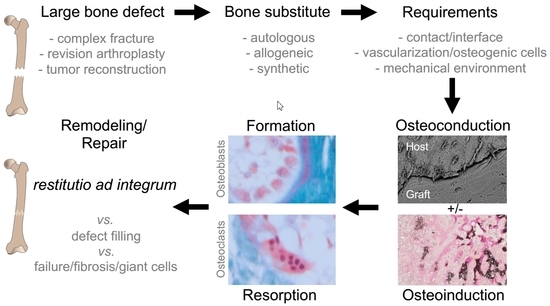
Cellular Mechanisms Responsible for Success and Failure of Bone Substitute Materials
Abstract
:1. Introduction
2. Bone Remodeling
3. Basic Cellular Concept of Bone Substitute Incorporation
4. Allograft Incorporation
5. Requirements for Synthetic Bone Substitutes
6. Bone Modeling on Implants
7. Limits and Failure of Bone Substitutes and Implants
8. Immune Responses to Bone Substitute Materials
9. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Rolvien, T.; Barvencik, F.; Klatte, T.O.; Busse, B.; Hahn, M.; Rueger, J.M.; Rupprecht, M. β-TCP bone substitutes in tibial plateau depression fractures. Knee 2017, 24, 1138–1145. [Google Scholar] [CrossRef] [PubMed]
- Sen, M.K.; Miclau, T. Autologous iliac crest bone graft: Should it still be the gold standard for treating nonunions? Injury 2007, 38, S75–S80. [Google Scholar] [CrossRef] [PubMed]
- Dawson, J.; Kiner, D.; Gardner, W.; Swafford, R.; Nowotarski, P.J. The reamer-irrigator-aspirator as a device for harvesting bone graft compared with iliac crest bone graft: Union rates and complications. J. Orthop. Trauma 2014, 28, 584–590. [Google Scholar] [CrossRef] [PubMed]
- Anselme, K. Osteoblast adhesion on biomaterials. Biomaterials 2000, 21, 667–681. [Google Scholar] [CrossRef]
- Kruyt, M.C.; van Gaalen, S.M.; Oner, F.C.; Verbout, A.J.; de Bruijn, J.D.; Dhert, W.J. Bone tissue engineering and spinal fusion: The potential of hybrid constructs by combining osteoprogenitor cells and scaffolds. Biomaterials 2004, 25, 1463–1473. [Google Scholar] [CrossRef]
- Glenske, K.; Donkiewicz, P.; Kowitsch, A.; Milosevic-Oljaca, N.; Rider, P.; Rofall, S.; Franke, J.; Jung, O.; Smeets, R.; Schnettler, R.; et al. . Applications of metals for bone regeneration. Int. J. Mol. Sci. 2018, 19, 826. [Google Scholar] [CrossRef] [PubMed]
- Buma, P.; Lamerigts, N.; Schreurs, B.W.; Gardeniers, J.; Versleyen, D.; Slooff, T.J. Impacted graft incorporation after cemented acetabular revision. Histological evaluation in 8 patients. Acta Orthop. Scand. 1996, 67, 536–540. [Google Scholar] [CrossRef] [PubMed]
- Van der Donk, S.; Buma, P.; Slooff, T.J.; Gardeniers, J.W.; Schreurs, B.W. Incorporation of morselized bone grafts: A study of 24 acetabular biopsy specimens. Clin. Orthop. Relat. Res. 2002, 396, 131–141. [Google Scholar] [CrossRef]
- Butscheidt, S.; Moritz, M.; Gehrke, T.; Puschel, K.; Amling, M.; Hahn, M.; Rolvien, T. Incorporation and remodeling of structural allografts in acetabular reconstruction: Multiscale, micro-morphological analysis of 13 pelvic explants. J. Bone Jt. Surg. Am. 2018, 100, 1406–1415. [Google Scholar] [CrossRef] [PubMed]
- Remes, A.; Williams, D.F. Immune response in biocompatibility. Biomaterials 1992, 13, 731–743. [Google Scholar] [CrossRef]
- Zaidi, M. Skeletal remodeling in health and disease. Nat. Med. 2007, 13, 791–801. [Google Scholar] [CrossRef] [PubMed]
- Bellido, T. Osteocyte-driven bone remodeling. Calcif. Tissue Int. 2014, 94, 25–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Einhorn, T.A.; Gerstenfeld, L.C. Fracture healing: Mechanisms and interventions. Nat. Rev. Rheumatol. 2015, 11, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Gugala, Z.; Lindsey, R.W.; Gogolewski, S. New approaches in the treatment of critical-size segmental defects in long bones. Macromol. Symp. 2007, 253, 147–161. [Google Scholar] [CrossRef]
