Surface Functionalization and Targeting Strategies of Liposomes in Solid Tumor Therapy: A Review
Abstract
:1. Introduction
2. Liposomes and Their Classification
3. Strategies for Targeting of Anticancer Agents at Requisite Site
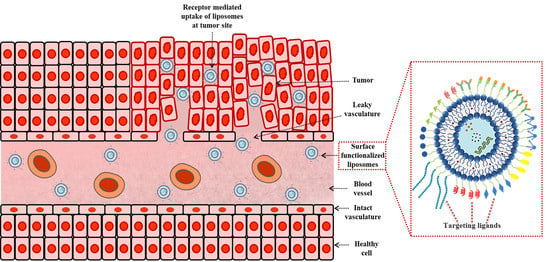
3.1. Enhanced Permeability and Retention (EPR) Effect and Its Application in Tumor Therapy
3.2. Active Targeting with Surface Engineered Liposomes, Functionalized with Targeting Ligands
3.2.1. Targeting of Over-Expressed Receptors on Cancer Cell’s Surface
Targeting of Epidermal Growth Factor Receptor (EGFR)
Targeting of Fibroblast Growth Factor Receptors (FGFRs)
Targeting of Folate Receptors (FRs)
Targeting of Transferrin Receptors (TfRs)
3.2.2. Targeting Directly to the Desired Organelle, Over-Expressed Receptors in Cytoplasm and Nucleus
Targeting Directly to the Desired Organelle
Targeting Over-Expressed Receptors in Cell Cytoplasm and Nucleus
3.2.3. Tumor Microenvironments Targeting
Targeting of Vascular Cell-Adhesion Molecules (VCAMs)
Targeting of Integrins
Targeting of Matrix-Metalloproteases (MMPs)
Targeting of Cluster-of-Differentiation 44 (CD44)
4. Targeting Ligands for Surface Functionalization of Liposomes in Tumor Therapy
4.1. Surface Modification of Liposomes with Antibody
4.2. Surface Modification of Liposomes with Peptides
4.3. Surface Modification of Liposomes with Aptamers
4.4. Surface Modification of Liposomes with Small Molecules
4.5. Surface Modification of Liposomes with Dual-Targeting Ligands
5. Stimuli Strategies for Enhanced Delivery of Anticancer Agents at Requisite Location Using Stimuli-Responsive Functionalized Liposomes
5.1. Temperature Responsive Liposomes
5.2. pH Responsive Liposomes
5.3. Magnetic-Field Responsive Liposomes
5.4. Ultrasound Responsive Liposomes
5.5. Other Stimuli Responsive Liposomes
5.6. Liposomes Responsive to Concurrent or Multiple Stimuli
6. Enhanced Delivery of Anticancer Agents at Tumor Site Using Dual Functionalized Liposomes Responsive to Stimuli and Grafted with Targeting Ligands
7. Challenges and Limitations of Functionalized Liposomes as a Carrier for Anticancer Agents
8. Conclusions and Future Perspective
Conflicts of Interest
References
- Sriraman, S.K.; Aryasomayajula, B.; Torchilin, V.P. Barriers to drug delivery in solid tumors. Tissue Barriers 2014, 2, e29528. [Google Scholar] [CrossRef] [PubMed]
- Millard, M.; Yakavets, I.; Zorin, V.; Kulmukhamedova, A.; Marchal, S.; Bezdetnaya, L. Drug delivery to solid tumors: The predictive value of the multicellular tumor spheroid model for nanomedicine screening. Int. J. Nanomed. 2017, 12, 7993–8007. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2017. CA Cancer J. Clin. 2017, 67, 7–30. [Google Scholar] [CrossRef] [PubMed]
- American Cancer Society. Cancer Facts & Figures 2016; American Cancer Society: Atlanta, GA, USA, 2016; pp. 1–9. [Google Scholar]
- Cancer Mortality Statistics|Cancer Research UK. Available online: http://www.cancerresearchuk.org/health-professional/cancer-statistics/mortality (accessed on 14 August 2017).
- Formulary Committee Joint. Malignant disease and immunosuppression. In British National Formulary 68 (September 2014–March 2015); BMJ Group and Pharmaceutical Press: London, UK, 2014; pp. 562–645. [Google Scholar]
- Drugs.com. Available online: https://www.drugs.com/ (accessed on 17 August 2017).
- RxList—The Internet Drug Index for Prescription Drugs, Medications and Pill Identifier. Available online: http://www.rxlist.com (accessed on 2 September 2017).
- Howard, D.H.; Bach, P.B.; Berndt, E.R.; Conti, R.M. Pricing in the Market for Anticancer Drugs; NBER Working Paper Series; National Bureau of Economic Research: Cambridge, MA, USA, 2015; Volume 53, pp. 1689–1699. [Google Scholar]
- Taşkın-Tok, T.; Gowder, S.J.T. Anticancer Drug—Friend or Foe. In Pharmacology and Therapeutics; InTech: Oakdale, CA, USA, 2014. [Google Scholar]
- Couvreur, P. Nanoparticles in drug delivery: Past, present and future. Adv. Drug Deliv. Rev. 2013, 65, 21–23. [Google Scholar] [CrossRef] [PubMed]
- Calixto, G.; Fonseca-Santos, B.; Chorilli, M.; Bernegossi, J. Nanotechnology-based drug delivery systems for treatment of oral cancer: A review. Int. J. Nanomed. 2014, 9, 3719. [Google Scholar] [CrossRef] [PubMed]
- Hoare, T.R.; Kohane, D.S. Hydrogels in drug delivery: Progress and challenges. Polymer 2008, 49, 1993–2007. [Google Scholar] [CrossRef]
- Goodman, C.M.; McCusker, C.D.; Yilmaz, T.; Rotello, V.M. Toxicity of gold nanoparticles functionalized with cationic and anionic side chains. Bioconjug. Chem. 2004, 15, 897–900. [Google Scholar] [CrossRef] [PubMed]
- Arvizo, R.; Bhattacharya, R.; Mukherjee, P. Gold nanoparticles: Opportunities and challenges in nanomedicine. Expert Opin. Drug Deliv. 2010, 7, 753–763. [Google Scholar] [CrossRef] [PubMed]
- Fenske, D.; Cullis, P. Liposomal nanomedicines. Expert Opin. Drug Deliv. 2008, 5, 25–44. [Google Scholar] [CrossRef] [PubMed]
- Suntres, Z.E. Liposomal antioxidants for protection against oxidant-induced damage. J. Toxicol. 2011, 2011, 152474. [Google Scholar] [CrossRef] [PubMed]
- Landi-Librandi, A.P.; Chrysostomo, T.N.; Caleiro Seixas Azzolini, A.E.; Marzocchi-Machado, C.M.; deOliveira, C.A.; Lucisano-Valim, Y.M. Study of quercetin-loaded liposomes as potential drug carriers: In vitro evaluation of human complement activation. J. Liposome Res. 2012, 22, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Mignet, N.; Seguin, J.; Chabot, G. Bioavailability of polyphenol liposomes: A challenge ahead. Pharmaceutics 2013, 5, 457–471. [Google Scholar] [CrossRef] [PubMed]
- Durymanov, M.O.; Rosenkranz, A.A.; Sobolev, A.S. Current approaches for improving intratumoral accumulation and distribution of nanomedicines. Theranostics 2015, 5, 1007–1020. [Google Scholar] [CrossRef] [PubMed]
- Gregoriadis, G. (Ed.) Liposome Technology, Entrapment of Drugs and Other Materials into Liposomes, 3rd ed.; Informa Healthcare: New York, NY, USA, 2007; Volume 2, ISBN 9780849388217. [Google Scholar]
