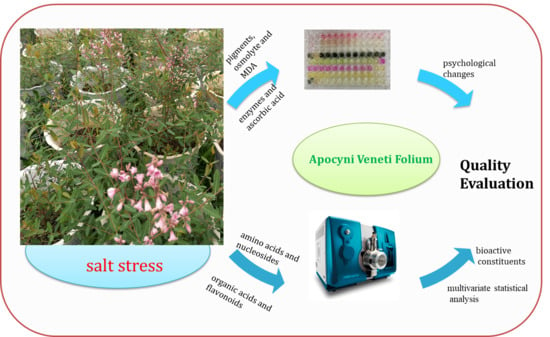
Variations in Physiology and Multiple Bioactive Constituents under Salt Stress Provide Insight into the Quality Evaluation of Apocyni Veneti Folium
Abstract
:1. Introduction
2. Results
2.1. Physiological Changes Affected by NaCl
2.1.1. Effects of Salt Stress on Photosynthetic Pigments
2.1.2. Effects of Salt Stress on Osmolytes and Lipid Peroxidation
2.1.3. Effects of Salt Stress on Antioxidant Enzyme and Ascorbic Acid
2.2. Determination of Multiple Bioactive Components
2.2.1. Optimization of Sample Preparation and UFLC-QTRAP-MS/MS Conditions
2.2.2. Method Validation
2.2.3. Sample Determination
2.2.4. Multivariate Statistical Analysis of Samples
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Salinity Treatments
4.2. Physiological Experiment
4.2.1. Extraction and Assay of Pigment
4.2.2. Osmolytes and MDA Assay
4.2.3. Enzyme Activities and Ascorbic Acid Assay
4.3. Multiple Bioactive Constituents Assay
4.3.1. Chemicals and Reagents
4.3.2. Sample Preparation
4.3.3. Chromatographic and Mass Spectrometric Conditions
4.3.4. Method Validation and Sample Determination
4.3.5. Multivariate Statistical Analysis
4.4. Data Processing
5. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Zhao, G.M.; Han, Y.; Sun, X.; Li, S.H.; Shi, Q.M.; Wang, C.H. Salinity stress increases secondary metabolites and enzyme activity in safflower. Ind. Crop. Prod. 2015, 64, 175–181. [Google Scholar]
- Zhou, Y.; Tang, N.Y.; Huang, L.J.; Zhao, Y.J.; Tang, X.Q.; Wang, K.C. Effects of Salt Stress on Plant Growth, Antioxidant Capacity, Glandular Trichome Density, and Volatile Exudates of Schizonepeta tenuifolia Briq. Int. J. Mol. Sci. 2018, 19, 252. [Google Scholar] [CrossRef] [PubMed]
- Aghaei, K.; Komatsu, S. Crop and medicinal plants proteomics in response to salt stress. Front. Plant Sci. 2013, 4, 8. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.L.; Xiao, Z.X.; Li, M.; Wong, F.; Yung, W.S.; Ku, Y.S.; Wang, Q.W.; Wang, X.; Xie, M.; Yim, A.K.; et al. Transcriptomic reprogramming in soybean seedlings under salt stress. Plant Cell Environ. 2018, 1–17. [Google Scholar]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef] [PubMed]
- Arbona, V.; Manzi, M.; Ollas, C.; Gómez-Cadenas, A. Metabolomics as a Tool to Investigate Abiotic Stress Tolerance in Plants. Int. J. Mol. Sci. 2013, 14, 4885–4911. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, B.; Liu, K.; Zheng, Y.; Wang, Y.; Wang, J.; Liao, H. Disruption of AtWNK8 Enhances Tolerance of Arabidopsis to Salt and Osmotic Stresses via Modulating Proline Content and Activities of Catalase and Peroxidase. Int. J. Mol. Sci. 2013, 14, 7032–7047. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaleel, C.A.; Riadh, K.; Gopi, R.; InèsHameed, M.; Inès, J.; Al-Juburi, H.; Zhao, C.X.; Shao, H.B.; Rajaram, P. Antioxidant defense responses: Physiological plasticity in higher plants under abiotic constraints. Acta Physiol. Plant. 2009, 31, 427–436. [Google Scholar] [CrossRef]