- Uchida, A.; Nade, S.; McCartney, E.; Ching, W. Bone ingrowth into three different porous ceramics implanted into the tibia of rats and rabbits. J. Orthop. Res. 1985, 3, 65–77. [Google Scholar] [CrossRef] [PubMed]
- Berger, G.; Gildenhaar, R.; Ploska, U. Rapid resorbable, glassy crystalline materials on the basis of calcium alkali orthophosphates. Biomaterials 1995, 16, 1241–1248. [Google Scholar] [CrossRef]
- Athanasiou, K.A.; Niederauer, G.G.; Agrawal, C.M. Sterilization, toxicity, biocompatibility and clinical applications of polylactic acid/polyglycolic acid copolymers. Biomaterials 1996, 17, 93–102. [Google Scholar] [CrossRef]
- Kaban, L.B.; Glowacki, J. Augmentation of rat mandibular ridge with demineralized bone implants. J. Dent. Res. 1984, 63, 998–1002. [Google Scholar] [CrossRef] [PubMed]
- Campana, V.; Milano, G.; Pagano, E.; Barba, M.; Cicione, C.; Salonna, G.; Lattanzi, W.; Logroscino, G. Bone substitutes in orthopaedic surgery: From basic science to clinical practice. J. Mater. Sci. Mater. Med. 2014, 25, 2445–2461. [Google Scholar] [CrossRef] [PubMed]
- Schilling, A.F.; Linhart, W.; Filke, S.; Gebauer, M.; Schinke, T.; Rueger, J.M.; Amling, M. Resorbability of bone substitute biomaterials by human osteoclasts. Biomaterials 2004, 25, 3963–3972. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, O.; Bouler, J.M.; Weiss, P.; Bosco, J.; Aguado, E.; Daculsi, G. Short-term effects of mineral particle sizes on cellular degradation activity after implantation of injectable calcium phosphate biomaterials and the consequences for bone substitution. Bone 1999, 25, 71S–74S. [Google Scholar] [CrossRef]
- Stevenson, S.; Emery, S.E.; Goldberg, V.M. Factors affecting bone graft incorporation. Clin. Orthop. Relat. Res. 1996, 324, 66–74. [Google Scholar] [CrossRef]
- Krause, M.; Oheim, R.; Catala-Lehnen, P.; Pestka, J.M.; Hoffmann, C.; Huebner, W.; Peters, F.; Barvencik, F.; Amling, M. Metaphyseal bone formation induced by a new injectable beta-tcp-based bone substitute: A controlled study in rabbits. J. Biomater. Appl. 2014, 28, 859–868. [Google Scholar] [CrossRef] [PubMed]
- Lucarelli, E.; Fini, M.; Beccheroni, A.; Giavaresi, G.; Di Bella, C.; Aldini, N.N.; Guzzardella, G.; Martini, L.; Cenacchi, A.; Di Maggio, N. Stromal stem cells and platelet-rich plasma improve bone allograft integration. Clin. Orthop. Relat. Res. 2005, 435, 62–68. [Google Scholar] [CrossRef]
- Kawano, M.; Ariyoshi, W.; Iwanaga, K.; Okinaga, T.; Habu, M.; Yoshioka, I.; Tominaga, K.; Nishihara, T. Mechanism involved in enhancement of osteoblast differentiation by hyaluronic acid. Biochem. Biophys. Res. Commun. 2011, 405, 575–580. [Google Scholar] [CrossRef] [PubMed]
- Daugaard, H.; Elmengaard, B.; Andreassen, T.T.; Baas, J.; Bechtold, J.E.; Soballe, K. The combined effect of parathyroid hormone and bone graft on implant fixation. J. Bone Jt. Surg. Br. 2011, 93, 131–139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolff, J. Das gesetz der transformation der knochen; Hirschwald: Berlin, Germany, 1892. [Google Scholar]
- Bonewald, L.F. Mechanosensation and transduction in osteocytes. Bonekey Osteovision 2006, 3, 7–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bauer, T.W.; Muschler, G.F. Bone graft materials. An overview of the basic science. Clin. Orthop. Relat. Res. 2000, 371, 10–27. [Google Scholar] [CrossRef]
