In Vivo Analysis of the Biocompatibility and Macrophage Response of a Non-Resorbable PTFE Membrane for Guided Bone Regeneration
Abstract
:1. Introduction
2. Results
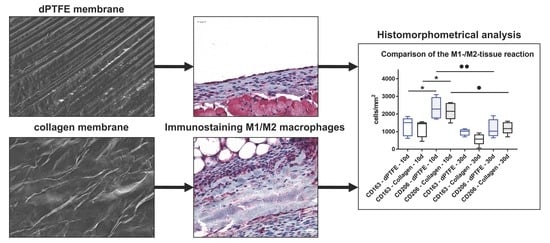
2.1. Histological (Qualitative) Analysis
2.2. Histomorphometrical (Quantitative) Analysis
3. Discussion
4. Materials and Methods
4.1. Barrier Membranes
4.1.1. dPTFE Membrane (Permamem®)
4.1.2. Pericardium-Based Collagen Membrane (Jason® membrane)
4.2. Scanning Electron Microscopy (SEM)
4.3. In Vivo Study Design, Subcutaneous Implantation, and Explantation Procedure
4.3.1. Histology and Immunohistochemistry
4.3.2. Histological Analysis
4.3.3. Histomorphometrical Analysis
4.3.4. Statistical Analyses
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Buser, D.; Dahlin, C.; Schenk, R.K. Guided bone regeneration in implant dentistry. In Guided Bone Regeneration; Chicago Quintessence: Bern, Switzerland, 1994. [Google Scholar]
- Jung, R.E.; Fenner, N.; Hämmerle, C.H.; Zitzmann, N.U. Long-term outcome of implants placed with guided bone regeneration (GBR) using resorbable and non-resorbable membranes after 12–14 years. Clin. Oral Implants Res. 2013, 24, 1065–1073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scantlebury, T.V. 1982–1992: A Decade of Technology Development for Guided Tissue Regeneration. J. Periodontol. 1993, 64, 1129–1137. [Google Scholar] [CrossRef] [PubMed]
- Hämmerle, C.H.; Karring, T. Guided bone regeneration at oral implant sites. Periodontology 1998, 17, 151–175. [Google Scholar] [CrossRef]
- Wessing, B.; Lettner, S.; Zechner, W. Guided Bone Regeneration with Collagen Membranes and Particulate Graft Materials: A Systematic Review and Meta-Analysis. Int. J. Oral Maxillofac. Implants 2018, 33, 87–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Q.; Humpe, A.; Kletsas, D.; Warnke, F.; Becker, S.T.; Douglas, T.; Sivananthan, S.; Warnke, P.H. Proliferation assessment of primary human mesenchymal stem cells on collagen membranes for guided bone regeneration. Int. J. Oral Maxillofac. Implants 2001, 26, 1004–1010. [Google Scholar]
- Imbronito, A.V.; Todescan, J.H.; Carvalho, C.V.; Arana-Chavez, V.E. Healing of alveolar bone in resorbable and non-resorbable membrane-protected defects. A histologic pilot study in dogs. Biomaterials 2002, 23, 4079–4086. [Google Scholar] [CrossRef]
- Liu, J.; Kerns, D.G. Mechanisms of guided bone regeneration: A review. Open Dent. J. 2014, 8, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Elgali, I.; Omar, O.; Dahlin, C.; Thomsen, P. Guided bone regeneration: Materials and biological mechanisms revisited. Eur. J. Oral Sci. 2017, 125, 315–337. [Google Scholar] [CrossRef] [PubMed]
- Hoornaert, A.; d’Arros, C.; Heymann, M.-F.; Layrolle, P. Biocompatibility, resorption and biofunctionality of a new synthetic biodegradable membrane for guided bone regeneration. Biomed. Mater. 2016, 11. [Google Scholar] [CrossRef] [PubMed]
- Caballé-Serrano, J.; Munar-Frau, A.; Ortiz-Puigpelat, O.; Soto-Penaloza, D.; Peñarrocha, M.; Hernández-Alfaro, F. On the search of the ideal barrier membrane for guided bone regeneration. J. Clin. Exp. Dent. 2018, 10, e477–e483. [Google Scholar] [CrossRef] [PubMed]
- Soldatos, N.K.; Stylianou, P.; Koidou, V.P.; Angelov, N.; Yukna, R.; Romanos, G.E. Limitations and options using resorbable versus nonresorbable membranes for successful guided bone regeneration. Quintessence Int. 2017, 48, 131–147. [Google Scholar] [CrossRef] [PubMed]
- Trobos, M.; Juhlin, A.; Shah, F.A.; Hoffman, M.; Sahlin, H.; Dahlin, C. In vitro evaluation of barrier function against oral bacteria of dense and expanded polytetrafluoroethylene (PTFE) membranes for guided bone regeneration. Clin. Implant Dent. Relat. Res. 2018. [Google Scholar] [CrossRef] [PubMed]