- Tran, L.S.; Urao, T.; Qin, F.; Maruyama, K.; Kakimoto, T.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Functional analysis of AHK1/ATHK1 and cytokinin receptor histidine kinases in response to abscisic acid, drought, and salt stress in Arabidopsis. Proc. Natl. Acad. Sci. USA 2007, 104, 20623–20638. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.L.; Mu, X.M.; Shao, H.B.; Wang, H.; Brestic, M. Global plant-responding mechanisms to salt stress: Physiological and molecular levels and implications in biotechnology. Crit. Rev. Biotechnol. 2015, 35, 425–437. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.H.; Liu, Z.X.; Zou, L.X.; Liu, X.H.; Chai, C.; Zhao, H.; Yan, Y.; Wang, C.C. Quality evaluation of Apocyni Veneti Folium from different habitats and commercial herbs based on simultaneous determination of multiple bioactive constituents combined with multivariate statistical analysis. Molecules 2018, 23, 573. [Google Scholar] [CrossRef] [PubMed]
- The Pharmacopoeia Committee of the Health Ministry of People’s Republic of China. Pharmacopoeia of People’s Republic of China; Guangdong Scientific Technologic Publisher: Guangzhou, China, 1995; p. 182.
- Pharmacopoeia Commission of the Ministry of Health of the People’s Republic of China. Pharmacopoeia of the People’s Republic of China; Part I; Medical Science and Technology Press: Beijing, China, 2015; pp. 211–212.
- Ksouri, R.; Megdiche, W.; Debez, A.; Falleh, H.; Grignon, C.; Abdelly, C. Salinity effects on polyphenol content and antioxidant activities in leaves of the halophyte Cakile maritima. Plant Physiol. Biochem. 2007, 45, 244–249. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.Z.; Gao, G.H.; Zhou, X.M.; Yu, D.; Chen, X.H.; Bi, K.S. Simultaneous determination of five active components in traditional Chinese medicine Apocynum venetum L. by RP-HPLC–DAD. J. Med. Plants Res. 2011, 5, 735–742. [Google Scholar]
- Liu, X.H.; Zhang, Y.C.; Li, S.J.; Wang, M.; Wang, L.J. Simultaneous Determination of Four Flavonoids in Folium Apocyni Veneti by HPCE-DAD. Chin. Pharmacol. J. 2010, 45, 464–467. [Google Scholar]
- An, H.J.; Wang, H.; Lan, Y.X.; Hashi, Y.; Chen, S.Z. Simultaneous qualitative and quantitative analysis of phenolic acids and flavonoids for the quality control of Apocynum venetum L. leaves by HPLC–DAD–ESI–IT–TOF–MS and HPLC–DAD. J. Pharmaceut. Biomed. 2013, 85, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Zhang, F.S.; Yang, N.Y.; Liu, X.H. Simultaneous Determination of 10 Nucleosides and Nucleobases in Antrodia camphorata Using QTRAP LC–MS/MS. J. Chromatogr. Sci. 2014, 52, 852–861. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.; Breitkopf, S.B.; Yang, X.; Asara, J.M. A positive/negative ion-switching, targeted mass spectrometry-based metabolomics platform for bodily fluids, cells, and fresh and fixed tissue. Nat. Protoc. 2012, 7, 872–881. [Google Scholar] [CrossRef] [PubMed]
- March, R.E. An introduction to quadrupole ion trap mass spectrometry. J. Mass Spectrom. 1997, 32, 351–369. [Google Scholar] [CrossRef]
- Shi, J.Y.; Li, G.L.; Zhang, R.; Zheng, J.; Suo, Y.R.; You, J.M.; Liu, Y.J. A validated HPLC-DAD-MS method for identifying and determining the bioactive components of two kinds of luobuma. J. Liq. Chromatogr. Relat. Technol. 2011, 34, 537–547. [Google Scholar] [CrossRef]
- Wahid, A.; Ghazanfar, A. Possible involvement of some secondary metabolites in salt tolerance of sugarcane. J. Plant Physiol. 2006, 163, 723–730. [Google Scholar] [CrossRef] [PubMed]
