Chitosan Hydrogel Beads Functionalized with Thymol-Loaded Solid Lipid–Polymer Hybrid Nanoparticles
Abstract
:1. Introduction
2. Results and Discussion
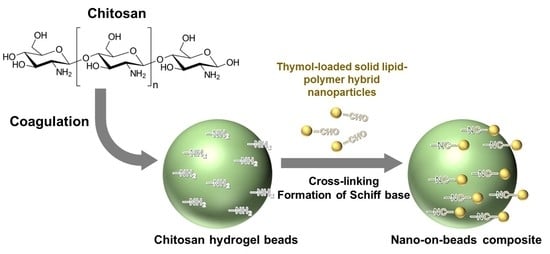
2.1. Functionalization of Chitosan Beads with SLPN
2.2. Effect of Chitosan Beads Dosage and Incubation Duration
2.3. Characterization of Composite Beads
2.4. Encapsulation of Thymol and Functionalization
3. Materials and Methods
3.1. Materials
3.2. Preparation of Chitosan Hydrogel Beads
3.3. Preparation of SLPN
3.4. Encapsulation of Thymol in SLPN
3.5. Functionalization of Chitosan Beads with SLPN
3.6. Characterization of SLPN Functionalized Chitosan Beads (Nano-on-Beads Composite)
3.7. Release Study
3.8. Statistical Analyses
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef] [PubMed]
- Caló, E.; Khutoryanskiy, V.V. Biomedical applications of hydrogels: A review of patents and commercial products. Eur. Polym. J. 2015, 65, 252–267. [Google Scholar] [CrossRef]
- Gaharwar, A.K.; Peppas, N.A.; Khademhosseini, A. Nanocomposite hydrogels for biomedical applications. Biotechnol. Bioeng. 2014, 111, 441–453. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.-W.; Zhong, L.-X.; Ren, J.-L.; Sun, R.-C. Highly effective adsorption of heavy metal ions from aqueous solutions by macroporous xylan-rich hemicelluloses-based hydrogel. J. Agric. Food Chem. 2012, 60, 3909–3916. [Google Scholar] [CrossRef] [PubMed]
- Dao, V.H.; Cameron, N.R.; Saito, K. Synthesis, properties and performance of organic polymers employed in flocculation applications. Polym. Chem. 2016, 7, 11–25. [Google Scholar] [CrossRef] [Green Version]
- Bolto, B.; Gregory, J. Organic polyelectrolytes in water treatment. Water Res. 2007, 41, 2301–2324. [Google Scholar] [CrossRef] [PubMed]
- Mi, F.-L.; Kuan, C.-Y.; Shyu, S.-S.; Lee, S.-T.; Chang, S.-F. The study of gelation kinetics and chain-relaxation properties of glutaraldehyde-cross-linked chitosan gel and their effects on microspheres preparation and drug release. Carbohydr. Polym. 2000, 41, 389–396. [Google Scholar] [CrossRef]
- Silva, A.K.; Juenet, M.; Meddahi-Pellé, A.; Letourneur, D. Polysaccharide-based strategies for heart tissue engineering. Carbohydr. Polym. 2015, 116, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Bhattarai, N.; Gunn, J.; Zhang, M. Chitosan-based hydrogels for controlled, localized drug delivery. Adv. Drug Deliv. Rev. 2010, 62, 83–99. [Google Scholar] [CrossRef] [PubMed]
- Thoniyot, P.; Tan, M.J.; Karim, A.A.; Young, D.J.; Loh, X.J. Nanoparticle–hydrogel composites: Concept, design, and applications of these promising, multi-functional materials. Adv. Sci. 2015, 2. [Google Scholar] [CrossRef] [PubMed]
- Tyliszczak, B.; Drabczyk, A.; Kudłacik-Kramarczyk, S.; Sobczak-Kupiec, A. Preparation, characterization, and in vitro cytotoxicity of chitosan hydrogels containing silver nanoparticles. J. Biomater. Sci. Polym. Ed. 2017, 28, 1665–1676. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, C.; Gupta, A.; Chaudhary, A.; Nandi, C.K. Gold nanoparticle chitosan composite hydrogel beads show efficient removal of methyl parathion from waste water. RSC Adv. 2014, 4, 39830–39838. [Google Scholar] [CrossRef]
- Saifuddin, N.; Nian, C.; Zhan, L.; Ning, K. Chitosan-silver nanoparticles composite as point-of-use drinking water filtration system for household to remove pesticides in water. Asian J. Biochem. 2011, 6, 142–159. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, L.; Chen, Q.; Chen, C. Cytotoxic potential of silver nanoparticles. Yonsei Med. J. 2014, 55, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Tyliszczak, B.; Drabczyk, A.; Kudłacik-Kramarczyk, S.; Bialik-Wąs, K.; Kijkowska, R.; Sobczak-Kupiec, A. Preparation and cytotoxicity of chitosan-based hydrogels modified with silver nanoparticles. Colloids Surf. B 2017, 160, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Korani, M.; Ghazizadeh, E.; Korani, S.; Hami, Z.; Mohammadi-Bardbori, A. Effects of silver nanoparticles on human health. Eur. J. Nanomed. 2015, 7, 51–62. [Google Scholar] [CrossRef]