- Weiner, N. Phospholipid liposomes: Properties and potential use in flavor encapsulation. In Flavor Technology; American Chemical Society: Washington, DC, USA, 1997; pp. 210–218. [Google Scholar]
- Bangham, A.D.; Horne, R.W. Negative staining of phospholipids and their structural modification by surface-active agents as observed in the electron microscope. J. Mol. Biol. 1964, 8. [Google Scholar] [CrossRef]
- Bangham, A.D.; Standish, M.M.; Watkins, J.C. Diffusion of univalent ions across the lamellae of swollen phospholipids. J. Mol. Biol. 1965, 13, 238–252. [Google Scholar] [CrossRef]
- Sessa, G.; Weissmann, G. Phospholipid spherules (liposomes) as a model for biological membranes. J. Lipid Res. 1968, 9, 310–318. [Google Scholar] [PubMed]
- Deamer, D.W. From “banghasomes” to liposomes: A memoir of Alec Bangham, 1921–2010. FASEB J. 2010, 24, 1308–1310. [Google Scholar] [CrossRef] [PubMed]
- Hatakeyama, H.; Akita, H.; Harashima, H. The polyethyleneglycol dilemma: Advantage and disadvantage of PEGylation of liposomes for systemic genes and nucleic acids delivery to tumors. Biol. Pharm. Bull. 2013, 36, 892–899. [Google Scholar] [CrossRef] [PubMed]
- Balazs, D.A.; Godbey, W.; Balazs, D.A.; Godbey, W. Liposomes for use in gene delivery. J. Drug Deliv. 2011, 2011. [Google Scholar] [CrossRef] [PubMed]
- Yuan, D.; Zong, T.; Gao, H.; He, Q. Cell penetrating peptide TAT and brain tumor targeting peptide T7 dual modified liposome preparation and in vitro targeting evaluation. Yao Xue Xue Bao 2015, 50, 104–110. [Google Scholar] [PubMed]
- Zhang, Y.; Zhai, M.; Chen, Z.; Han, X.; Yu, F.; Li, Z.; Xie, X.; Han, C.; Yu, L.; Yang, Y.; et al. Dual-modified liposome codelivery of doxorubicin and vincristine improve targeting and therapeutic efficacy of glioma. Drug Deliv. 2017, 24, 1045–1055. [Google Scholar] [CrossRef] [PubMed]
- Portnoy, E.; Lecht, S.; Lazarovici, P.; Danino, D.; Magdassi, S. Cetuximab-labeled liposomes containing near-infrared probe for in vivo imaging. Nanomed. Nanotechnol. Biol. Med. 2011, 7, 480–488. [Google Scholar] [CrossRef] [PubMed]
- Al-Jamal, W.T.; Al-Jamal, K.T.; Bomans, P.H.; Frederik, P.M.; Kostarelos, K. Functionalized-Quantum-Dot-Liposome Hybrids as Multimodal Nanoparticles for Cancer. Small 2008, 4, 1406–1415. [Google Scholar] [CrossRef] [PubMed]
- Erdogan, S.; Torchilin, V.P. Gadolinium-loaded polychelating polymer-containing tumor-targeted liposomes. In Methods in Molecular Biology (Clifton, N.J.); Springer: Berlin, Germany, 2017; Volume 1522, pp. 179–192. [Google Scholar]
- Li, S.; Goins, B.; Zhang, L.; Bao, A. Novel multifunctional theranostic liposome drug delivery system: Construction, characterization, and multimodality MR, near-infrared fluorescent, and nuclear imaging. Bioconjug. Chem. 2012, 23, 1322–1332. [Google Scholar] [CrossRef] [PubMed]
- Fonslow, B.R.; Stein, B.D.; Webb, K.J.; Xu, T.; Choi, J.; Park, S.K.; Yates, J.R., 3rd. Digestion and depletion of abundant proteins improves proteomic coverage. Nat. Methods 2013, 10, 54–56. [Google Scholar] [CrossRef] [PubMed]
- Sercombe, L.; Veerati, T.; Moheimani, F.; Wu, S.Y.; Sood, A.K.; Hua, S. Advances and challenges of liposome assisted drug delivery. Front. Pharmacol. 2015, 6. [Google Scholar] [CrossRef] [PubMed]
- Riaz, M. Review article: Stability and uses of liposomes. Pak. J. Pharm. Sci. 1995, 8, 69–79. [Google Scholar] [PubMed]
- Sonali; Singh, R.P.; Sharma, G.; Kumari, L.; Koch, B.; Singh, S.; Bharti, S.; Rajinikanth, P.S.; Pandey, B.L.; Muthu, M.S. RGD-TPGS decorated theranostic liposomes for brain targeted delivery. Colloids Surf. B Biointerfaces 2016, 147, 129–141. [Google Scholar] [CrossRef] [PubMed]
- Abolfazl, A.; Rogaie, R.-S.; Davaran, S.; Joo, S.W.; Zarghami, N.; Hanifehpour, Y.; Samiei, M.; Kouhi, M.; Nejati-Koshki, K. Liposome: Classification, preparation, and applications. Nanoscale Res. Lett. 2013, 8, 102. [Google Scholar] [CrossRef]
- Pillai, G. Nanomedicines for cancer therapy : An update of FDA approved and those under various stages of development. SOJ Pharm. Pharm. Sci. 2014, 1. [Google Scholar] [CrossRef]
- Bulbake, U.; Doppalapudi, S.; Kommineni, N.; Khan, W. Liposomal formulations in clinical use: An updated review. Pharmaceutics 2017, 9, 12. [Google Scholar] [CrossRef] [PubMed]
- Abu Lila, A.S.; Ishida, T. Liposomal delivery systems: Design optimization and current applications. Biol. Pharm. Bull. 2017, 40, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Arias, J.L. Drug targeting strategies in cancer treatment: An overview. Mini-Rev. Med. Chem. 2011, 11, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Sawant, R.R.; Torchilin, V.P. Challenges in development of targeted liposomal therapeutics. AAPS J. 2012, 14, 303–315. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, P.P.; Biswas, S.; Torchilin, V.P. Current trends in the use of liposomes for tumor targeting. Nanomedicine 2014, 8, 1509–1528. [Google Scholar] [CrossRef] [PubMed]
- Piktel, E.; Niemirowicz, K.; Wątek, M.; Wollny, T.; Deptuła, P.; Bucki, R. Recent insights in nanotechnology-based drugs and formulations designed for effective anti-cancer therapy. J. Nanobiotechnol. 2016, 14. [Google Scholar] [CrossRef] [PubMed]
- Hagimori, M.; Fuchigami, Y.; Kawakami, S. Peptide-based cancer-targeted DDS and molecular imaging. Chem. Pharm. Bull. 2017, 65, 618–624. [Google Scholar] [CrossRef] [PubMed]
- Zylberberg, C.; Matosevic, S. Pharmaceutical liposomal drug delivery: A review of new delivery systems and a look at the regulatory landscape. Drug Deliv. 2016, 23, 3319–3329. [Google Scholar] [CrossRef] [PubMed]
- Barenholz, Y. Doxil®—The first FDA-approved nano-drug: Lessons learned. J. Control. Release 2012, 160, 117–134. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Nakamura, H.; Fang, J. The EPR effect for macromolecular drug delivery to solid tumors: Improvement of tumor uptake, lowering of systemic toxicity, and distinct tumor imaging in vivo. Adv. Drug Deliv. Rev. 2013, 65, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.B.; Banerjee, A.; Önyüksel, H. Improvement of drug safety by the use of lipid- based nanocarriers. J. Control. Release 2012, 163, 34–45. [Google Scholar] [CrossRef] [PubMed]