- Mateos-Naranjo, E.; Andrades-Moreno, L.; Davy, A.J. Silicon alleviates deleterious effects of high salinity on the halophytic grass Spartina densiflora. Plant Physiol. Biochem. 2013, 63, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Khan, A.L.; Kim, D.H.; Lee, S.Y.; Kim, K.M.; Waqas, M.; Jung, H.Y.; Shin, J.H.; Kim, J.G.; Lee, I.J. Silicon mitigates heavy metal stress by regulating P-type heavy metal ATPases, Oryza sativa low silicon genes, and endogenous phytohormones. BMC Plant Biol. 2014, 14, 13. [Google Scholar] [CrossRef] [PubMed]
- Flowers, T.J.; Colmer, T.D. Salinity tolerance in halophytes. New Phytol. 2008, 179, 945–963. [Google Scholar] [CrossRef] [PubMed]
- Parihar, P.; Singh, S.; Singh, R.; Singh, V.P.; Prasad, S.M. Effect of salinity stress on plants and its tolerance strategies: A review. Environ. Sci. Pollut. Res. Int. 2015, 22, 4056–4075. [Google Scholar] [CrossRef] [PubMed]
- Deinlein, U.; Stephan, A.B.; Horie, T.; Luo, W.; Xu, G.; Schroeder, J.I. Plant salt-tolerance mechanisms. Trends Plant Sci. 2014, 19, 371–379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Golldack, D.; Li, C.; Mohan, H.; Probst, N. Tolerance to drought and salts tress in plants: Unraveling the signaling networks. Front. Plant Sci. 2014, 5, 151. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.J.; Negrão, S.; Tester, M. Salt resistant crop plants. Curr. Opin. Biotechnol. 2014, 26, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Gururani, M.A.; Venkatesh, J.; Tran, L.S.P. Regulation of photosynthesis during abiotic stress-induced photoinhibition. Mol. Plant 2015, 8, 1304–1320. [Google Scholar] [CrossRef] [PubMed]
- Gururani, M.A.; Mohanta, T.K.; Bae, H. Current understanding of the interplay between phytohormones and photosynthesis under environmental stress. Int. J. Mol. Sci. 2015, 16, 19055–19085. [Google Scholar] [CrossRef] [PubMed]
- Flowers, T.J.; Munns, R.; Colmer, T.D. Sodium chloride toxicity and the cellular basis of salt tolerance in halophytes. Ann. Bot. 2015, 115, 419–431. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, M.R.; Devaraj, V.R. Biochemical responses of Hyacinth bean (Lablab purpureus) to salinity stress. Acta Physiol. Plant. 2010, 32, 341–353. [Google Scholar]
- Rosa, M.; Prado, C.; Podazza, G.; Interdonato, R.; Gonzalez, J.A.; Hilal, M.; Prado, F.E. Soluble sugars: Metabolism, sensing and abiotic stress. A complex network in the life of plants. Plant Signal. Behav. 2009, 4, 388–393. [Google Scholar] [CrossRef] [PubMed]
- Abbaspour, H.; Afshari, H.; Abdel-Wahhab, A. Influence of salt stress on growth, pigments, soluble sugars and ion accumulation in three pistachio cultivars. J. Med. Plants Res. 2012, 6, 2468–2473. [Google Scholar] [CrossRef]
- Mittal, S.; Kumari, N.; Sharma, V. Differential response of salt stress on Brassica juncea: Photosynthetic performance, pigment, proline, D1 and antioxidant enzymes. Plant Physiol. Biochem. 2012, 54, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Gengmao, Z.; Quanmei, S.; Yu, H.; Shihui, L.; Changhai, W. The physiological and biochemical responses of a medicinal plant (Salvia miltiorrhiza L.) to stress caused by various concentrations of NaCl. PLoS ONE 2014, 9, e89624. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Verslues, P.E. Mechanisms independent of abscisic acid (ABA) or proline feedback have a predominant role in transcriptional regulation of proline metabolism during low water potential and stress recovery. Plant Cell Environ. 2010, 33, 1838–1851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szabados, L.; Savoure, A. Proline: A multifunctional amino acid. Trends Plant Sci. 2010, 15, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.G.; Reddy, A.M.; Sudhakar, C. NaCl effects on proline metabolism in two high yielding genotypes of mulberry (Morus alba L.) with contrasting salt tolerance. Plant Sci. 2003, 165, 1245–1251. [Google Scholar] [CrossRef]