- Hooten, J.P., Jr.; Engh, C.A.; Heekin, R.D.; Vinh, T.N. Structural bulk allografts in acetabular reconstruction. Analysis of two grafts retrieved at post-mortem. J. Bone Jt. Surg. Br. 1996, 78, 270–275. [Google Scholar] [CrossRef]
- Malinin, T.I.; Carpenter, E.M.; Temple, H.T. Particulate bone allograft incorporation in regeneration of osseous defects; importance of particle sizes. Open Orthop. J. 2007, 1, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Schimmel, J.W.; Buma, P.; Versleyen, D.; Huiskes, R.; Slooff, T.J. Acetabular reconstruction with impacted morselized cancellous allografts in cemented hip arthroplasty: A histological and biomechanical study on the goat. J. Arthroplasty 1998, 13, 438–448. [Google Scholar] [CrossRef]
- Lanza, R.; Langer, R.; Vacanti, J. Tissue engineering. Science 1993, 260, 920–926. [Google Scholar]
- Khan, W.S.; Rayan, F.; Dhinsa, B.S.; Marsh, D. An osteoconductive, osteoinductive, and osteogenic tissue-engineered product for trauma and orthopaedic surgery: How far are we? Stem Cells Int. 2012, 2012, 236231. [Google Scholar] [CrossRef] [PubMed]
- Tadic, D.; Epple, M. A thorough physicochemical characterisation of 14 calcium phosphate-based bone substitution materials in comparison to natural bone. Biomaterials 2004, 25, 987–994. [Google Scholar] [CrossRef]
- Low, K.L.; Tan, S.H.; Zein, S.H.S.; Roether, J.A.; Mouriño, V.; Boccaccini, A.R. Calcium phosphate-based composites as injectable bone substitute materials. J. Biomed. Mater. Res. Part B Appl. Biomater. 2010, 94B, 273–286. [Google Scholar] [CrossRef] [PubMed]
- Giannoudis, P.V.; Einhorn, T.A.; Marsh, D. Fracture healing: The diamond concept. Injury 2007, 38, S3–S6. [Google Scholar] [CrossRef]
- Alam, M.I.; Asahina, I.; Ohmamiuda, K.; Takahashi, K.; Yokota, S.; Enomoto, S. Evaluation of ceramics composed of different hydroxyapatite to tricalcium phosphate ratios as carriers for rhbmp-2. Biomaterials 2001, 22, 1643–1651. [Google Scholar] [CrossRef]
- Seidenstuecker, M.; Ruehe, J.; Suedkamp, N.P.; Serr, A.; Wittmer, A.; Bohner, M.; Bernstein, A.; Mayr, H.O. Composite material consisting of microporous β-tcp ceramic and alginate for delayed release of antibiotics. Acta Biomater. 2017, 51, 433–446. [Google Scholar] [CrossRef] [PubMed]
- Faigle, G.; Bernstein, A.; Suedkamp, N.; Mayr, H.; Peters, F.; Huebner, W.; Seidenstuecker, M. Release behavior of van from four types of cap-ceramic granules using various loading methods at two different degrees of acidity. J. Mater. Sci. Mater. Med. 2018, 29, 12. [Google Scholar] [CrossRef] [PubMed]
- Bobyn, J.D.; Pilliar, R.M.; Binnington, A.G.; Szivek, J.A. The effect of proximally and fully porous-coated canine hip stem design on bone modeling. J. Orthop. Res. 1987, 5, 393–408. [Google Scholar] [CrossRef] [PubMed]
- Li, J.P.; Habibovic, P.; van den Doel, M.; Wilson, C.E.; de Wijn, J.R.; van Blitterswijk, C.A.; de Groot, K. Bone ingrowth in porous titanium implants produced by 3d fiber deposition. Biomaterials 2007, 28, 2810–2820. [Google Scholar] [CrossRef] [PubMed]
- Konttinen, Y.T.; Zhao, D.; Beklen, A.; Ma, G.; Takagi, M.; Kivela-Rajamaki, M.; Ashammakhi, N.; Santavirta, S. The microenvironment around total hip replacement prostheses. Clin. Orthop. Relat. Res. 2005, 430, 28–38. [Google Scholar] [CrossRef]
