5-Azacytidine: A Promoter of Epigenetic Changes in the Quest to Improve Plant Somatic Embryogenesis
Abstract
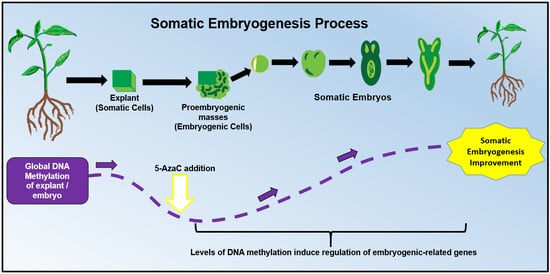
:1. Introduction
2. DNA Methylation: A Key Player During Somatic Embryogenesis
2.1. Hypomethylation Promotes Embryogenic Capacity
2.2. The Role of Auxins in DNA Methylation
3. The Use of 5-Azacytidine During Somatic Embryogenesis
5-AzaC and 2,4-D Can Work Together During SE
- Test the effects of different concentrations of 5-AzaC to know the minimum levels to observe the differential impact and maximum concentrations so that they are not toxic to the explants.
- Select the timing of the process for adding the inhibitor, as the effect could make the embryogenic process more efficient or inhibit it, depending on whether 5-AzaC is applied before/during the induction or development of the somatic embryos.
- If the culture medium includes reagents that sequester substances, such as activated carbon [111], higher concentrations of the inhibitor should be applied than in culture media without this type of reagent. Another option is to use a pre-treatment with the inhibitor for a specific time and then transfer the explant to the conventional culture medium if it contains activated charcoal.
- Take into consideration the pH and temperature of the culture medium at the time the inhibitor is applied. It has been reported that 5-AzaC is moderately stable in acidic solutions while rapidly decomposing in alkaline media and that degradation is accelerated dramatically with increasing temperature [112].
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shahzad, A.; Sharma, S.; Parveen, S.; Saeed, T.; Shaheen, A.; Akhtar, R.; Yadav, V.; Upadhyay, A.; Ahmad, Z. Historical perspective and basic principles of plant tissue culture. In Plant Biotechnology: Principles and Applications; Springer: Singapore, Singapore, 2017; pp. 1–36. [Google Scholar]
- Hussain, A.; Qarshi, I.A.; Nazir, H.; Ullah, I.; Leva, A.; Rinaldi, L. Recent advances in plant in vitro culture. Chapter 2012, 1, 1–28. [Google Scholar]
- Von Arnold, S.; Sabala, I.; Bozhkov, P.; Dyachok, J.; Filonova, L. Developmental pathways of somatic embryogenesis. Plant Cell Tissue Organ Cult. 2002, 69, 233–249. [Google Scholar] [CrossRef]
- Thorpe, T.A.; Stasolla, C. Somatic embryogenesis. In Current Trends in the Eembryology of Angiosperms; Springer: Dordrecht, The Netherlands, 2001; pp. 279–336. [Google Scholar]
- Williams, E.; Maheswaran, G. Somatic embryogenesis: Factors influencing coordinated behaviour of cells as an embryogenic group. Ann. Bot. 1986, 57, 443–462. [Google Scholar] [CrossRef]
- Jain, S.M.; Gupta, P. Step Wise Protocols for Somatic Embryogenesis of Important Woody Plants; Springer: Cham, Switzerland, 2018; pp. i–xvi. [Google Scholar]
- Lee, K.; Seo, P.J. Dynamic Epigenetic Changes during Plant Regeneration. Trends Plant Sci. 2018. [Google Scholar] [CrossRef] [PubMed]
- Loyola-Vargas, V.M. The History of somatic embryogenesis. In Somatic Embryogenesis: Fundamental Aspects and Applications; Springer: Cham, Switzerland, 2016; pp. 11–22. [Google Scholar]
- Heringer, A.S.; Santa-Catarina, C.; Silveira, V. Insights from proteomic studies into plant somatic embryogenesis. Proteomics 2018, 18, 1700265. [Google Scholar] [CrossRef] [PubMed]
- Góngora-Castillo, E.; Nic-Can, G.I.; Galaz-Ávalos, R.M.; Loyola-Vargas, V.M. Elaboration of Transcriptome During the Induction of Somatic Embryogenesis. In Plant Cell Culture Protocols; Humana Press: New York, NY, USA, 2018; pp. 411–427. [Google Scholar]