- Cuin, T.A.; Shabala, S. Compatible solutes reduce ROS-induced potassium efflux in Arabidopsis roots. Plant Cell Environ. 2007, 30, 875–885. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Çoban, Ö.; Baydar, N.G. Brassinosteroid effects on some physical and biochemical properties and secondary metabolite accumulation in peppermint (Mentha piperita L.) under salt stress. Ind. Crops Prod. 2016, 86, 251–258. [Google Scholar] [CrossRef]
- Bajji, M.; Kinet, J.M.; Lutts, S. The use of the electrolyte leakage method for assessing cell membrane stability as a water stress tolerance test in durum wheat. Plant Growth Regul. 2002, 36, 61–70. [Google Scholar] [CrossRef]
- Yamauchi, Y.; Sugimoto, Y. Effect of protein modification by malondialdehyde on the interaction between the oxygen-evolving complex 33 kDa protein and photosystem II core proteins. Planta 2010, 231, 1077–1088. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.H.; Liang, X.; Dong, Y.J.; Xu, L.L.; Zhang, X.W.; Kong, J.; Liu, S. Effects of exogenous salicylic acid and nitric oxide on physiological characteristics of perennial ryegrass under cadmium stress. J. Plant Growth Regul. 2013, 32, 721–731. [Google Scholar] [CrossRef]
- Tasgin, E.; Atici, O.; Nalbantoglu, B.; Popova, L.P. Effects of salicylic acid and cold treatments on protein levels and on the activities of antioxidant enzymes in the apoplast of winter wheat leaves. Phytochemistry 2006, 67, 710–715. [Google Scholar] [CrossRef] [PubMed]
- Kang, G.Z.; Wang, C.H.; Sun, G.C.; Wang, Z.X. Salicylic acid changes activities of H2O2-metabolizing enzymes and increases the chilling tolerance of banana seedlings. Environ. Exp. Bot. 2003, 50, 9–15. [Google Scholar] [CrossRef]
- Nwugo, C.C.; Huerta, A.J. The effect of silicon on the leaf proteome of rice (Oryza sativa L.) Plants under Cadmium-Stress. J. Proteome Res. 2011, 10, 518–528. [Google Scholar] [CrossRef] [PubMed]
- Melo, A.M.P.; Roberts, T.H.; Moller, I.M. Evidence for the presence of two rotenone-insensitive NAD(P)H dehydrogenases on the inner surface of the inner membrane of potato tuber mitochondria. Biochim. Biophys. Acta 1996, 1276, 133–139. [Google Scholar] [CrossRef]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.Y.; Xiong, L.; Li, W.; Zhu, J.K.; Zhu, J. The plant cuticle is required for osmotic stress regulation of abscisic acid biosynthesis and osmotic stress tolerance in Arabidopsis. Plant Cell 2011, 23, 1971–1984. [Google Scholar] [CrossRef] [PubMed]
- Moradi, F.; Ismail, A.M. Responses of photosynthesis, chlorophyll fluorescence and ROS-Scavenging systems to salt stress during seedling and reproductive stages in rice. Ann. Bot. 2007, 99, 1161–1173. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Patel, M.K.; Jha, B. Non-targeted metabolomics and scavenging activity of reactive oxygen species reveal the potential of Salicornia brachiata as a functional food. J. Funct. Foods 2015, 13, 21–31. [Google Scholar] [CrossRef]