- Torchilin, V.P. Passive and active drug targeting: Drug delivery to tumors as an example. In Drug Delivery; Schäfer-Korting, M., Ed.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 3–53. ISBN 978-3-642-00477-3. [Google Scholar]
- Patel, J.D.; O’Carra, R.; Jones, J.; Woodward, J.G.; Mumper, R.J. Preparation and characterization of nickel nanoparticles for binding to HIS-TAG proteins and antigens. Pharm. Res. 2007, 24, 343–352. [Google Scholar] [CrossRef] [PubMed]
- Torchilin, V.P. Recent approaches to intracellular delivery of drugs and DNA and organelle targeting. Annu. Rev. Biomed. Eng. 2006, 8, 343–375. [Google Scholar] [CrossRef] [PubMed]
- Saul, J.M.; Annapragada, A.; Natarajan, J.V.; Bellamkonda, R.V. Controlled targeting of liposomal doxorubicin via the folate receptor in vitro. J. Control. Release 2003, 92, 49–67. [Google Scholar] [CrossRef]
- Ye, J.; Liu, E.; Yu, Z.; Pei, X.; Chen, S.; Zhang, P.; Shin, M.-C.; Gong, J.; He, H.; Yang, V. CPP-assisted intracellular drug delivery, what is next? Int. J. Mol. Sci. 2016, 17, 1892. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Mumper, R.J. A critical review of lipid-based nanoparticles for taxane delivery. Cancer Lett. 2013, 334, 157–175. [Google Scholar] [CrossRef] [PubMed]
- Benhabbour, S.R.; Luft, J.C.; Kim, D.; Jain, A.; Wadhwa, S.; Parrott, M.C.; Liu, R.; DeSimone, J.M.; Mumper, R.J. In vitro and in vivo assessment of targeting lipid-based nanoparticles to the epidermal growth factor-receptor (EGFR) using a novel Heptameric ZEGFR domain. J. Control. Release 2012, 158, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Fath, I.S.; Oyelere, A.K. Liposomal drug delivery systems for targeted cancer therapy: Is active targeting the best choice? Future Med. Chem. 2016, 8, 2091–2112. [Google Scholar] [CrossRef] [PubMed]
- Riaz, M.K.; Tyagi, D.; Yang, Z. Surface engineering: Incorporation of bioactive compound. In Bioactivity of Engineered Nanoparticles; Yan, B., Zhou, H., Gardea-Torresdey, J.L., Eds.; Springer: Singapore, 2017; pp. 111–143. ISBN 978-981-10-5864-6. [Google Scholar]
- Mamot, C.; Drummond, D.C.; Noble, C.O.; Kallab, V.; Guo, Z.; Hong, K.; Kirpotin, D.B.; Park, J.W. Epidermal growth factor receptor-targeted immunoliposomes significantly enhance the efficacy of multiple anticancer drugs in vivo. Cancer Res. 2005, 65, 11631–11638. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.K.; Lee, T.S.; Song, I.H.; Jeong, H.Y.; Kang, S.J.; Kim, M.W.; Ryu, S.H.; Jung, I.H.; Kim, J.S.; Park, Y.S. Inhibition of pulmonary cancer progression by epidermal growth factor receptor-targeted transfection with Bcl-2 and survivin siRNAs. Cancer Gene Ther. 2015, 22, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Park, J.W.; Hong, K.; Kirpotin, D.B.; Colbern, G.; Shalaby, R.; Baselga, J.; Shao, Y.; Nielsen, U.B.; Marks, J.D.; Moore, D.; et al. Anti-HER2 immunoliposomes: Enhanced efficacy attributable to targeted delivery 1. Clin. Cancer Res. 2002, 8, 1172–1181. [Google Scholar] [PubMed]
- Chi, B.; Wong, K.; Qin, L. Carbonic anhydrase IX-directed immunoliposomes for targeted drug delivery to human lung cancer cells in vitro. Drug Des. Dev. Ther. 2014, 8, 993–1001. [Google Scholar] [CrossRef]
- Hatakeyama, H.; Akita, H.; Ishida, E.; Hashimoto, K.; Kobayashi, H.; Aoki, T.; Yasuda, J.; Obata, K.; Kikuchi, H.; Ishida, T.; et al. Tumor targeting of doxorubicin by anti-MT1-MMP antibody-modified PEG liposomes. Int. J. Pharm. 2007, 342, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Eloy, J.O.; Petrilli, R.; Chesca, D.L.; Saggioro, F.P.; Lee, R.J.; Marchetti, J.M. Anti-HER2 immunoliposomes for co-delivery of paclitaxel and rapamycin for breast cancer therapy. Eur. J. Pharm. Biopharm. 2017, 115, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Wicki, A.; Rochlitz, C.; Orleth, A.; Ritschard, R.; Albrecht, I.; Herrmann, R.; Christofori, G.; Mamot, C. Targeting tumor-associated endothelial cells: Anti-VEGFR2 immunoliposomes mediate tumor vessel disruption and inhibit tumor growth. Clin. Cancer Res. 2012, 18, 454–464. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.; Dodwadkar, N.S.; Deshpande, P.P.; Torchilin, V.P. Liposomes loaded with paclitaxel and modified with novel triphenylphosphonium-PEG-PE conjugate possess low toxicity, target mitochondria and demonstrate enhanced antitumor effects in vitro and in vivo. J. Control. Release 2012, 159, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.; Dodwadkar, N.S.; Sawant, R.R.; Koshkaryev, A.; Torchilin, V.P. Surface modification of liposomes with rhodamine-123-conjugated polymer results in enhanced mitochondrial targeting. J. Drug Target. 2011, 19, 552–561. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Li, L.; He, X.; Yi, Q.; He, B.; Cao, J.; Pan, W.; Gu, Z. Overcoming drug-resistant lung cancer by paclitaxel loaded dual-functional liposomes with mitochondria targeting and pH-response. Biomaterials 2015, 52, 126–139. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, N.; Wu, J.; Xu, X.; Kamaly, N.; Farokhzad, O.C. Cancer nanotechnology: The impact of passive and active targeting in the era of modern cancer biology. Adv. Drug Deliv. Rev. 2014, 66, 2–25. [Google Scholar] [CrossRef] [PubMed]
- Krasinskas, A.M. EGFR Signaling in Colorectal Carcinoma. Pathol. Res. Int. 2011, 2011. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, N.; Iqbal, N. Human epidermal growth factor receptor 2 (HER2) in cancers: Overexpression and therapeutic implications. Mol. Biol. Int. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Limasale, Y.D.P.; Tezcaner, A.; Özen, C.; Keskin, D.; Banerjee, S. Epidermal growth factor receptor-targeted immunoliposomes for delivery of celecoxib to cancer cells. Int. J. Pharm. 2015, 479, 364–373. [Google Scholar] [CrossRef] [PubMed]
- Breastcancer.org—Breast Cancer Information and Awareness. Available online: http://www.breastcancer.org/ (accessed on 15 September 2017).