- Mabry, T.M.; Hanssen, A.D. The role of stems and augments for bone loss in revision knee arthroplasty. J. Arthroplasty 2007, 22, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Levine, B.R.; Sporer, S.; Poggie, R.A.; Della Valle, C.J.; Jacobs, J.J. Experimental and clinical performance of porous tantalum in orthopedic surgery. Biomaterials 2006, 27, 4671–4681. [Google Scholar] [CrossRef] [PubMed]
- Bobyn, J.D.; Toh, K.K.; Hacking, S.A.; Tanzer, M.; Krygier, J.J. Tissue response to porous tantalum acetabular cups: A canine model. J. Arthroplasty 1999, 14, 347–354. [Google Scholar] [CrossRef]
- Unger, A.S.; Duggan, J.P. Midterm results of a porous tantalum monoblock tibia component clinical and radiographic results of 108 knees. J. Arthroplasty 2011, 26, 855–860. [Google Scholar] [CrossRef] [PubMed]
- Breer, S.; Hahn, M.; Kendoff, D.; Krause, M.; Koehne, T.; Haasper, C.; Gehrke, T.; Amling, M.; Gebauer, M. Histological ex vivo analysis of retrieved human tantalum augmentations. Int. Orthop. 2012, 36, 2269–2274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knight, J.L.; Fujii, K.; Atwater, R.; Grothaus, L. Bone-grafting for acetabular deficiency during primary and revision total hip arthroplasty. A radiographic and clinical analysis. J. Arthroplasty 1993, 8, 371–382. [Google Scholar] [CrossRef]
- Deakin, D.E.; Bannister, G.C. Graft incorporation after acetabular and femoral impaction grafting with washed irradiated allograft and autologous marrow. J. Arthroplasty 2007, 22, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Ullmark, G.; Sorensen, J.; Nilsson, O. Bone healing of severe acetabular defects after revision arthroplasty. Acta Orthop. 2009, 80, 179–183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schreurs, B.W.; Keurentjes, J.C.; Gardeniers, J.W.; Verdonschot, N.; Slooff, T.J.; Veth, R.P. Acetabular revision with impacted morsellised cancellous bone grafting and a cemented acetabular component: A 20- to 25-year follow-up. J. Bone Jt. Surg. Br. 2009, 91, 1148–1153. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.D.; Frierson, K.E.; Keller, T.S.; Cook, C.; Scheinberg, R.; Zerwekh, J.; Meyers, L.; Sciadini, M.F. Porous ceramics as bone graft substitutes in long bone defects: A biomechanical, histological, and radiographic analysis. J. Orthop. Res. 1996, 14, 351–369. [Google Scholar] [CrossRef] [PubMed]
- Linhart, W.; Briem, D.; Amling, M.; Rueger, J.; Windolf, J. Mechanical failure of porous hydroxyapatite ceramics 7.5 years after implantation in the proximal tibial. Der Unfallchirurg 2004, 107, 154–157. [Google Scholar] [CrossRef] [PubMed]
- Engelbrecht, E.; von Foerster, G.; Delling, G. Ionogran in revision arthroplasty. J. Bone Jt. Surg. Br. 2000, 82, 192–199. [Google Scholar] [CrossRef]
- Mountziaris, P.M.; Mikos, A.G. Modulation of the inflammatory response for enhanced bone tissue regeneration. Tissue Eng. Part B Rev. 2008, 14, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.M.; Rodriguez, A.; Chang, D.T. Foreign body reaction to biomaterials. In Seminars in Immunology; Elsevier: New York, NY, USA, 2008; pp. 86–100. [Google Scholar]
- Hu, W.J.; Eaton, J.W.; Ugarova, T.P.; Tang, L. Molecular basis of biomaterial-mediated foreign body reactions. Blood 2001, 98, 1231–1238. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Eaton, J.W. Fibrin(ogen) mediates acute inflammatory responses to biomaterials. J. Exp. Med. 1993, 178, 2147–2156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbeck, M.; Motta, A.; Migliaresi, C.; Sader, R.; Kirkpatrick, C.J.; Ghanaati, S. Heterogeneity of biomaterial-induced multinucleated giant cells: Possible importance for the regeneration process? J. Biomed. Mater. Res. A 2016, 104, 413–418. [Google Scholar] [CrossRef] [PubMed]