- Kohal, R.J.; Trejo, P.M.; Wirsching, C.; Hürzeler, M.B.; Caffesse, R.G. Comparison of bioabsorbable and bioinert membranes for guided bone regeneration around non-submerged implants: An experimental study in the mongrel dog. Clin. Oral Implants Res. 1999, 10, 226–237. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.M.; Rodriguez, A.; Chang, D.T. Foreign body reaction to biomaterials. Semin. Immunol. 2008, 20, 86–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lamichhane, S.; Anderson, J.A.; Vierhout, T.; Remund, T.; Sun, H.; Kelly, P. Polytetrafluoroethylene topographies determine the adhesion, activation, and foreign body giant cell formation of macrophages. J. Biomed. Mater. Res. A 2017, 105, 2441–2450. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.A.; Chang, D.T.; Meyerson, H.; Colton, E.; Kwon, I.K.; Matsuda, T.; Anderson, J.M. Proteomic analysis and quantification of cytokines and chemokines from biomaterial surface-adherent macrophages and foreign body giant cells. J. Biomed. Mater. Res. A 2007, 83, 585–596. [Google Scholar] [CrossRef] [PubMed]
- Brodbeck, W.G.; Nakayama, Y.; Matsuda, E.; Colton, T.; Ziats, N.P.; Anderson, J.M. Biomaterial surface chemistry dictates adherent monocyte/macrophage cytokine expression in vitro. Cytokine 2002, 18, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Brown, B.N.; Valentin, J.E.; Stewart-Akers, A.M.; McCabe, G.P.; Badylak, S.F. Macrophage phenotype and remodeling outcomes in response to biologic scaffolds with and without a cellular component. Biomaterials 2009, 30, 1482–1491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Badylak, S.F.; Valentin, J.E.; Ravindra, A.K.; McCabe, G.P.; Stewart-Akers, A.M. Macrophage Phenotype as a Determinant of Biologic Scaffold Remodeling. Tissue Eng. A 2008, 14, 1835–1842. [Google Scholar] [CrossRef] [PubMed]
- Rothamel, F.; Schwarz, D.; Fienitz, T.; Smeets, R.; Dreiseidler, T.; Ritter, L.; Happe, A.; Zöller, J. Biocompatibility and biodegradation of a native porcine pericardium membrane: Results of in vitro and in vivo examinations. Int. J. Oral Maxillofac. Implants 2012, 27, 146–154. [Google Scholar] [PubMed]
- Papagiannoulis, N.; Daum, O.; Tadic, D.; Steigmann, M. Vergleich von allogenem und alloplastischem Knochenregenerationsmaterial mit Pericardium Membran in der horizontalen gesteuerten Augmentation von Alveolardefekten. Dent. Implantol. 2012, 16, 360–369. [Google Scholar]
- Rothamel, D.; Schwarz, F.; Smeets, R.; Happe, A.; Fienitz, T.; Mazor, Z.; Zöller, J. Sinus floor elevation using a sintered, natural bone mineral. A histological case report study. Z. Zahnärztl. Implantol. 2011, 27, 60–70. [Google Scholar]
- Barbeck, M.; Dard, M.; Kokkinopoulou, M.; Markl, J.; Booms, P.; Sader, R.A.; Kirkpatrick, C.J.; Ghanaati, S. Small-sized granules of biphasic bone substitutes support fast implant bed vascularization. Biomatter 2015, 5, e1056943. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbeck, M.; Udeabor, S.E.; Lorenz, J.; Kubesch, A.; Choukroun, J.; Sader, R.A.; Kirkpatrick, C.J.; Ghanaati, S. Induction of multinucleated giant cells in response to small sized bovine bone substitute (Bio-OssTM) results in an enhanced early implantation bed vascularization. Ann. Maxillofac. Surg. 2014, 4, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Barbeck, M.; Serra, T.; Booms, P.; Stojanovic, S.; Najman, S.; Engel, E.; Sader, R.; Kirkpatrick, C.J.; Navarro, M.; Ghanaati, S. Analysis of the in vitro degradation and the in vivo tissue response to bi-layered 3D-printed scaffolds combining PLA and biphasic PLA/bioglass components—Guidance of the inflammatory response as basis for osteochondral regeneration. Bioact. Mater. 2017, 2, 208–223. [Google Scholar] [CrossRef] [PubMed]