- Lin, C.; Ng, H.; Pan, W.; Chen, H.; Zhang, G.; Bian, Z.; Lu, A.; Yang, Z. Exploring different strategies for efficient delivery of colorectal cancer therapy. Int. J. Mol. Sci. 2015, 16, 26936–26952. [Google Scholar] [CrossRef] [PubMed]
- Haugsten, E.M.; Wiedlocha, A.; Olsnes, S.; Wesche, J. Roles of fibroblast growth factor receptors in carcinogenesis. Mol. Cancer Res. 2010, 8, 1439–1452. [Google Scholar] [CrossRef] [PubMed]
- Rusnati, M.; Dell’Era, P.; Urbinati, C.; Tanghetti, E.; Massardi, M.L.; Nagamine, Y.; Monti, E.; Presta, M. A distinct basic fibroblast growth factor (FGF-2)/FGF receptor interaction distinguishes urokinase-type plasminogen activator induction from mitogenicity in endothelial cells. Mol. Biol. Cell 1996, 7, 369–381. [Google Scholar] [CrossRef] [PubMed]
- Low, P.S.; Henne, W.A.; Doorneweerd, D.D. Discovery and development of folic-acid-based receptor targeting for imaging and therapy of cancer and inflammatory diseases. Acc. Chem. Res. 2008, 41, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Heath, J.L.; Weiss, J.M.; Lavau, C.P.; Wechsler, D.S. Iron deprivation in cancer-potential therapeutic implications. Nutrients 2013, 5, 2836–2859. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ding, L.; Xu, Y.; Wang, Y.; Ping, Q. Targeted delivery of doxorubicin using stealth liposomes modified with transferrin. Int. J. Pharm. 2009, 373, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Zhai, G.; Wu, J.; Yu, B.; Guo, C.; Yang, X.; Lee, R.J. A transferrin receptor-targeted liposomal formulation for docetaxel. J. Nanosci. Nanotechnol. 2010, 10, 5129–5136. [Google Scholar] [CrossRef] [PubMed]
- Koshkaryev, A.; Piroyan, A.; Torchilin, V.P. Increased apoptosis in cancer cells in vitro and in vivo by ceramides in transferrin-modified liposomes. Cancer Biol. Ther. 2012, 13, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Sarraf, P.; Mueller, E.; Jones, D.; King, F.J.; DeAngelo, D.J.; Partridge, J.B.; Holden, S.A.; Chen, L.B.; Singer, S.; Fletcher, C.; et al. Differentiation and reversal of malignant changes in colon cancer through PPARγ. Nat. Med. 1998, 4, 1046–1052. [Google Scholar] [CrossRef] [PubMed]
- Pablos, J.L.; Santiago, B.; Carreira, P.E.; Galindo, M.; Gomez-Reino, J.J. Cyclooxygenase-1 and -2 are expressed by human T cells. Clin. Exp. Immunol. 1999, 115, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Srivastava, M.; Ahmad, N.; Bostwick, D.G.; Mukhtar, H. Over-expression of cyclooxygenase-2 in Human prostate adenocarcinoma Over-Expression of Cyclooxygenase-2 in human. Prostate 2000, 42, 73–78. [Google Scholar] [CrossRef]
- Bertagnolli, M.M.; Eagle, C.J.; Zauber, A.G.; Redston, M.; Breazna, A.; Kim, K.; Tang, J.; Rosenstein, R.B.; Umar, A.; Bagheri, D.; et al. Five-year efficacy and safety analysis of the adenoma prevention with celecoxib trial. Cancer Prev. Res. 2009, 2, 310–321. [Google Scholar] [CrossRef] [PubMed]
- Mikhaylova, M.; Stasinopoulos, I.; Kato, Y.; Artemov, D.; Bhujwalla, Z.M. Imaging of cationic multifunctional liposome-mediated delivery of COX-2 siRNA. Cancer Gene Ther. 2009, 16, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Linton, S.S.; Sherwood, S.G.; Drews, K.C.; Kester, M. Targeting cancer cells in the tumor microenvironment: Opportunities and challenges in combinatorial nanomedicine. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2016, 8, 208–222. [Google Scholar] [CrossRef] [PubMed]
- Staal-van den Brekel, A.J.; Thunnissen, F.B.; Buurman, W.A.; Wouters, E.F. Expression of E-selectin, intercellular adhesion molecule (ICAM)-1 and vascular cell adhesion molecule (VCAM)-1 in non-small-cell lung carcinoma. Virchows Arch. 1996, 428, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Chiu, G.N.C.; Bally, M.B.; Mayer, L.D. Targeting of antibody conjugated, phosphatidylserine-containing liposomes to vascular cell adhesion molecule 1 for controlled thrombogenesis. Biochim. Biophys. Acta Biomembr. 2003, 1613, 115–121. [Google Scholar] [CrossRef]
- Gosk, S.; Moos, T.; Gottstein, C.; Bendas, G. VCAM-1 directed immunoliposomes selectively target tumor vasculature in vivo. Biochim. Biophys. Acta Biomembr. 2008, 1778, 854–863. [Google Scholar] [CrossRef] [PubMed]
- Meng, S.; Su, B.; Li, W.; Ding, Y.; Tang, L.; Zhou, W.; Song, Y.; Caicun, Z. Integrin-targeted paclitaxel nanoliposomes for tumor therapy. Med. Oncol. 2011, 28, 1180–1187. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Deng, J.; Zhao, Y.; Tao, T. Cyclic RGD peptide-modified liposomal drug delivery system: Enhanced cellular uptake in vitro and improved pharmacokinetics in rats. Int. J. Nanomed. 2012, 7, 3803–3811. [Google Scholar] [CrossRef] [PubMed]
- Handsley, M.M.; Edwards, D.R. Metalloproteinases and their inhibitors in tumor angiogenesis. Int. J. Cancer 2005, 115, 849–860. [Google Scholar] [CrossRef] [PubMed]
- Cathcart, J.; Pulkoski-Gross, A.; Cao, J. Targeting matrix metalloproteinases in cancer: Bringing new life to old ideas. Genes Dis. 2015, 2, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Jambhrunkar, S.; Thorn, P.; Chen, J.; Gu, W.; Yu, C. Hyaluronic acid modified mesoporous silica nanoparticles for targeted drug delivery to CD44-overexpressing cancer cells. Nanoscale 2013, 5, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Alshaer, W.; Vergnaud, J.; Ismail, S.; Fattal, E. Functionalizing Liposomes with anti-CD44 Aptamer for Selective Targeting of Cancer Cells. Bioconjug. Chem. 2015, 26, 1307–1313. [Google Scholar] [CrossRef] [PubMed]
- Torchilin, V.K.A. Coupling and labeling of phospholipids. In Phospholipid Handbook; Gregor, C., Ed.; Marcel Dekker: New York, NY, USA, 1993; pp. 293–322. [Google Scholar]
- Maruyama, K.; Takizawa, T.; Yuda, T.; Kennel, S.J.; Huang, L.; Iwatsuru, M. Targetability of novel immunoliposomes modified with amphipathic poly(ethylene glycol) s conjugated at their distal terminals to monoclonal antibodies. Biochim. Biophys. Acta Biomembr. 1995, 1234, 74–80. [Google Scholar] [CrossRef]
- Keitaro, S.; Taro, E.; Shinji, T.; Tsuchida, E. Poly(ethylene glycol)-modification of the phospholipid vesicles by using the spontaneous incorporation of poly(ethylene glycol)-lipid into the vesicles. Bioconjug. Chem. 2000, 11, 372–379. [Google Scholar] [CrossRef]
- Mirafzali, Z. Immunoliposomes. Available online: http://www.liposomes.org/2011/09/immunoliposomes.html (accessed on 21 September 2017).
- Manjappa, A.S.; Chaudhari, K.R.; Venkataraju, M.P.; Dantuluri, P.; Nanda, B.; Sidda, C.; Sawant, K.K.; Ramachandra Murthy, R.S. Antibody derivatization and conjugation strategies: Application in preparation of stealth immunoliposome to target chemotherapeutics to tumor. J. Control. Release 2011, 150, 2–22. [Google Scholar] [CrossRef] [PubMed]
- Carbonic Anhydrase|Enzyme|Britannica.com. Available online: https://global.britannica.com/science/carbonic-anhydrase (accessed on 8 September 2017).
- Carbonic Anhydrase. Available online: https://en.wikipedia.org/wiki/Carbonic_anhydrase (accessed on 8 September 2017).