- Barbeck, M.; Udeabor, S.; Lorenz, J.; Schlee, M.; Holthaus, M.G.; Raetscho, N.; Choukroun, J.; Sader, R.; Kirkpatrick, C.J.; Ghanaati, S. High-temperature sintering of xenogeneic bone substitutes leads to increased multinucleated giant cell formation: In vivo and preliminary clinical results. J. Oral Implantol. 2015, 41, e212–e222. [Google Scholar] [CrossRef] [PubMed]
- Barbeck, M.; Udeabor, S.E.; Lorenz, J.; Kubesch, A.; Choukroun, J.; Sader, R.A.; Kirkpatrick, C.J.; Ghanaati, S. Induction of multinucleated giant cells in response to small sized bovine bone substitute (bio-oss) results in an enhanced early implantation bed vascularization. Ann. Maxillofac. Surg. 2014, 4, 150–157. [Google Scholar] [PubMed]
- Barbeck, M.; Booms, P.; Unger, R.; Hoffmann, V.; Sader, R.; Kirkpatrick, C.J.; Ghanaati, S. Multinucleated giant cells in the implant bed of bone substitutes are foreign body giant cells—New insights into the material—Mediated healing process. J. Biomed. Mater. Res. A 2017, 105, 1105–1111. [Google Scholar] [CrossRef] [PubMed]
- Barbeck, M.; Dard, M.; Kokkinopoulou, M.; Markl, J.; Booms, P.; Sader, R.A.; Kirkpatrick, C.J.; Ghanaati, S. Small-sized granules of biphasic bone substitutes support fast implant bed vascularization. Biomatter 2015, 5, e1056943. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbeck, M.; Serra, T.; Booms, P.; Stojanovic, S.; Najman, S.; Engel, E.; Sader, R.; Kirkpatrick, C.J.; Navarro, M.; Ghanaati, S. Analysis of the in vitro degradation and the in vivo tissue response to bi-layered 3d-printed scaffolds combining pla and biphasic pla/bioglass components–guidance of the inflammatory response as basis for osteochondral regeneration. Bioact. Mater. 2017, 2, 208–223. [Google Scholar] [CrossRef] [PubMed]
- Brown, B.N.; Ratner, B.D.; Goodman, S.B.; Amar, S.; Badylak, S.F. Macrophage polarization: An opportunity for improved outcomes in biomaterials and regenerative medicine. Biomaterials 2012, 33, 3792–3802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McNally, A.K.; Anderson, J.M. Macrophage fusion and multinucleated giant cells of inflammation. In Cell Fusion in Health and Disease; Springer: Berlin, Germany, 2011; pp. 97–111. [Google Scholar]
- Ghanaati, S.; Barbeck, M.; Orth, C.; Willershausen, I.; Thimm, B.W.; Hoffmann, C.; Rasic, A.; Sader, R.A.; Unger, R.E.; Peters, F.; et al. Influence of beta-tricalcium phosphate granule size and morphology on tissue reaction in vivo. Acta Biomater. 2010, 6, 4476–4487. [Google Scholar] [CrossRef] [PubMed]
- Ghanaati, S.; Barbeck, M.; Detsch, R.; Deisinger, U.; Hilbig, U.; Rausch, V.; Sader, R.; Unger, R.E.; Ziegler, G.; Kirkpatrick, C.J. The chemical composition of synthetic bone substitutes influences tissue reactions in vivo: Histological and histomorphometrical analysis of the cellular inflammatory response to hydroxyapatite, beta-tricalcium phosphate and biphasic calcium phosphate ceramics. Biomed. Mater. 2012, 7, 015005. [Google Scholar] [PubMed]
- Barbeck, M; Jung, O; Wenisch, S; Schnettler, R. Pro- and anti-inflammation are needed for synchronous defect healing and degradation of bone substitutes. J. Biomed. Mater. Res. A. submitted.