- Fehér, A. Somatic embryogenesis—Stress-induced remodeling of plant cell fate. Biochim. Biophys. Acta 2015, 1849, 385–402. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, V.M. Involvement of Plant Hormones and Plant Growth Regulators on in vitro Somatic Embryogenesis. Plant Growth Regul. 2005, 47, 91–110. [Google Scholar] [CrossRef]
- Zavattieri, M.A.; Frederico, A.M.; Lima, M.; Sabino, R.; Arnholdt-Schmitt, B. Induction of somatic embryogenesis as an example of stress-related plant reactions. Electron. J. Biotechnol. 2010, 13, 12–13. [Google Scholar] [CrossRef]
- Karami, O.; Aghavaisi, B.; Mahmoudi Pour, A. Molecular aspects of somatic-to-embryogenic transition in plants. J. Chem. Biol. 2009, 2, 177–190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elhiti, M.; Stasolla, C.; Wang, A. Molecular regulation of plant somatic embryogenesis. In Vitro Cell. Dev. Biol. Plant 2013, 49, 631–642. [Google Scholar] [CrossRef]
- Verdeil, J.-L.; Alemanno, L.; Niemenak, N.; Tranbarger, T.J. Pluripotent versus totipotent plant stem cells: Dependence versus autonomy? Trends Plant Sci. 2007, 12, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Toonen, M.A.; Hendriks, T.; Schmidt, E.D.; Verhoeven, H.A.; van Kammen, A.; de Vries, S.C. Description of somatic-embryo-forming single cells in carrot suspension cultures employing video cell tracking. Planta 1994, 194, 565–572. [Google Scholar] [CrossRef]
- Fehér, A. Why somatic plant cells start to form embryos? In Somatic Embryogenesis; Springer: Berlin, Gerlin, 2005; pp. 85–101. [Google Scholar]
- Mahdavi-Darvari, F.; Noor, N.M.; Ismanizan, I. Epigenetic regulation and gene markers as signals of early somatic embryogenesis. Plant Cell Tissue Organ Cult. 2015, 120, 407–422. [Google Scholar] [CrossRef]
- Kumar, V.; Van Staden, J. New insights into plant somatic embryogenesis: An epigenetic view. Acta Physiol. Plant. 2017, 39, 194. [Google Scholar] [CrossRef]
- De-la-Peña, C.; Nic-Can, G.I.; Galaz-Ávalos, R.M.; Avilez-Montalvo, R.; Loyola-Vargas, V.M. The role of chromatin modifications in somatic embryogenesis in plants. Front. Plant Sci. 2015, 6, 635. [Google Scholar] [CrossRef] [PubMed]
- Bhojwani, S.S.; Razdan, M.K. Plant Tissue Culture: Theory and Practice; Elsevier: Amsterdam, The Netherlands, 1986; Volume 5, pp. 91–112. [Google Scholar]
- Karim, R.; Tan, Y.S.; Singh, P.; Khalid, N.; Harikrishna, J.A. Expression and DNA methylation of SERK, BBM, LEC2 and WUS genes in in vitro cultures of Boesenbergia rotunda (L.) Mansf. Physiol. Mol. Biol. Plants 2018, 24, 741–751. [Google Scholar] [CrossRef] [PubMed]
- Karim, R.; Nuruzzaman, M.; Khalid, N.; Harikrishna, J. Importance of DNA and histone methylation in in vitro plant propagation for crop improvement: A review. Ann. Appl. Biol. 2016, 169, 1–16. [Google Scholar] [CrossRef]
- Razin, A.; Riggs, A.D. DNA methylation and gene function. Science 1980, 210, 604–610. [Google Scholar] [CrossRef] [PubMed]
- Taylor, S.M.; Jones, P.A. Mechanism of action of eukaryotic DNA methyltransferase: Use of 5-azacytosine-containing DNA. J. Mol. Biol. 1982, 162, 679–692. [Google Scholar] [CrossRef]
- Jones, P.A.; Taylor, S.M. Cellular differentiation, cytidine analogs and DNA methylation. Cell 1980, 20, 85–93. [Google Scholar] [CrossRef]