- Shuchismita, D.; David, G. Carbonic Anhydrase; RCSB Protein Data Bank: La Jolla, CA, USA, 2004. [Google Scholar]
- Mahon, B.P.; Pinard, M.A.; McKenna, R. Targeting carbonic anhydrase IX activity and expression. Molecules 2015, 20, 2323–2348. [Google Scholar] [CrossRef] [PubMed]
- Helena Ng, H.L.; Lu, A.; Lin, G.; Qin, L.; Yang, Z. The potential of liposomes with carbonic anhydrase IX to deliver anticancer ingredients to cancer cells in Vivo. Int. J. Mol. Sci. 2014, 16, 230–255. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Wong, B.C.K.; Chen, H.; Bian, Z.; Zhang, G.; Zhang, X.; Kashif Riaz, M.; Tyagi, D.; Lin, G.; Zhang, Y.; et al. Pulmonary delivery of triptolide-loaded liposomes decorated with anti-carbonic anhydrase IX antibody for lung cancer therapy. Sci. Rep. 2017, 7, 1097. [Google Scholar] [CrossRef] [PubMed]
- Koren, E.; Torchilin, V.P. Cell-penetrating peptides: Breaking through to the other side. Trends Mol. Med. 2012, 18, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Munyendo, W.L.; Lv, H.; Benza-Ingoula, H.; Baraza, L.D.; Zhou, J. Cell penetrating peptides in the delivery of biopharmaceuticals. Biomolecules 2012, 2, 187–202. [Google Scholar] [CrossRef] [PubMed]
- Dissanayake, S.; Denny, W.A.; Gamage, S.; Sarojini, V. Recent developments in anticancer drug delivery using cell penetrating and tumor targeting peptides. J. Control. Release 2017, 250, 62–76. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Lin, C.; Lu, A.; Lin, G.; Chen, H.; Liu, Q.; Yang, Z.; Zhang, H. Liposomes equipped with cell penetrating peptide BR2 enhances chemotherapeutic effects of cantharidin against hepatocellular carcinoma. Drug Deliv. 2017, 24, 986–998. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Yao, L.; Mei, J.; Li, F. Development of synthetic of peptide-functionalized liposome for enhanced targeted ovarian carcinoma therapy. Int. J. Clin. Exp. Pathol. 2015, 8, 207–216. [Google Scholar] [PubMed]
- Xie, Y.; Ding, Y.; Sun, D.; Wang, G.; Yang, H.; Xu, H.; Wang, Z.; Chen, J. An efficient PEGylated liposomal nanocarrier containing cell-penetrating peptide and pH-sensitive hydrazone bond for enhancing tumor-targeted drug delivery. Int. J. Nanomed. 2015, 10, 6199–6214. [Google Scholar] [CrossRef] [PubMed]
- Patra, S.; Roy, E.; Madhuri, R.; Sharma, P.K. The next generation cell-penetrating peptide and carbon dot conjugated nano-liposome for transdermal delivery of curcumin. Biomater. Sci. 2016, 4, 418–429. [Google Scholar] [CrossRef] [PubMed]
- Catuogno, S.; Esposito, C.L.; deFranciscis, V. Aptamer-mediated targeted delivery of therapeutics: An update. Pharmaceuticals 2016, 9, 69. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Tong, R.; Mishra, A.; Xu, W.; Wong, G.C.L.; Cheng, J.; Lu, Y. Reversible cell-specific drug delivery with aptamer-functionalized liposomes. Angew. Chem. Int. Ed. 2009, 48, 6494–6498. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; O’Donoghue, M.B.; Liu, H.; Tan, W.A. liposome-based nanostructure for aptamer directed delivery. Chem. Commun. 2010, 46, 249–251. [Google Scholar] [CrossRef] [PubMed]
- Baek, S.E.; Lee, K.H.; Park, Y.S.; Oh, D.-K.; Oh, S.; Kim, K.-S.; Kim, D.-E. RNA aptamer-conjugated liposome as an efficient anticancer drug delivery vehicle targeting cancer cells in vivo. J. Control. Release 2014, 196, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Stuart, C.H.; Singh, R.; Smith, T.L.; D’Agostino, R.; Caudell, D.; Balaji, K.; Gmeiner, W.H. Prostate-specific membrane antigen-targeted liposomes specifically deliver the Zn2+ chelator TPEN inducing oxidative stress in prostate cancer cells. Nanomedicine 2016, 11, 1207–1222. [Google Scholar] [CrossRef] [PubMed]
- Moosavian, S.A.; Abnous, K.; Badiee, A.; Jaafari, M.R. Improvement in the drug delivery and anti-tumor efficacy of PEGylated liposomal doxorubicin by targeting RNA aptamers in mice bearing breast tumor model. Colloids Surf. B Biointerfaces 2016, 139, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Powell, D.; Chandra, S.; Dodson, K.; Shaheen, F.; Wiltz, K.; Ireland, S.; Syed, M.; Dash, S.; Wiese, T.; Mandal, T.; et al. Aptamer-functionalized hybrid nanoparticle for the treatment of breast cancer. Eur. J. Pharm. Biopharm. 2017, 114, 108–118. [Google Scholar] [CrossRef] [PubMed]
- Sriraman, S.K.; Salzano, G.; Sarisozen, C.; Torchilin, V. Anti-cancer activity of doxorubicin-loaded liposomes co-modified with transferrin and folic acid. Eur. J. Pharm. Biopharm. 2016, 105, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Alavizadeh, S.H.; Akhtari, J.; Badiee, A.; Golmohammadzadeh, S.; Jaafari, M.R. Improved therapeutic activity of HER2 Affibody-targeted cisplatin liposomes in HER2-expressing breast tumor models. Expert Opin. Drug Deliv. 2016, 13, 325–336. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.H.; Yoo, H.J.; Kwon, Y.H.; Yoon, H.Y.; Lee, S.G.; Kim, S.R.; Yeom, D.W.; Kang, M.J.; Choi, Y.W. Design of multifunctional liposomal nanocarriers for folate receptor-specific intracellular drug delivery. Mol. Pharm. 2015, 12, 4200–4213. [Google Scholar] [CrossRef] [PubMed]
- Zong, T.; Mei, L.; Gao, H.; Cai, W.; Zhu, P.; Shi, K.; Chen, J.; Wang, Y.; Gao, F.; He, Q. Synergistic dual-ligand doxorubicin liposomes improve targeting and therapeutic efficacy of brain glioma in animals. Mol. Pharm. 2014, 11, 2346–2357. [Google Scholar] [CrossRef] [PubMed]
- Movahedi, F.; Hu, R.G.; Becker, D.L.; Xu, C. Stimuli-responsive liposomes for the delivery of nucleic acid therapeutics. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 1575–1584. [Google Scholar] [CrossRef] [PubMed]
- David, N.; Park, J.-Y.; Alexander, M.W.; Tong, J. Materials characterization of the low temperature sensitive liposome (LTSL): Effects of the lipid composition (lysolipid and DSPE–PEG2000) on the thermal transition and release of doxorubicin. Faraday Discuss. 2013, 161, 515–534. [Google Scholar] [CrossRef]
- Simoes, S.; Moreira, N.J.; Fonseca, C.; Duzgunes, N.; Pedroso De Lima, M.C. On the formulation of pH-sensitive liposomes with long circulation times. Adv. Drug Deliv. Rev. 2004, 56, 947–965. [Google Scholar] [CrossRef] [PubMed]
- Madni, M.A.; Sarfraz, M.; Rehman, M.; Ahmad, M.; Akhtar, N.; Ahmad, S.; Tahir, N.; Ijaz, S.; Al-Kassas, R.; Lobenberg, R. Liposomal drug delivery: A versatile platform for challenging clinical applications. J. Pharm. Pharm. Sci. 2014, 17, 401–426. [Google Scholar] [CrossRef] [PubMed]
- Felber, A.E.; Dufresne, M.-H.; Leroux, J.-C. pH-sensitive vesicles, polymeric micelles, and nanospheres prepared with polycarboxylates. Adv. Drug Deliv. Rev. 2012, 64, 979–992. [Google Scholar] [CrossRef] [PubMed]
- Lipid Products|18:1 DAP|890850. Available online: https://avantilipids.com/product/890850/ (accessed on 5 October 2017).