- Murillo-Amador, B.; Córdoba-Matson, M.V.; Villegas-Espinoza, J.A.; Hernández-Montiel, L.G.; Troyo-Diéguez, E.; García-Hernández, J.L. Mineral content and biochemical variables of Aloe vera L. under salt stress. PLoS ONE 2014, 9, e9487. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.; Bressan, R.A.; Hasegawa, P.M.; Pardo, J.M. Ion homeostasis in NaCl stress environments. Plant Physiol. 1995, 109, 735–742. [Google Scholar] [CrossRef] [PubMed]
- Qi, Z.; Xiong, L. Characterization of a Purine Permease Family Gene OsPUP7 Involved in Growth and Development Control in Rice. Chin. Bull. Botany 2013, 55, 1119–1135. [Google Scholar]
- Zhao, X.; Wang, W.; Zhang, F.; Deng, J.; Li, Z.; Fu, B. Comparative metabolite profiling of two rice genotypes with contrasting salt stress tolerance at the seedling stage. PLoS ONE 2014, 29, e108020. [Google Scholar] [CrossRef] [PubMed]
- Yokozawa, T.; Kashiwada, Y.; Hattori, M.; Chung, H.Y. Study on the components of Luobuma with peroxynitrite-scavenging activity. Biol. Pharm. Bull. 2002, 25, 748–752. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.H.; Park, K.J.; Kim, B.K.; Jeong, J.W.; Kim, H.J. Effect of salinity stress on phenolic compounds and carotenoids in buckwheat (Fagopyrum esculentum M.) sprout. Food Chem. 2012, 135, 1065–1070. [Google Scholar] [CrossRef] [PubMed]
- Chisari, M.; Todaro, A.; Barbagallo, R.N.; Spagna, G. Salinity effects on enzymatic browning and antioxidant capacity of fresh-cut baby Romaine lettuce (Lactuca sativa L. cv. Duende). Food Chem. 2010, 119, 1502–1506. [Google Scholar] [CrossRef]
- Nichenametla, S.N.; Taruscio, T.G.; Barney, D.L.; Exon, J.H. A review of the effects and mechanisms of polyphenolics in cancer. Crit. Rev. Food Sci. Nutr. 2006, 46, 161–183. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.H.; Xu, H.; Liu, X.H.; Zou, L.S.; Wang, M.; Liu, Z.X.; Fu, X.S.; Zhao, H.; Yan, Y. Site-specific accumulation and dynamic change of flavonoids in Apocyni Veneti Folium. Microsc. Res. Tech. 2017, 80, 1315–1322. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.H.; Jeong, S.M.; Lee, J.H.; Kim, J.H.; Yoon, I.S.; Lee, J.H.; Choi, S.H.; Lee, S.M.; Chang, C.G.; Kim, H.C.; et al. Quercetin inhibits the 5-hydroxytryptamine type 3 receptor-mediated ion current by interacting with pre-transmembrane domain I. Mol. Cells 2005, 20, 69–73. [Google Scholar] [PubMed]
- Kim, Y.H.; Lee, Y.J. RAIL apoptosis is enhanced by quercetin through Akt dephosphorylation. J. Cell Biochem. 2007, 100, 998–1009. [Google Scholar] [CrossRef] [PubMed]
- Winkel-Shirley, B. Biosynthesis of flavonoids and effects of stress. Curr. Opin. Plant Biol. 2002, 5, 218–223. [Google Scholar] [CrossRef]
- Bettaieb, I.; Knioua, S.; Hamrouni, I.; Limam, F.; Marzouk, B. Water-deficit impact on fatty acid and essential oil composition and antioxidant activities of cumin (Cuminum cyminum L.) aerial parts. J. Agr. Food Chem. 2011, 59, 328–334. [Google Scholar] [CrossRef] [PubMed]
- Close, D.C.; McArthor, C. Rethinking the role of many plant phenolics—Protection against photodamage not herbivores? OIKOS 2002, 99, 166–172. [Google Scholar] [CrossRef]