- Niwa, O.; Sugahara, T. 5-Azacytidine induction of mouse endogenous type C virus and suppression of DNA methylation. Proc. Natl. Acad. Sci. USA 1981, 78, 6290–6294. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, J.; Steffen, D.; Gusella, J.; Tabin, C.; Bird, S.; Cowing, D.; Weinberg, R. DNA methylation affecting the expression of murine leukemia proviruses. J. Virol. 1982, 44, 144–157. [Google Scholar] [PubMed]
- Groudine, M.; Eisenman, R.; Weintraub, H. Chromatin structure of endogenous retroviral genes and activation by an inhibitor of DNA methylation. Nature 1981, 292, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Hepburn, A.; Clarke, L.; Pearson, L.; White, J. The role of cytosine methylation in the control of nopaline synthase gene expression in a plant tumor. J. Mol. Appl. Genet. 1983, 2, 315–329. [Google Scholar] [PubMed]
- Santi, D.V.; Garrett, C.E.; Barr, P.J. On the mechanism of inhibition of DNA-cytosine methyltransferases by cytosine analogs. Cell 1983, 33, 9–10. [Google Scholar] [CrossRef]
- Li, J.; Wang, M.; Li, Y.; Zhang, Q.; Lindsey, K.; Daniell, H.; Jin, S.; Zhang, X. Multi-omics analyses reveal epigenomics basis for cotton somatic embryogenesis through successive regeneration acclimation (SRA) process. Plant Biotechnol. J. 2018. [Google Scholar] [CrossRef] [PubMed]
- Grzybkowska, D.; Morończyk, J.; Wójcikowska, B.; Gaj, M.D. Azacitidine (5-AzaC)-treatment and mutations in DNA methylase genes affect embryogenic response and expression of the genes that are involved in somatic embryogenesis in Arabidopsis. Plant Growth Regul. 2018, 85, 243–256. [Google Scholar] [CrossRef] [Green Version]
- Quiroz-Figueroa, F.R.; Rojas-Herrera, R.; Galaz-Avalos, R.M.; Loyola-Vargas, V.M. Embryo production through somatic embryogenesis can be used to study cell differentiation in plants. Plant Cell Tissue Organ Cult. 2006, 86, 285. [Google Scholar] [CrossRef]
- Quiroz-Figueroa, F.; Fuentes-Cerda, C.; Rojas-Herrera, R.; Loyola-Vargas, V. Histological studies on the developmental stages and differentiation of two different somatic embryogenesis systems of Coffea arabica. Plant Cell Rep. 2002, 20, 1141–1149. [Google Scholar]
- Birnbaum, K.D.; Roudier, F. Epigenetic memory and cell fate reprogramming in plants. Regeneration 2017, 4, 15–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ikeuchi, M.; Iwase, A.; Sugimoto, K. Control of plant cell differentiation by histone modification and DNA methylation. Curr. Opin. Plant Biol. 2015, 28, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Jullien, P.E.; Susaki, D.; Yelagandula, R.; Higashiyama, T.; Berger, F. DNA methylation dynamics during sexual reproduction in Arabidopsis thaliana. Curr. Biol. 2012, 22, 1825–1830. [Google Scholar] [CrossRef] [PubMed]
- Diez, C.M.; Roessler, K.; Gaut, B.S. Epigenetics and plant genome evolution. Curr. Opin. Plant Biol. 2014, 18, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Matzke, M.A.; Mosher, R.A. RNA-directed DNA methylation: An epigenetic pathway of increasing complexity. Nat. Rev. Genet. 2014, 15, 394–408. [Google Scholar] [CrossRef] [PubMed]
- Turck, F.; Coupland, G. Natural variation in epigenetic gene regulation and its effects on plant developmental traits. Evolution 2014, 68, 620–631. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X. Comparative epigenomics: A powerful tool to understand the evolution of DNA methylation. New Phytol. 2016, 210, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Vanyushin, B. Replicative DNA methylation in animals and higher plants. Curr. Top. Microbiol. Immunol. 1984, 108, 99–114. [Google Scholar] [PubMed]