- Zhao, Y.; Ren, W.; Zhong, T.; Zhang, S.; Huang, D.; Guo, Y.; Yao, X.; Wang, C.; Zhang, W.Q.; Zhang, X.; et al. Tumor-specific pH-responsive peptide-modified pH-sensitive liposomes containing doxorubicin for enhancing glioma targeting and anti-tumor activity. J. Control. Release 2016, 222, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Yoshizaki, Y.; Yuba, E.; Sakaguchi, N.; Koiwai, K.; Harada, A.; Kono, K. Potentiation of pH-sensitive polymer-modified liposomes with cationic lipid inclusion as antigen delivery carriers for cancer immunotherapy. Biomaterials 2014, 35, 8186–8196. [Google Scholar] [CrossRef] [PubMed]
- Yoshizaki, Y.; Yuba, E.; Komatsu, T.; Udaka, K.; Harada, A.; Kono, K. Improvement of peptide-based tumor immunotherapy using pH-sensitive fusogenic polymer-modified liposomes. Molecules 2016, 21, 1284. [Google Scholar] [CrossRef] [PubMed]
- Clares, B.; Biedma-Ortiz, R.A.; Sáez-Fernández, E.; Prados, J.C.; Melguizo, C.; Cabeza, L.; Ortiz, R.; Arias, J.L. Nano-engineering of 5-fluorouracil-loaded magnetoliposomes for combined hyperthermia and chemotherapy against colon cancer. Eur. J. Pharm. Biopharm. 2013, 85, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Torchilin, V.P. Stimulus-responsive nanopreparations for tumor targeting. Integr. Biol. 2013, 5, 96–107. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.-L. Ultrasound-Responsive Liposomes; Humana Press: New York, NY, USA, 2010; pp. 113–128. [Google Scholar]
- Han, H.D.; Jeon, Y.W.; Kwon, H.J.; Jeon, H.N.; Byeon, Y.; Lee, C.O.; Cho, S.H.; Shin, B.C. Therapeutic efficacy of doxorubicin delivery by a CO2 generating liposomal platform in breast carcinoma. Acta Biomater. 2015, 24, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Chi, Y.; Yin, X.; Sun, K.; Feng, S.; Liu, J.; Chen, D.; Guo, C.; Wu, Z. Redox-sensitive and hyaluronic acid functionalized liposomes for cytoplasmic drug delivery to osteosarcoma in animal models. J. Control. Release 2017, 261, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yang, X.; Zhou, Z.; Wang, K.; Li, C.; Qiao, H.; Oupicky, D.; Sun, M. Near-infrared light-triggered drug release from a multiple lipid carrier complex using an all-in-one strategy. J. Control. Release 2017, 261, 126–137. [Google Scholar] [CrossRef] [PubMed]
- Ta, T.; Bartolak-Suki, E.; Park, E.J.; Karrobi, K.; McDannold, N.J.; Porter, T.M. Localized delivery of doxorubicin in vivo from polymer-modified thermosensitive liposomes with MR-guided focused ultrasound-mediated heating. J. Control. Release 2014, 194, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Yang, Y.; Xie, X.; Xu, X.; Xia, X.; Wang, H.; Li, L.; Dong, W.; Ma, P.; Liu, Y. Dual stimulus of hyperthermia and intracellular redox environment triggered release of siRNA for tumor-specific therapy. Int. J. Pharm. 2016, 506, 158–173. [Google Scholar] [CrossRef] [PubMed]
- Al-Ahmady, Z.S.; Chaloin, O.; Kostarelos, K. Monoclonal antibody-targeted, temperature-sensitive liposomes: In vivo tumor chemotherapeutics in combination with mild hyperthermia. J. Control. Release 2014, 196, 332–343. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Liu, M.; Tong, X.; Sun, N.; Zhou, L.; Cao, Y.; Wang, J.; Zhang, H.; Pei, R. Aptamer-modified temperature-sensitive liposomal contrast agent for magnetic resonance imaging. Biomacromolecules 2015, 16, 2618–2623. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Tang, Q.; Zhang, P.; Wang, Z.; Zhao, T.; Zhou, J.; Li, H.; Ding, Q.; Li, W.; Hu, F.; et al. Human epidermal growth factor receptor-2 antibodies enhance the specificity and anticancer activity of light-sensitive doxorubicin-labeled liposomes. Biomaterials 2015, 57, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Amari, T.; Semba, K.; Yamamoto, T.; Takeoka, S. Construction and evaluation of pH-sensitive immunoliposomes for enhanced delivery of anticancer drug to ErbB2 over-expressing breast cancer cells. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 1219–1227. [Google Scholar] [CrossRef] [PubMed]
- Zuo, T.; Guan, Y.; Chang, M.; Zhang, F.; Lu, S.; Wei, T.; Shao, W.; Lin, G. RGD(Arg-Gly-Asp) internalized docetaxel-loaded pH sensitive liposomes: Preparation, characterization and antitumor efficacy in vivo and in vitro. Colloids Surf. B Biointerfaces 2016, 147, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, P.; Giri, J.; Rieken, F.; Koch, C.; Mykhaylyk, O.; Döblinger, M.; Banerjee, R.; Bahadur, D.; Plank, C. Targeted temperature sensitive magnetic liposomes for thermo-chemotherapy. J. Control. Release 2010, 142, 108–121. [Google Scholar] [CrossRef] [PubMed]
- Zang, X.; Ding, H.; Zhao, X.; Li, X.; Du, Z.; Hu, H.; Qiao, M.; Chen, D.; Deng, Y. Anti-EphA10 antibody-conjugated pH-sensitive liposomes for specific intracellular delivery of siRNA. Int. J. Nanomed. 2016, 3951–3967. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.; Stellacci, F. Effect of surface properties on nanoparticle-cell interactions. Small 2010, 6, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Albanese, A.; Tang, P.S.; Chan, W.C.W. The Effect of Nanoparticle Size, Shape, and Surface Chemistry on Biological Systems. Annu. Rev. Biomed. Eng. 2012, 14, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Barenholz, Y. Liposome application: Problems and prospects. Curr. Opin. Colloid Interface Sci. 2001, 6, 66–77. [Google Scholar] [CrossRef]
- Stohrer, M.; Boucher, Y.; Stangassinger, M.; Jain, R. Oncotic pressure in solid tumors is elevated. Cancer Res. 2000, 60, 4251–4255. [Google Scholar] [PubMed]
- Cheng, Z.; AlZaki, A.; Hui, J.Z.; Muzykantov, V.R.; Tsourkas, A. Multifunctional nanoparticles: Cost versus benefit of adding targeting and imaging capabilities. Science 2012, 338, 903–910. [Google Scholar] [CrossRef] [PubMed]
- Salvati, A.; Pitek, A.S.; Monopoli, M.P.; Prapainop, K.; Bombelli, F.B.; Hristov, D.R.; Kelly, P.M.; Åberg, C.; Mahon, E.; Dawson, K.A. Transferrin-functionalized nanoparticles lose their targeting capabilities when a biomolecule corona adsorbs on the surface. Nat. Nanotechnol. 2013, 8, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Dokka, S.; Toledo, D.; Shi, X.; Castranova, V.; Rojanasakul, Y. Oxygen Radical-Mediated Pulmonary Toxicity Induced by Some Cationic Liposomes. Pharm. Res. 2000, 17, 521–525. [Google Scholar] [CrossRef] [PubMed]
- Danhier, F.; Préat, V. Strategies to improve the EPR effect for the delivery of anti-cancer nanomedicines. Cancer Cell Microenviron. 2015, 2. [Google Scholar] [CrossRef]
- Paolinelli, R.; Corada, M.; Orsenigo, F.; Dejana, E. The molecular basis of the blood brain barrier differentiation and maintenance. Is it still a mystery? Pharmacol. Res. 2011, 63, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Perche, F.; Torchilin, V.P. Recent trends in multifunctional liposomal nanocarriers for enhanced tumor targeting. J. Drug Deliv. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Aillon, K.L.; Xie, Y.; El-Gendy, N.; Berkland, C.J.; Forrest, M.L. Effects of nanomaterial physicochemical properties on in vivo toxicity. Adv. Drug Deliv. Rev. 2009, 61, 457–466. [Google Scholar] [CrossRef] [PubMed]