- Hirayama, T.; Shinozaki, K. Research on plant abiotic stress responses in the post-genome era: Past, present and future. Plant J. 2010, 61, 1041–1052. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.H.; Huang, J.L. Principles and Techniques of Plant Physiological Biochemical Experiment, 3rd ed.; Higher Education Press: Beijing, China, 2015. [Google Scholar]
- Zeng, J.W.; Chen, A.M.; Li, D.D.; Yi, B.; Wu, W. Effects of Salt Stress on the Growth, Physiological Responses, and Glycoside Contents of Stevia rebaudiana Bertoni. J. Agric. Food Chem. 2013, 61, 5720–5726. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.B.; Zhang, G.P.; Dominy, P. Four barley genotypes respond differently to cadmium: Lipid peroxidation and activities of antioxidant capacity. Environ. Exp. Bot. 2003, 50, 67–78. [Google Scholar] [CrossRef]
- Zenki, M.; Tanishita, A.; Yokoyama, T. Repetitive determination of ascorbic acid using iron(III)-1.10-phenanthroline-peroxodisulfate system in a circulatory flow injection method. Talanta 2004, 64, 1273–1277. [Google Scholar] [CrossRef] [PubMed]
- Hua, Y.J.; Wang, S.N.; Chai, C.; Liu, Z.S.; Liu, X.H.; Zou, L.S.; Wu, Q.N.; Zhao, H.; Yan, Y. Quality Evaluation of Pseudostellariae Radix Based on Simultaneous Determination of Multiple Bioactive Components Combined with Grey Relational Analysis. Molecules 2016, 22, 13. [Google Scholar] [CrossRef] [PubMed]




| Treatments | Pigment Content | ||||
|---|---|---|---|---|---|
| Chl a (mg g−1 FW) | Chl b (mg g−1 FW) | total Chl (mg g−1 FW) | Chl a/b | Carotenoids (mg g−1 FW) | |
| 0 mM | 2.35 ± 0.11 c | 0.49 ± 0.06 ab | 2.84 ± 0.05 c | 4.77 ± 0.82 c | 0.86 ± 0.07 c |
| 100 mM | 3.66 ± 0.19 a | 0.55 ± 0.04 a | 4.21 ± 0.22 a | 6.63 ± 0.14 a | 1.25 ± 0.01 a |
| 200 mM | 3.00 ± 0.20 b | 0.48 ± 0.01 ab | 3.47 ± 0.20 b | 6.30 ± 0.49 ab | 1.04 ± 0.02 b |
| 300 mM | 2.31 ± 0.12 c | 0.43 ± 0.01 b | 2.74 ± 0.11 c | 5.33 ± 0.40 bc | 0.83 ± 0.01 c |
| Treatments | ri | Quality-Ranking |
|---|---|---|
| 0 mM | 0.3984 | 4 |
| 100 mM | 0.5253 | 2 |
| 200 mM | 0.6363 | 1 |
| 300 mM | 0.4827 | 3 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.; Wang, C.; Liu, Z.; Liu, X.; Zou, L.; Shi, J.; Chen, S.; Chen, J.; Tan, M. Variations in Physiology and Multiple Bioactive Constituents under Salt Stress Provide Insight into the Quality Evaluation of Apocyni Veneti Folium. Int. J. Mol. Sci. 2018, 19, 3042. https://doi.org/10.3390/ijms19103042
Chen C, Wang C, Liu Z, Liu X, Zou L, Shi J, Chen S, Chen J, Tan M. Variations in Physiology and Multiple Bioactive Constituents under Salt Stress Provide Insight into the Quality Evaluation of Apocyni Veneti Folium. International Journal of Molecular Sciences. 2018; 19(10):3042. https://doi.org/10.3390/ijms19103042
Chicago/Turabian StyleChen, Cuihua, Chengcheng Wang, Zixiu Liu, Xunhong Liu, Lisi Zou, Jingjing Shi, Shuyu Chen, Jiali Chen, and Mengxia Tan. 2018. "Variations in Physiology and Multiple Bioactive Constituents under Salt Stress Provide Insight into the Quality Evaluation of Apocyni Veneti Folium" International Journal of Molecular Sciences 19, no. 10: 3042. https://doi.org/10.3390/ijms19103042
APA StyleChen, C., Wang, C., Liu, Z., Liu, X., Zou, L., Shi, J., Chen, S., Chen, J., & Tan, M. (2018). Variations in Physiology and Multiple Bioactive Constituents under Salt Stress Provide Insight into the Quality Evaluation of Apocyni Veneti Folium. International Journal of Molecular Sciences, 19(10), 3042. https://doi.org/10.3390/ijms19103042