- Tawil, G.; Barbeck, M.; Unger, R.; Tawil, P.; Witte, F. Sinus Floor Elevation Using the Lateral Approach and Window Repositioning and a Xenogeneic Bone Substitute as a Grafting Material: A. Histologic, Histomorphometric, and Radiographic Analysis. Int. J. Oral Maxillofac. Implants 2018, 33, 1089–1096. [Google Scholar] [CrossRef] [PubMed]
- Barbeck, M.; Unger, R.E.; Booms, P.; Dohle, E.; Sader, R.A.; Kirkpatrick, C.J.; Ghanaati, S. Monocyte preseeding leads to an increased implant bed vascularization of biphasic calcium phosphate bone substitutes via vessel maturation. J. Biomed. Mater. Res. A 2016, 104, 2928–2935. [Google Scholar] [CrossRef] [PubMed]
- Uludağ, H. Grand challenges in biomaterials. Front. Bioeng. Biotechnol. 2014, 2. [Google Scholar] [CrossRef] [PubMed]
- Ghensi, P.; Stablum, W.; Bettio, E.; Soldini, M.C.; Tripi, T.R.; Soldini, C. Management of the exposure of a dense PTFE (d-PTFE) membrane in guided bone regeneration (GBR): A case report. Oral Implantol. 2017, 10, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Hammerle, C.H.F.; Jung, R.E.; Feloutzis, A. A systematic review of the survival of implants in bone sites augmented with barrier membranes (guided bone regeneration) in partially edentulous patients. J. Clin. Periodontol. 2002, 29, 226–231. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, A.-S.-F.; Macedo, L.-G.-S.; Macedo, N.-L.; Balducci, I. Polyurethane and PTFE membranes for guided bone regeneration: Histopathological and ultrastructural evaluation. Med. Oral Patol. Oral Cir. Bucal 2010, 15, e401–e406. [Google Scholar] [CrossRef] [PubMed]
- Carbonell, J.M.; Martín, I.S.; Santos, A.; Pujol, A.; Sanz-Moliner, J.D.; Nart, J. High-density polytetrafluoroethylene membranes in guided bone and tissue regeneration procedures: A literature review. Int. J. Oral Maxillofa. Surg. 2014, 43, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Sridharan, R.; Cameron, A.R.; Kelly, D.J.; Kearney, C.J.; O’Brien, F.J. Biomaterial based modulation of macrophage polarization: A review and suggested design principles. Mater. Today 2015, 18, 313–325. [Google Scholar] [CrossRef]
- Chattopadhyay, S.; Raines, R.T. Review collagen-based biomaterials for wound healing. Biopolymers 2014, 101, 821–833. [Google Scholar] [CrossRef] [Green Version]
- Onuki, Y.; Bhardwaj, U.; Papadimitrakopoulos, F.; Burgess, D.J. A Review of the Biocompatibility of Implantable Devices: Current Challenges to Overcome Foreign Body Response. J. Diabetes Sci. Technol. 2008, 2, 1003–1015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parenteau-Bareil, R.; Gauvin, R.; Berthod, F. Collagen-based biomaterials for tissue engineering applications. Materials 2010, 3, 1863–1887. [Google Scholar] [CrossRef]
- Micke, P.; Ostman, A.; Lundeberg, J.; Ponten, F. Laser-assisted cell microdissection using the PALM system. Meth. Mol. Biol. 2005, 293, 151–166. [Google Scholar]
- Fink, L.; Kinfe, T.; Stein, M.M.; Ermert, L.; Hänze, J.; Kummer, W.; Seeger, W.; Bohle, R.M. Immunostaining and laser-assisted cell picking for mRNA analysis. Lab. Investig. 2000, 80, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, X.; Shi, B.; Miron, R.J. Membranes for guided tissue and bone regeneration. Ann. Oral Maxillofac. Surg. 2013, 1. [Google Scholar] [CrossRef] [Green Version]
- Permamem®—Botiss Dental | Botiss Biomaterials GmbH. (n.d.). Available online: https://botiss-dental.com/products/permamem/ (accessed on 22 August 2018).