| Targeting Ligand | Anticancer Agent | Targeting Site | Surface Engineering Technique Used | Drug Loading | Tumor Treated | Reference |
|---|---|---|---|---|---|---|
| Fab′ fragments of mAb C225 (cetuximab) | Doxorubicin | EGFR | FabV fragments were covalently linked to the maleimide (MAL) group of DSPE-PEG-MAL. Incorporation of DSPE-PEG-MAL-FabV into preformed liposomes by coincubation at 55 °C for 30 min. | Passive loading | Breast cancer | [61] |
| Anti EGFR antibody | Small interfering RNA (siRNA) | EGFR | Thiolated antibody were conjugated to the MAL group at distal end of DSPE-PEG-MAL chains of preformed liposomes (0.2:1, Ab and MAL molar ratio) by over-night incubation at 4 °C with mild shaking. | Active loading | Lung cancer | [62] |
| Anti HER2 antibody i.e., fragments of trastuzumab-mAb | Doxorubicin | HER2 | Covalent conjugation of Fab or scFv to drug loaded liposomes by thioether linkage of free thiol of Ab-fragment and MAL group or alternatively thiol group was conjugated to terminal MAL group present on surface of liposomes. | Active loading by ammonium-sulfate gradient | Breast cancer | [63] |
| CA-IX Antibody | Docetaxel | CA-IX | Antibody was reduced by reaction with DTT. Conjugation of DSPE-PEG-MAL micelles with the reduced antibody was done by incubation at room temperature for 24 h. DSPE-PEG-MAL-Ab micelles were incubated with preformed liposomes at 60 °C for 2 h (post insertion technique) to formulate CA-IX directed liposomes. | Passive loading | Lung cancer | [64] |
| Anti-MT1-MMP Fab | Doxorubicin | MT1-MMP | Fab fragments were conjugated to the MAL moiety in preformed liposomes at a molar ratio of 1:3 respectively in an incubation at 4 °C for 20 h. | Active loading by ammonium-sulfate gradient. | Fibrosarcoma, i.e., HT-1080 cancer cells | [65] |
| Trastuzumab (Anti-HER2) | Paclitaxel and Rapamycin | HER2 | Thiolated antibody was conjugated to the MAL group of DSPE-PEG-MAL in preformed liposomes by overnight incubation and purification with CL-4B column. | Passive loading | HER2 (+) breast cancer, RAP acts synergistically | [66] |
| Anti VEGFR2 | Doxorubicin | VEGFR2 | Fab′-Mal-PEG-DSPE was incorporated into the preformed liposomes by coincubation at 55 °C for 30 min. This step was followed by purification with gel filtration. | Passive loading | Colon Cancer | [67] |
| Liposomes for Intracellular organelle targeting | ||||||
| Triphenyl-phosphonium (tpp) | Paclitaxel | Mitochondrial targeting; higher accumulation of payload in mitochondria | CTPP was incubated for 2 h with triethylamine, EDCI and NHS in choroform at 25 °C with stirring. NH2-PEG-PE prepared was added to the chloroform solution, stirred overnight and chloroform was evaporated. Purification of reaction mixture was done by dialysis with water. Freeze drying was done to get the purified TPP-PEG-PE polymer. The polymer was used in preparation of TPP directed liposomes by film hydration method. | Passive loading | Breast Cancer | [68] |
| Rhodamine-123 | Paclitaxel | Mitochondria | DOPE, NPC-PEG were dissolved in chloroform in presence of triethylamine with overnight stirring. Chloroform was removed from reaction mixture and then reaction mixture was freeze dried. The reaction mixture was hydrated with water to get pNP-PEG-DOPE micelles. Rh123-PEG3400-DOPE was synthesized by dissolution of Rh123 (a fluorescent probe) in methanol and triethylamine solution. Addition of the solution was done to the pNP-PEG-DOPE in chloroform, followed by incubation at 25 °C with stirring for 4 h. Liposomes were prepared using the above polymer by film hydration method. | Passive loading | Cervical cancer | [69] |
| KLA-peptide having terminal cysteine | Paclitaxel | Mitochondrial targeting of A549 cancer cells and pH responsive liposomal system | In first step, DSPE-KLA was obtained by conjugation of DSPE-Mal with KLA-Cys peptide in chloroform and methanol mixture. Further, DSPE-KLA-DMA was synthesized by dissolution of DSPE-KLA in dichloromethane containing N, N-Diisopropylethylamine (DIPEA) and 2,3-dimethylmaleic-anhydride (DMA) and agitation in ice and water bath for 24 h. Film hydration method was used for preparation of DSPE-KLA-DMA (DKD) directed liposomes. | Passive loading | Lung cancer (A549 cell-line) | [70] |
| Stimuli Used | Anticancer Agent | Targeting Site | Targeting Ligand | Lipids Used | Technique Used for Functionalization of Liposomes | Liposome Formulation Method | Drug Loading | Tumor Treated | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Temperature | Doxorubicin | - | - | DPPC:MSPC:DSPE-PEG(2000) (86.5:9.7:3.8, mol %) | Liposomes were formulated by film hydration method by formation of lipid film, followed by hydration with citrate-buffer at 50 °C for 10 min. | Film hydration method | pH gradient | Ovarian Cancer | [129] |
| pH | Doxorubicin | - | H7K(R2)2, a pH-sensitive peptide | DOPE, DSPE-PEG-H7K(R2)2 | DSPE-PEG-H7K(R2)2 was prepared by reaction of DSPE-PEG-NHS with H7K(R2)2. H7K(R2)2-targeted liposomes were prepared by film hydration method. | Film hydration method | Ammonium-sulfate gradient | Glioma (C6-cells) and glioblastoma (U87-MG cells) | [134] |
| Magnetic field | 5-Fluorouracil | - | - | Phosphatidylcholine (PC) | Film hydration method was used to prepare magnetoliposomes. Lipid film of PC solution in chloroform was prepared by evaporation under vacuum, followed by hydration with Fe3O4 suspension in water. | Film hydration method | Passive loading | Human colon carcinoma T-84 cell lines | [137] |
| Temperature (for drug release) and ultrasound waves (for drug release monitoring) | Doxorubicin | - | - | DPPC:MSPC:DSPE-mPEG(2000) (21.6:2.6:1.0, molar ratio) | Thermo-responsive liposomes (capable of producing CO2 bubbles on hyperthermia) by hydrating the dried lipid film with citrate buffer (300 mM, pH 4). | Film hydration method | Ammonium-sulfate gradient | Breast tumor (MDA-MB-23) | [140] |
| Redox | Doxorubicin | CD44 receptor | Hyaluronic acid | SPC, DOPE, DOTAP, Chol-SS-mPEG or Chol-mPEG | Solvent-injection method was used to prepare cationic liposomes by dissolution of lipids in ethanol, followed by addition of ethanolic solution to the PBS with stirring. HA (negatively charged) solution was coated on the cationic liposomes with 4 h stirring. An ammonium-sulfate gradient was used for loading doxorubicin in the liposomes. | Solvent injection method | Ammonium-sulfate gradient | Osteosarcoma (MG63 cell-line) | [141] |
| Laser irradiation | AMD3100 and IR780 (a dye) | CXCR4-receptors | - | Soybean-phosphatidylcholine (SPC) | Multiple lipid-carrier complex consisting of a nanostructured lipid-carrier (NLC) within liposomes have been formulated. IR780-loaded NLC have been formulated by film-dispersion method. The dried lipid film for liposome formulation was hydrated with water containing IR780-loaded NLC and AMD3100. | Preparation of NLC by film-dispersion method and formulation of liposomes by film hydration method | Passive loading | Osteosarcoma (U20S cell-line) and Breast cancer (4 T1-luc cell-line) | [142] |