- Ahamed, M.; AlSalhi, M.S.; Siddiqui, M. Silver nanoparticle applications and human health. Clin. Chim. Acta 2010, 411, 1841–1848. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Hu, Q.; Lee, J.; Luo, Y. Solid lipid-polymer hybrid nanoparticles by in-situ conjugation for oral delivery of astaxanthin. J. Agric. Food Chem. 2018, 66, 9473–9480. [Google Scholar] [CrossRef] [PubMed]
- Jóźwiak, T.; Filipkowska, U.; Szymczyk, P.; Rodziewicz, J.; Mielcarek, A. Effect of ionic and covalent crosslinking agents on properties of chitosan beads and sorption effectiveness of Reactive Black 5 dye. React. Funct. Polym. 2017, 114, 58–74. [Google Scholar] [CrossRef]
- Ruan, C.-Q.; Strømme, M.; Lindh, J. Preparation of porous 2,3-dialdehyde cellulose beads crosslinked with chitosan and their application in adsorption of Congo red dye. Carbohydr. Polym. 2018, 181, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Kim, U.-J.; Kim, H.J.; Choi, J.W.; Kimura, S.; Wada, M. Cellulose-chitosan beads crosslinked by dialdehyde cellulose. Cellulose 2017, 24, 5517–5528. [Google Scholar] [CrossRef]
- Hoffmann, B.; Seitz, D.; Mencke, A.; Kokott, A.; Ziegler, G. Glutaraldehyde and oxidised dextran as crosslinker reagents for chitosan-based scaffolds for cartilage tissue engineering. J. Mater. Sci. Mater. Med. 2009, 20, 1495–1503. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, M.; Segura, R.L.; Abian, O.; Betancor, L.; Hidalgo, A.; Mateo, C.; Fernandez-Lafuente, R.; Guisan, J.M. Determination of protein-protein interactions through aldehyde-dextran intermolecular cross-linking. Proteomics 2004, 4, 2602–2607. [Google Scholar] [CrossRef] [PubMed]
- Bautista, M.C.; Bomati-Miguel, O.; del Puerto Morales, M.; Serna, C.J.; Veintemillas-Verdaguer, S. Surface characterisation of dextran-coated iron oxide nanoparticles prepared by laser pyrolysis and coprecipitation. J. Magn. Magn. Mater. 2005, 293, 20–27. [Google Scholar] [CrossRef]
- Fernandes Queiroz, M.; Melo, K.R.T.; Sabry, D.A.; Sassaki, G.L.; Rocha, H.A.O. Does the use of chitosan contribute to oxalate kidney stone formation? Mar. Drugs 2014, 13, 141–158. [Google Scholar] [CrossRef] [PubMed]
- Bhattarai, S.R.; Kc, R.B.; Kim, S.Y.; Sharma, M.; Khil, M.S.; Hwang, P.H.; Chung, G.H.; Kim, H.Y. N-hexanoyl chitosan stabilized magnetic nanoparticles: Implication for cellular labeling and magnetic resonance imaging. J. Nanobiotechnol. 2008, 6, 1. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Wang, T.; Zhou, M.; Xue, J.; Luo, Y. In vitro antioxidant-activity evaluation of gallic-acid-grafted chitosan conjugate synthesized by free-radical-induced grafting method. J. Agric. Food Chem. 2016, 64, 5893–5900. [Google Scholar] [CrossRef] [PubMed]
- Clougherty, L.; Sousa, J.; Wyman, G. C=N stretching frequency in infrared spectra of aromatic azomethines. J. Org. Chem. 1957, 22, 462. [Google Scholar] [CrossRef]
- Monier, M.; Ayad, D.; Wei, Y.; Sarhan, A. Immobilization of horseradish peroxidase on modified chitosan beads. Int. J. Biol. Macromol. 2010, 46, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, P.K.; Anand, A. Immobilization of acid phosphatase from Vigna aconitifolia seeds on chitosan beads and its characterization. Int. J. Biol. Macromol. 2014, 64, 150–154. [Google Scholar] [CrossRef] [PubMed]
