clear cell renal cell carcinoma via the Akt/GSK-3beta/beta-catenin signaling pathway. Oncogenesis 2017, 6, e375. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Liang, F.; Cheng, W.; Zhou, R.; Wu, X.; Feng, Y.; Wang, Y. The mechanisms for lung cancer risk of PM2.5: Induction of epithelial-mesenchymal transition and cancer stem cell properties in human non-small cell lung cancer cells. Environ. Toxicol. 2017, 32, 2341–2351. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.S.; Jiang, J.; Liang, X.H.; Tang, Y.L. Links between cancer stem cells and epithelial-mesenchymal transition. Onco Targets Ther. 2015, 8, 2973–2980. [Google Scholar] [PubMed]
- Heerboth, S.; Housman, G.; Leary, M.; Longacre, M.; Byler, S.; Lapinska, K.; Willbanks, A.; Sarkar, S. EMT and tumor metastasis. Clin. Transl. Med. 2015, 4, 6. [Google Scholar] [CrossRef] [PubMed]
- In, S.J.; Kim, S.H.; Go, R.E.; Hwang, K.A.; Choi, K.C. Benzophenone-1 and nonylphenol stimulated MCF-7 breast cancer growth by regulating cell cycle and metastasis-related genes via an estrogen receptor alpha-dependent pathway. J. Toxicol. Environ. Health A 2015, 78, 492–505. [Google Scholar] [CrossRef] [PubMed]
- Garcia, M.; Platet, N.; Liaudet, E.; Laurent, V.; Derocq, D.; Brouillet, J.P.; Rochefort, H. Biological and clinical significance of cathepsin D in breast cancer metastasis. Stem Cells 1996, 14, 642–650. [Google Scholar] [CrossRef] [PubMed]
- Bretschneider, N.; Kangaspeska, S.; Seifert, M.; Reid, G.; Gannon, F.; Denger, S. E2-mediated cathepsin D (CTSD) activation involves looping of distal enhancer elements. Mol. Oncol. 2008, 2, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.; Go, R.E.; Kim, C.W.; Hwang, K.A.; Nam, K.H.; Choi, K.C. Effect of benzophenone-1 and octylphenol on the regulation of epithelial-mesenchymal transition via an estrogen receptor-dependent pathway in estrogen receptor expressing ovarian cancer cells. Food Chem. Toxicol. 2016, 93, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Choi, H.G.; Lee, H.M.; Lee, G.A.; Hwang, K.A.; Choi, K.C. Effects of bisphenol compounds on the growth and epithelial mesenchymal transition of MCF-7 CV human breast cancer cells. J. Biomed. Res. 2017, 31, 358–369. [Google Scholar] [PubMed]
- Karadag, H.; Ozhan, F. Effect of cyprodinil and fludioxonil pesticides on bovine liver catalase activity. Biotechnol. Biotechnol. Equip. 2015, 29, 40–44. [Google Scholar] [CrossRef] [PubMed]
- Kanetis, L.; Forster, H.; Jones, C.A.; Borkovich, K.A.; Adaskaveg, J.E. Characterization of genetic and biochemical mechanisms of fludioxonil and pyrimethanil resistance in field isolates of Penicillium digitatum. Phytopathology 2008, 98, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Go, R.E.; Kim, C.W.; Choi, K.C. Effect of fenhexamid and cyprodinil on the expression of cell cycle- and metastasis-related genes via an estrogen receptor-dependent pathway in cellular and xenografted ovarian cancer models. Toxicol. Appl. Pharmacol. 2015, 289, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Jin, Y.; Zhou, D.; Xu, G.; Huang, J.; Shen, L. IQGAP1 modulates the proliferation and migration of vascular smooth muscle cells in response to estrogen. Int. J. Mol. Med. 2015, 35, 1460–1466. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.C.; Chen, F.Y.; Chen, C.R.; Liu, C.C.; Wong, L.C.; Liu, Y.W.; Su, J.G. Cyprodinil as an activator of aryl hydrocarbon receptor. Toxicology 2013, 304, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Tamm-Rosenstein, K.; Simm, J.; Suhorutshenko, M.; Salumets, A.; Metsis, M. Changes in the transcriptome of the human endometrial Ishikawa cancer cell line induced by estrogen, progesterone, tamoxifen, and mifepristone (RU486) as detected by RNA-sequencing. PLoS ONE 2013, 8, e68907. [Google Scholar] [CrossRef] [PubMed]
- Guan, X. Cancer metastases: challenges and opportunities. Acta Pharm. Sin. B 2015, 5, 402–418. [Google Scholar] [CrossRef] [PubMed]
- Lo, U.G.; Lee, C.F.; Lee, M.S.; Hsieh, J.T. The Role and Mechanism of Epithelial-to-Mesenchymal Transition in Prostate Cancer Progression. Int. J. Mol. Sci. 2017, 18, 2079. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.M.; Hwang, K.A.; Choi, K.C. Diverse pathways of epithelial mesenchymal transition related with cancer progression and metastasis and potential effects of endocrine disrupting chemicals on epithelial mesenchymal transition process. Mol. Cell. Endocrinol. 2017, 457, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Hwang, K.A.; Choi, K.C. Treatment with kaempferol suppresses breast cancer cell growth caused by estrogen and triclosan in cellular and xenograft breast cancer models. J. Nutr. Biochem. 2016, 28, 70–82. [Google Scholar] [CrossRef] [PubMed]
- Scsukova, S.; Rollerova, E.; Bujnakova Mlynarcikova, A. Impact of endocrine disrupting chemicals on onset and development of female reproductive disorders and hormone-related cancer. Reprod. Biol. 2016, 16, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Choi, K.C.; Hwang, K.A. Genistein suppressed epithelial-mesenchymal transition and migration efficacies of BG-1 ovarian cancer cells activated by estrogenic chemicals via estrogen receptor pathway and downregulation of TGF-beta signaling pathway. Phytomedicine 2015, 22, 993–999. [Google Scholar] [CrossRef] [PubMed]
- Xiao, D.; He, J. Epithelial mesenchymal transition and lung cancer. J. Thorac. Dis. 2010, 2, 154–159. [Google Scholar] [PubMed]
- Zeisberg, M.; Neilson, E.G. Biomarkers for epithelial-mesenchymal transitions. J. Clin. Investig. 2009, 119, 1429–1437. [Google Scholar] [CrossRef] [PubMed]
- Hazan, R.B.; Phillips, G.R.; Qiao, R.F.; Norton, L.; Aaronson, S.A. Exogenous expression of N-cadherin in breast cancer cells induces cell migration, invasion, and metastasis. J. Cell Biol. 2000, 148, 779–790. [Google Scholar] [CrossRef] [PubMed]
- Herrera, A.; Herrera, M.; Pena, C. The emerging role of Snail1 in the tumor stroma. Clin. Transl. Oncol. 2016, 18, 872–877. [Google Scholar] [CrossRef] [PubMed]
- Brzozowa, M.; Michalski, M.; Wyrobiec, G.; Piecuch, A.; Dittfeld, A.; Harabin-Slowinska, M.; Boron, D.; Wojnicz, R. The role of Snail1 transcription factor in colorectal cancer progression and metastasis. Contemp. Oncol. (Pozn.) 2015, 19, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, S.; Doi, R.; Toyoda, E.; Tsuji, S.; Wada, M.; Koizumi, M.; Tulachan, S.S.; Ito, D.; Kami, K.; Mori, T.; et al. N-cadherin expression and epithelial-mesenchymal transition in pancreatic carcinoma. Clin. Cancer Res. 2004, 10, 4125–4133. [Google Scholar] [CrossRef] [PubMed]