- Barbeck, A.; Motta, M.; Migliaresi, C.; Sader, R.; Kirkpatrick, C.J.; Ghanaati, S. Heterogeneity of biomaterial-induced multinucleated giant cells: Possible importance for the regeneration process? J. Biomed. Mater. Res. A 2016, 104, 413–418. [Google Scholar] [CrossRef] [PubMed]
- Ghanaati, S.; Barbeck, M.; Detsch, R.; Deisinger, U.; Hilbig, U.; Rausch, V.; Sader, R.; Unger, R.E.; Ziegler, G.; Kirkpatrick, C.J. The chemical composition of synthetic bone substitutes influences tissue reactions in vivo: Histological and histomorphometrical analysis of the cellular inflammatory response to hydroxyapatite, beta-tricalcium phosphate and biphasic calcium phosphate ceramics. Biomed. Mater. 2012, 7. [Google Scholar] [CrossRef]
- Barbeck, M.; Booms, P.; Unger, R.; Hoffmann, V.; Sader, R.; Kirkpatrick, C.J.; Ghanaati, S. Multinucleated giant cells in the implant bed of bone substitutes are foreign body giant cells-New insights into the material-mediated healing process. J. Biomed. Mater. Res. A 2017, 105, 1105–1111. [Google Scholar] [CrossRef] [PubMed]




© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Korzinskas, T.; Jung, O.; Smeets, R.; Stojanovic, S.; Najman, S.; Glenske, K.; Hahn, M.; Wenisch, S.; Schnettler, R.; Barbeck, M. In Vivo Analysis of the Biocompatibility and Macrophage Response of a Non-Resorbable PTFE Membrane for Guided Bone Regeneration. Int. J. Mol. Sci. 2018, 19, 2952. https://doi.org/10.3390/ijms19102952
Korzinskas T, Jung O, Smeets R, Stojanovic S, Najman S, Glenske K, Hahn M, Wenisch S, Schnettler R, Barbeck M. In Vivo Analysis of the Biocompatibility and Macrophage Response of a Non-Resorbable PTFE Membrane for Guided Bone Regeneration. International Journal of Molecular Sciences. 2018; 19(10):2952. https://doi.org/10.3390/ijms19102952
Chicago/Turabian StyleKorzinskas, Tadas, Ole Jung, Ralf Smeets, Sanja Stojanovic, Stevo Najman, Kristina Glenske, Michael Hahn, Sabine Wenisch, Reinhard Schnettler, and Mike Barbeck. 2018. "In Vivo Analysis of the Biocompatibility and Macrophage Response of a Non-Resorbable PTFE Membrane for Guided Bone Regeneration" International Journal of Molecular Sciences 19, no. 10: 2952. https://doi.org/10.3390/ijms19102952
APA StyleKorzinskas, T., Jung, O., Smeets, R., Stojanovic, S., Najman, S., Glenske, K., Hahn, M., Wenisch, S., Schnettler, R., & Barbeck, M. (2018). In Vivo Analysis of the Biocompatibility and Macrophage Response of a Non-Resorbable PTFE Membrane for Guided Bone Regeneration. International Journal of Molecular Sciences, 19(10), 2952. https://doi.org/10.3390/ijms19102952