| Liposomes responsive to multiple stimuli | |||||||||
| pH and Temperature | Doxorubicin | - | - | pH-sensitive polymer (2-PAA) and temperature sensitive-polymer (NIPAAm) | Film hydration method was used to prepare liposomes and doxorubicin was loaded by pH gradient. MR-guided focused ultrasound was used to heat specific tissues and trigger local drug release. | Film Hydration Method | pH gradient | Breast tumor (MCF-7 cells) | [143] |
| Cellular redox-environment and temperature | CPP-siRNA conjugate. | c-myc gene | NGR-Peptide | DPPC:MSPC:DSPE-PEG(2000)-NGR (87:3:10 weight ratio) | siRNA-CPP conjugate was prepared by conjugation of siRNA with CPP through disulfide-linkage. NGR directed temperature-responsive liposomes were developed by dissolution of DPPC:MSPC: DSPE-PEG(2000)-NGR (87:3:10, weight ratio) in chloroform with subsequent evaporation to form lipid film and then the film was hydrated with HEPES buffer. | Film hydration method | Passive loading | Fibrosarcoma-cells (HT-1080 cell line) | [144] |
| Targeting Ligand | Stimuli Used | Anticancer Agent | Targeting Site | Lipids Used | Techniques Used in Functionalization of Liposomes with Stimuli and Targeting Ligand | Liposome Formulation Method | Drug Loading | Tumor Treated | Reference |
|---|---|---|---|---|---|---|---|---|---|
| hCTMO1 antibody | Temperature | Doxorubicin | MUC1-gene | DPPC, HSPC, DSPE-PEG(2000) | DSPE-PEG-MAL-hCTMO1 micelles were prepared by reaction of thiolated antibody with MAL group. Thermosensitive targeted-liposomes were formulated using postinsertion technique by incubation of thermosensitive liposomes with DSPE-PEG-MAL-hCTMO1 micelles for 1 h at 60 °C. | Film hydration method | Ammonium-sulfate gradient | Breast Cancer | [145] |
| AS1411 aptamer | Temperature | Gd-DTPA | Nucleolin Receptor | DPPC, MSPC, DSPE-PEG(2000)-COOH | Thermosensitive liposomes (TSL) were conjugated with the AS1411-aptamer by utilizing terminal –COOH group present on formulated liposomes. Addition of TSL was carried out with stirring into MES buffer at pH 6 containing sulfo-NHS and EDC. Subsequently, AS1411 (aptamer) was added and stirring was done for 6 h. | Film hydration method | Passive loading | Breast cancer | [146] |
| HER-2 antibody | Near-infrared light | Doxorubicin and hollow-gold nanospheres (HAuNS) | HER2 | HSPC, DPPC, DSPE-PEG(2000)NH-MAL | DSPE-PEG(2000)-NH-MAL, HSPC, DPPC and cholesterol were dissolved in chloroform. A solution of OMP (Octa-decyl-3-mercaptopionate) modified HAuNS in dichlormethane was added into the above lipid mixture dissolved in chloroform. Subsequently, a dry lipid film was formed and hydrated. Finally, HER2 targeted liposomes were formulated by HER-2 antibody overnight incubation (at 4 °C) of preformed liposomes with HER2-antibody. | Film hydration method | Ammonium-sulfate gradient | Ovarian cancer (SKOV3 cells), Breast cancer (BT474 cells) | [147] |
| Fab′fragment of ErbB2 antibody | pH | Doxorubicin | HER2 Receptor | GGLG, PEG-DSPE, Fab′-MAL-PEG-Glu2C18 | pH responsive liposomes were formulated by dissolution of lipids in t-butyl alcohol at a temperature of 60 °C. This step was followed by freeze drying, yielding a mixture of dried lipid powder subsequently hydrated with 30 mM citrate-solution for 2 h. Immunoliposomes were formulated by a covalent (thioether) linkage between thiol group of Fab′ and terminal MAL group present on preformed liposomes. Preformed pH-responsive liposomes were incubated with the Fab′ with stirring at room temperature for 6 h. | Lipid powder mixture preparation by lyophilization and hydration with PBS at 60 °C | Active loading | Breast Cancer (HCC1954 cell-line) | [148] |
| RGD-peptide | pH | Docetaxel | αVβ3 integrin receptor | PE, linoleic acid (LA), RGD-PEG-LA | Cholesterol, phospahtidyl-ethanolamine (PE), Docetaxel, linoleic acid (LA) and RGD-PEG-LA were dissolved in chloroform and a thin lipid film was formed by evaporation under vacuum using a rotary-evaporator. Subsequently, hydration of the lipid film was done with PBS (pH 7.4). | Film hydration method | Passive loading | Breast tumor (MCF-7 cells) | [149] |
| Folate | Temperature | Doxorubicin | Folate receptor | DPPC, DSPE-PEG(2000), DSPE-PEG-Folate | DSPE-PEG-FA was prepared by the carbodiimide mediated conjugation of folic acid with DSPE-PEG-NH2. Folate directed thermosensitive liposomes were formulated by film hydration method. A thin lipid-film was prepared after evaporation of lipids, i.e., DPPC:DSPE-PEG(2000):DSPE-PEG-Folate, and cholesterol dissolved in a chloroform:methanol mixture in a round-bottom flask. Subsequently, lipid-film was hydrated and extruded. Liposomes were loaded with doxorubicin using modified ammonium-sulfate gradient. | Film hydration method | Modified ammonium-sulfate gradient | Cervical cancer (HeLa cells) and Cervical-adenocarcinoma (KB cells) | [150] |
| Anti-EphA10 antibody | pH | MDR1-siRNA | EphA10receptor | PC, DOPE, DOTAP, Chol-SIB-PEG | Lipids, cholesterol and Chol-SIB-PEG were dissolved in dochloromethane and evaporated under vacuum to form a thin film. The film was hydrated with water, sonicated for four minutes and passed through 0.2 μm membrane to formulate Chol-SIB-PEG-modified liposomes (PSL). Surface modification of PSL with anti-EphA10 antibody was done by addition of sulfo-NHS and EDCI solution in PBS (pH 7.4) to the liposomal suspension with stirring for 2 h. This step was followed by addition of anti-EphA10 antibody to the liposomal suspension and overnight incubation at 4 °C. | Modified film-dispersion hydration method | Active loading | Multi-drug resistant breast tumor (MCF7/ADR cells) | [151] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Riaz, M.K.; Riaz, M.A.; Zhang, X.; Lin, C.; Wong, K.H.; Chen, X.; Zhang, G.; Lu, A.; Yang, Z. Surface Functionalization and Targeting Strategies of Liposomes in Solid Tumor Therapy: A Review. Int. J. Mol. Sci. 2018, 19, 195. https://doi.org/10.3390/ijms19010195
Riaz MK, Riaz MA, Zhang X, Lin C, Wong KH, Chen X, Zhang G, Lu A, Yang Z. Surface Functionalization and Targeting Strategies of Liposomes in Solid Tumor Therapy: A Review. International Journal of Molecular Sciences. 2018; 19(1):195. https://doi.org/10.3390/ijms19010195
Chicago/Turabian StyleRiaz, Muhammad Kashif, Muhammad Adil Riaz, Xue Zhang, Congcong Lin, Ka Hong Wong, Xiaoyu Chen, Ge Zhang, Aiping Lu, and Zhijun Yang. 2018. "Surface Functionalization and Targeting Strategies of Liposomes in Solid Tumor Therapy: A Review" International Journal of Molecular Sciences 19, no. 1: 195. https://doi.org/10.3390/ijms19010195
APA StyleRiaz, M. K., Riaz, M. A., Zhang, X., Lin, C., Wong, K. H., Chen, X., Zhang, G., Lu, A., & Yang, Z. (2018). Surface Functionalization and Targeting Strategies of Liposomes in Solid Tumor Therapy: A Review. International Journal of Molecular Sciences, 19(1), 195. https://doi.org/10.3390/ijms19010195