- Finnegan, E.; Kovac, K. Plant DNA methyltransferases. Plant Mol. Biol. 2000, 43, 189–201. [Google Scholar] [CrossRef] [PubMed]
- Goll, M.G.; Bestor, T.H. Eukaryotic cytosine methyltransferases. Annu. Rev. Biochem. 2005, 74, 481–514. [Google Scholar] [CrossRef] [PubMed]
- Bird, A. DNA methylation de novo. Science 1999, 286, 2287–2288. [Google Scholar] [CrossRef] [PubMed]
- Lindroth, A.M.; Cao, X.; Jackson, J.P.; Zilberman, D.; McCallum, C.M.; Henikoff, S.; Jacobsen, S.E. Requirement of CHROMOMETHYLASE3 for maintenance of CpXpG methylation. Science 2001, 292, 2077–2080. [Google Scholar] [CrossRef] [PubMed]
- Law, J.A.; Jacobsen, S.E. Establishing, maintaining and modifying DNA methylation patterns in plants and animals. Nat. Rev. Genet. 2010, 11, 204–220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, J.; Zhong, X.; Bernatavichute, Y.V.; Stroud, H.; Feng, S.; Caro, E.; Vashisht, A.A.; Terragni, J.; Chin, H.G.; Tu, A. Dual binding of chromomethylase domains to H3K9me2-containing nucleosomes directs DNA methylation in plants. Cell 2012, 151, 167–180. [Google Scholar] [CrossRef] [PubMed]
- Köhler, C.; Wolff, P.; Spillane, C. Epigenetic mechanisms underlying genomic imprinting in plants. Ann. Rev. Plant Biol. 2012, 63, 331–352. [Google Scholar] [CrossRef] [PubMed]
- Finnegan, E.J.; Dennis, E.S. Isolation and identification by sequence homology of a putative cytosine methyltransferase from Arabidopsis thaliana. Nucleic Acids Res. 1993, 21, 2383–2388. [Google Scholar] [CrossRef] [PubMed]
- Cokus, S.J.; Feng, S.; Zhang, X.; Chen, Z.; Merriman, B.; Haudenschild, C.D.; Pradhan, S.; Nelson, S.F.; Pellegrini, M.; Jacobsen, S.E. Shotgun bisulphite sequencing of the Arabidopsis genome reveals DNA methylation patterning. Nature 2008, 452, 215–219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lister, R.; O′Malley, R.C.; Tonti-Filippini, J.; Gregory, B.D.; Berry, C.C.; Millar, A.H.; Ecker, J.R. Highly integrated single-base resolution maps of the epigenome in Arabidopsis. Cell 2008, 133, 523–536. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Springer, N.M.; Muszynski, M.G.; Phillips, R.L.; Kaeppler, S.; Jacobsen, S.E. Conserved plant genes with similarity to mammalian de novo DNA methyltransferases. Proc. Natl. Acad. Sci. USA 2000, 97, 4979–4984. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, X.-J.; Chen, T.; Zhu, J.-K. Regulation and function of DNA methylation in plants and animals. Cell Res. 2011, 21, 442–465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Milutinovic, S.; Zhuang, Q.; Niveleau, A.; Szyf, M. Epigenomic stress response knockdown of DNA methyltransferase 1 triggers an intra-S-phase arrest of DNA replication and induction of stress response genes. J. Biol. Chem. 2003, 278, 14985–14995. [Google Scholar] [CrossRef] [PubMed]
- Berdasco, M.; Alcázar, R.; García-Ortiz, M.V.; Ballestar, E.; Fernández, A.F.; Roldán-Arjona, T.; Tiburcio, A.F.; Altabella, T.; Buisine, N.; Quesneville, H. Promoter DNA hypermethylation and gene repression in undifferentiated Arabidopsis cells. PLoS ONE 2008, 3, e3306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, W.; Liu, H.; Cheng, Z.J.; Su, Y.H.; Han, H.N.; Zhang, Y.; Zhang, X.S. DNA methylation and histone modifications regulate de novo shoot regeneration in Arabidopsis by modulating WUSCHEL expression and auxin signaling. PLoS Genet. 2011, 7, e1002243. [Google Scholar] [CrossRef] [PubMed]