- Dash, S.; Murthy, P.N.; Nath, L.; Chowdhury, P. Kinetic modeling on drug release from controlled drug delivery systems. Acta Pol. Pharm. 2010, 67, 217–223. [Google Scholar] [PubMed]
- Barzegar-Jalali, M.; Adibkia, K.; Valizadeh, H.; Shadbad, M.R.S.; Nokhodchi, A.; Omidi, Y.; Mohammadi, G.; Nezhadi, S.H.; Hasan, M. Kinetic analysis of drug release from nanoparticles. J. Pharm. Pharm. Sci. 2008, 11, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Mith, H.; Dure, R.; Delcenserie, V.; Zhiri, A.; Daube, G.; Clinquart, A. Antimicrobial activities of commercial essential oils and their components against food-borne pathogens and food spoilage bacteria. Food Sci. Nutr. 2014, 2, 403–416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Upadhyay, A.; Upadhyaya, I.; Kollanoor-Johny, A.; Venkitanarayanan, K. Antibiofilm effect of plant derived antimicrobials on Listeria monocytogenes. Food Microbiol. 2013, 36, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Niu, H.; Zhang, W.; Mu, H.; Sun, C.; Duan, J. Synergy among thymol, eugenol, berberine, cinnamaldehyde and streptomycin against planktonic and biofilm-associated food-borne pathogens. Lett. Appl. Microbiol. 2015, 60, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Džamić, A.; Nikolić, B.; Giweli, A.; Mitić-Ćulafić, D.; Soković, M.; Ristić, M.; Knežević-Vukčević, J.; Marin, P. Libyan Thymus capitatus essential oil: Antioxidant, antimicrobial, cytotoxic and colon pathogen adhesion-inhibition properties. J. Appl. Microbiol. 2015, 119, 389–399. [Google Scholar] [CrossRef] [PubMed]
- Drobchenko, S.N.; Isaeva-Ivanova, L.S.; Kleiner, A.R.; Lomakin, A.V.; Kolker, A.R.; Noskin, V.A. An investigation of the structure of periodate-oxidised dextran. Carbohydr. Res. 1993, 241, 189–199. [Google Scholar] [CrossRef]
- Wang, T.; Bae, M.; Lee, J.-Y.; Luo, Y. Solid lipid-polymer hybrid nanoparticles prepared with natural biomaterials: A new platform for oral delivery of lipophilic bioactives. Food Hydrocoll. 2018, 84, 581–592. [Google Scholar] [CrossRef]





| Group | Empty | T-SLPN1 | T-SLPN2 | T-SLPN3 |
|---|---|---|---|---|
| Thymol loading (v/v) | 0 | 0.05 | 0.075 | 0.1 |
| Particle size (nm) | 136.0 ± 5.6 | 141.1 ± 8.4 | 140.0 ± 5.6 | 144.1 ± 3.0 |
| PDI | 0.238 ± 0.011 | 0.241 ± 0.025 | 0.246 ± 0.032 | 0.275 ± 0.028 |
| Zeta potential (mV) | −14.2 ± 1.8 | −14.3 ± 1.6 | −14.4 ± 0.9 | −16.3 ± 2.4 |
| EE (%) | NA | 93.2 ± 1.6A | 91.1 ± 1.9A | 78.3 ± 8.3B |
| Group | Zero-Order Model | Higuchi Model | Korsmeyer–Peppas Model | |||
|---|---|---|---|---|---|---|
| Equation | R2 | Equation | R2 | Equation | R2 | |
| 200B-30m | y = 0.14x | 0.927 | y = 2.20x − 1.19 | 0.945 | y = 0.67x − 0.06 | 0.921 |
| 200B-60m | y = 0.16x | 0.920 | y = 2.44x + 1.18 | 0.982 | y = 0.45x + 0.51 | 0.974 |
| 200B-90m | y = 0.18x | 0.914 | y = 2.65x + 2.19 | 0.984 | y = 0.45x + 0.57 | 0.977 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, T.; Luo, Y. Chitosan Hydrogel Beads Functionalized with Thymol-Loaded Solid Lipid–Polymer Hybrid Nanoparticles. Int. J. Mol. Sci. 2018, 19, 3112. https://doi.org/10.3390/ijms19103112
Wang T, Luo Y. Chitosan Hydrogel Beads Functionalized with Thymol-Loaded Solid Lipid–Polymer Hybrid Nanoparticles. International Journal of Molecular Sciences. 2018; 19(10):3112. https://doi.org/10.3390/ijms19103112
Chicago/Turabian StyleWang, Taoran, and Yangchao Luo. 2018. "Chitosan Hydrogel Beads Functionalized with Thymol-Loaded Solid Lipid–Polymer Hybrid Nanoparticles" International Journal of Molecular Sciences 19, no. 10: 3112. https://doi.org/10.3390/ijms19103112
APA StyleWang, T., & Luo, Y. (2018). Chitosan Hydrogel Beads Functionalized with Thymol-Loaded Solid Lipid–Polymer Hybrid Nanoparticles. International Journal of Molecular Sciences, 19(10), 3112. https://doi.org/10.3390/ijms19103112