- Paksoy, M.; Hardal, U.; Caglar, C. Expression of cathepsin D and E-cadherin in primary laryngeal cancers correlation with neck lymph node involvement. J. Cancer Res. Clin. Oncol. 2011, 137, 1371–1377. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xu, J.; Xing, G. Lycorine inhibits the growth and metastasis of breast cancer through the blockage of STAT3 signaling pathway. Acta Biochim. Biophys. Sin. (Shanghai) 2017, 49, 771–779. [Google Scholar] [CrossRef] [PubMed]
- Auborn, K.J.; Fan, S.; Rosen, E.M.; Goodwin, L.; Chandraskaren, A.; Williams, D.E.; Chen, D.; Carter, T.H. Indole-3-carbinol is a negative regulator of estrogen. J. Nutr. 2003, 133 (Suppl. S7), 2470S–2475S. [Google Scholar] [PubMed]
- Wang, T.T.; Milner, M.J.; Milner, J.A.; Kim, Y.S. Estrogen receptor alpha as a target for indole-3-carbinol. J. Nutr. Biochem. 2006, 17, 659–664. [Google Scholar] [CrossRef] [PubMed]
- Rajoria, S.; Suriano, R.; George, A.; Shanmugam, A.; Schantz, S.P.; Geliebter, J.; Tiwari, R.K. Estrogen induced metastatic modulators MMP-2 and MMP-9 are targets of 3,3′-diindolylmethane in thyroid cancer. PLoS ONE 2011, 6, e15879. [Google Scholar] [CrossRef] [PubMed]







| Protein | Company | Cat No. | Description | Dilution Ratio |
|---|---|---|---|---|
| E-cadherin | Abcam | Ab15148 | Rabbit pAb | 1:500 |
| Occludin | Santa Cruz | Sc-5562 | Rabbit pAb | 1:1000 |
| N-cadherin | Abcom | Ab98952 | Mouse mAb | 1:2000 |
| Snail | Cell signaling | 3895S | Mouse mAb | 1:1000 |
| Cathepsin D | Abcam | Ab75852 | Rabbit mAb | 1:2000 |
| MMP-9 | Abcam | Ab76003 | Rabbit mAb | 1:1000 |
| GAPDH | Abcam | Ab8245 | Mouse mAb | 1:2000 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, B.-G.; Kim, J.-W.; Kim, S.-M.; Go, R.-E.; Hwang, K.-A.; Choi, K.-C. 3,3′-Diindolylmethane Suppressed Cyprodinil-Induced Epithelial-Mesenchymal Transition and Metastatic-Related Behaviors of Human Endometrial Ishikawa Cells via an Estrogen Receptor-Dependent Pathway. Int. J. Mol. Sci. 2018, 19, 189. https://doi.org/10.3390/ijms19010189
Kim B-G, Kim J-W, Kim S-M, Go R-E, Hwang K-A, Choi K-C. 3,3′-Diindolylmethane Suppressed Cyprodinil-Induced Epithelial-Mesenchymal Transition and Metastatic-Related Behaviors of Human Endometrial Ishikawa Cells via an Estrogen Receptor-Dependent Pathway. International Journal of Molecular Sciences. 2018; 19(1):189. https://doi.org/10.3390/ijms19010189
Chicago/Turabian StyleKim, Bo-Gyoung, Jin-Wook Kim, Soo-Min Kim, Ryeo-Eun Go, Kyung-A Hwang, and Kyung-Chul Choi. 2018. "3,3′-Diindolylmethane Suppressed Cyprodinil-Induced Epithelial-Mesenchymal Transition and Metastatic-Related Behaviors of Human Endometrial Ishikawa Cells via an Estrogen Receptor-Dependent Pathway" International Journal of Molecular Sciences 19, no. 1: 189. https://doi.org/10.3390/ijms19010189
APA StyleKim, B. -G., Kim, J. -W., Kim, S. -M., Go, R. -E., Hwang, K. -A., & Choi, K. -C. (2018). 3,3′-Diindolylmethane Suppressed Cyprodinil-Induced Epithelial-Mesenchymal Transition and Metastatic-Related Behaviors of Human Endometrial Ishikawa Cells via an Estrogen Receptor-Dependent Pathway. International Journal of Molecular Sciences, 19(1), 189. https://doi.org/10.3390/ijms19010189