- Nic-Can, G.I.; López-Torres, A.; Barredo-Pool, F.; Wrobel, K.; Loyola-Vargas, V.M.; Rojas-Herrera, R.; De-la-Peña, C. New Insights into Somatic Embryogenesis: LEAFY COTYLEDON1, BABY BOOM1 and WUSCHEL-RELATED HOMEOBOX4 Are Epigenetically Regulated in Coffea canephora. PLoS ONE 2013, 8, e72160. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez López, C.M.; Wetten, A.C.; Wilkinson, M.J. Progressive erosion of genetic and epigenetic variation in callus-derived cocoa (Theobroma cacao) plants. New Phytol. 2010, 186, 856–868. [Google Scholar] [CrossRef] [PubMed]
- Palmgren, G.; Mattsson, O.; Okkels, F.T. Specific levels of DNA methylation in various tissues, cell lines, and cell types of Daucus carota. Plant Physiol. 1991, 95, 174–178. [Google Scholar] [CrossRef] [PubMed]
- Noceda, C.; Salaj, T.; Pérez, M.; Viejo, M.; Cañal, M.J.; Salaj, J.; Rodriguez, R. DNA demethylation and decrease on free polyamines is associated with the embryogenic capacity of Pinus nigra Arn. cell culture. Trees (Berl. West) 2009, 23, 1285–1293. [Google Scholar] [CrossRef]
- Corredoira, E.; Cano, V.; Bárány, I.; Solís, M.-T.; Rodríguez, H.; Vieitez, A.-M.; Risueño, M.C.; Testillano, P.S. Initiation of leaf somatic embryogenesis involves high pectin esterification, auxin accumulation and DNA demethylation in Quercus alba. J. Plant Physiol. 2017, 213, 42–54. [Google Scholar] [CrossRef] [PubMed]
- LoSchiavo, F.; Pitto, L.; Giuliano, G.; Torti, G.; Nuti-Ronchi, V.; Marazziti, D.; Vergara, R.; Orselli, S.; Terzi, M. DNA methylation of embryogenic carrot cell cultures and its variations as caused by mutation, differentiation, hormones and hypomethylating drugs. Theor. Appl. Genet. 1989, 77, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Viejo, M.; Rodríguez, R.; Valledor, L.; Pérez, M.; Cañal, M.J.; Hasbún, R. DNA methylation during sexual embryogenesis and implications on the induction of somatic embryogenesis in Castanea sativa Miller. Sex. Plant Reprod. 2010, 23, 315–323. [Google Scholar] [CrossRef] [PubMed]
- El-Tantawy, A.-A.; Solís, M.-T.; Risueño, M.C.; Testillano, P.S. Changes in DNA methylation levels and nuclear distribution patterns after microspore reprogramming to embryogenesis in barley. Cytogenet. Genome Res. 2014, 143, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Us-Camas, R.; Rivera-Solís, G.; Duarte-Aké, F.; De-la-Pena, C. In vitro culture: An epigenetic challenge for plants. Plant Cell Tissue Organ Cult. 2014, 118, 187–201. [Google Scholar] [CrossRef]
- Von Aderkas, P.; Bonga, J.M. Influencing micropropagation and somatic embryogenesis in mature trees by manipulation of phase change, stress and culture environment. Tree Physiol. 2000, 20, 921–928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ayil-Gutiérrez, B.; Galaz-Ávalos, R.M.; Peña-Cabrera, E.; Loyola-Vargas, V.M. Dynamics of the concentration of IAA and some of its conjugates during the induction of somatic embryogenesis in Coffea canephora. Plant Signal. Behav. 2013, 8, e26998. [Google Scholar] [CrossRef] [PubMed]
- Munksgaard, D.; Mattsson, O.; Okkels, F.T. Somatic embryo development in carrot is associated with an increase in levels of S-adenosylmethionine, S-adenosylhomocysteine and DNA methylation. Physiol. Plant. 1995, 93, 5–10. [Google Scholar] [CrossRef]