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive oxygen species in inflammation and tissue injury. Antioxid. Redox Signal. 2014, 20, 1126–1167. [Google Scholar] [CrossRef] [PubMed]
- Burchardt, H. The biology of bone graft repair. Clin. Orthop. Relat. Res. 1983, 174, 28–42. [Google Scholar] [CrossRef]
- Hing, K.A. Bioceramic bone graft substitutes: Influence of porosity and chemistry. Int. J. Appl. Ceram. Technol. 2005, 2, 184–199. [Google Scholar] [CrossRef]
- Lindahl, P.; Hellstrom, M.; Kalen, M.; Betsholtz, C. Endothelial-perivascular cell signaling in vascular development: Lessons from knockout mice. Curr. Opin. Lipidol. 1998, 9, 407–411. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.J.; Kurz, H.; Christ, B.; Wilting, J. Platelet-derived growth factor-b induces transformation of fibrocytes into spindle-shaped myofibroblasts in vivo. Histochem. Cell Biol. 1998, 109, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Kouri, J.; Ancheta, O. Transformation of macrophages into fibroblasts. Exp. Cell Res. 1972, 71, 168–176. [Google Scholar] [CrossRef]
- Iwano, M.; Plieth, D.; Danoff, T.M.; Xue, C.; Okada, H.; Neilson, E.G. Evidence that fibroblasts derive from epithelium during tissue fibrosis. J. Clin. Investig. 2002, 110, 341–350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campbell, J.H.; Efendy, J.L.; Han, C.; Girjes, A.A.; Campbell, G.R. Haemopoietic origin of myofibroblasts formed in the peritoneal cavity in response to a foreign body. J. Vasc. Res. 2000, 37, 364–371. [Google Scholar] [CrossRef] [PubMed]
- McAnulty, R.J. Fibroblasts and myofibroblasts: Their source, function and role in disease. Int. J. Biochem. Cell Biol. 2007, 39, 666–671. [Google Scholar] [CrossRef] [PubMed]
- Morais, J.M.; Papadimitrakopoulos, F.; Burgess, D.J. Biomaterials/tissue interactions: Possible solutions to overcome foreign body response. AAPS J. 2010, 12, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, Y.; Zuo, Y.; Li, J.; Ma, S.; Cheng, L. Biocompatibility and osteogenesis of biomimetic nano-hydroxyapatite/polyamide composite scaffolds for bone tissue engineering. Biomaterials 2007, 28, 3338–3348. [Google Scholar] [CrossRef] [PubMed]
- Das, D.; Zhang, Z.; Winkler, T.; Mour, M.; Günter, C.I.; Morlock, M.M.; Machens, H.-G.; Schilling, A.F. Bioresorption and degradation of biomaterials. In Tissue Engineering III: Cell-Surface Interactions for Tissue Culture; Springer: Berlin, Germany, 2011; pp. 317–333. [Google Scholar]









© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rolvien, T.; Barbeck, M.; Wenisch, S.; Amling, M.; Krause, M. Cellular Mechanisms Responsible for Success and Failure of Bone Substitute Materials. Int. J. Mol. Sci. 2018, 19, 2893. https://doi.org/10.3390/ijms19102893
Rolvien T, Barbeck M, Wenisch S, Amling M, Krause M. Cellular Mechanisms Responsible for Success and Failure of Bone Substitute Materials. International Journal of Molecular Sciences. 2018; 19(10):2893. https://doi.org/10.3390/ijms19102893
Chicago/Turabian StyleRolvien, Tim, Mike Barbeck, Sabine Wenisch, Michael Amling, and Matthias Krause. 2018. "Cellular Mechanisms Responsible for Success and Failure of Bone Substitute Materials" International Journal of Molecular Sciences 19, no. 10: 2893. https://doi.org/10.3390/ijms19102893
APA StyleRolvien, T., Barbeck, M., Wenisch, S., Amling, M., & Krause, M. (2018). Cellular Mechanisms Responsible for Success and Failure of Bone Substitute Materials. International Journal of Molecular Sciences, 19(10), 2893. https://doi.org/10.3390/ijms19102893