- Fki, L.; Kriaa, W.; Nasri, A.; Baklouti, E.; Chkir, O.; Masmoudi, R.B.; Rival, A.; Drira, N. Indirect somatic embryogenesis of date palm using juvenile leaf explants and low 2, 4-D concentration. In Date Palm Biotechnology Protocols Volume I; Humana Press: New York, NY, USA, 2017; pp. 99–106. [Google Scholar]
- Steinmacher, D.A.; Heringer, A.S.; Jiménez, V.M.; Quoirin, M.G.; Guerra, M.P. Somatic Embryogenesis in Peach-Palm (Bactris gasipaes) Using Different Explant Sources. In Vitro Embryogenesis in Higher Plants; Humana Press: New York, NY, USA, 2016; pp. 279–288. [Google Scholar]
- Moon, H.-K.; Lee, H.; Paek, K.-Y.; Park, S.-Y. Osmotic stress and strong 2, 4-D shock stimulate somatic-to-embryogenic transition in Kalopanax septemlobus (Thunb.) Koidz. Acta Physiol. Plant. 2015, 37, 1710. [Google Scholar] [CrossRef]
- Ali, M.; Mujib, A.; Tonk, D.; Zafar, N. Plant regeneration through somatic embryogenesis and genome size analysis of Coriandrum sativum L. Protoplasma 2017, 254, 343–352. [Google Scholar] [CrossRef] [PubMed]
- Wójcikowska, B.; Botor, M.; Morończyk, J.; Wójcik, A.M.; Nodzyński, T.; Karcz, J.; Gaj, M.D. Trichostatin A Triggers an Embryogenic Transition in Arabidopsis Explants via an Auxin-Related Pathway. Front. Plant Sci. 2018, 9, 1353. [Google Scholar] [CrossRef] [PubMed]
- Klimaszewska, K.; Noceda, C.; Pelletier, G.; Label, P.; Rodriguez, R.; Lelu-Walter, M.A. Biological Characterization of Young and Aged Embryogenic Cultures of Pinus pinaster (Ait.). In Vitro Cell. Dev. Biol. Plant 2009, 45, 20–33. [Google Scholar] [CrossRef]
- Solís, M.-T.; El-Tantawy, A.-A.; Cano, V.; Risueño, M.C.; Testillano, P.S. 5-azacytidine promotes microspore embryogenesis initiation by decreasing global DNA methylation, but prevents subsequent embryo development in rapeseed and barley. Front. Plant Sci. 2015, 6, 472. [Google Scholar] [CrossRef] [PubMed]
- Quinga, L.A.P.; de Freitas Fraga, H.P.; do Nascimento Vieira, L.; Guerra, M.P. Epigenetics of long-term somatic embryogenesis in Theobroma cacao L.: DNA methylation and recovery of embryogenic potential. Plant Cell Tissue Organ Cult. 2017, 131, 295–305. [Google Scholar] [CrossRef]
- Cristofolini, C.; do Nascimento Vieira, L.; de Freitas Fraga, H.P.; da Costa, I.R.; Guerra, M.P.; Pescador, R. DNA methylation patterns and karyotype analysis of off-type and normal phenotype somatic embryos of feijoa. Theor. Exp. Plant Physiol. 2014, 26, 217–224. [Google Scholar] [CrossRef]
- Fraga, H.P.; Vieira, L.N.; Caprestano, C.A.; Steinmacher, D.A.; Micke, G.A.; Spudeit, D.A.; Pescador, R.; Guerra, M.P. 5-Azacytidine combined with 2, 4-D improves somatic embryogenesis of Acca sellowiana (O. Berg) Burret by means of changes in global DNA methylation levels. Plant Cell Rep. 2012, 31, 2165–2176. [Google Scholar] [CrossRef] [PubMed]
- Fraga, H.P.; Vieira, L.N.; Heringer, A.S.; Puttkammer, C.C.; Silveira, V.; Guerra, M.P. DNA methylation and proteome profiles of Araucaria angustifolia (Bertol.) Kuntze embryogenic cultures as affected by plant growth regulators supplementation. Plant Cell Tissue Organ Cult. 2016, 125, 353–374. [Google Scholar] [CrossRef]
- Heringer, A.S.; Steinmacher, D.A.; Fraga, H.P.F.; Vieira, L.N.; Ree, J.F.; Guerra, M.P. Global DNA methylation profiles of somatic embryos of peach palm (Bactris gasipaes Kunth) are influenced by cryoprotectants and droplet-vitrification cryopreservation. Plant Cell Tissue Organ Cult. 2013, 114, 365–372. [Google Scholar] [CrossRef]
- Betekhtin, A.; Milewska-Hendel, A.; Chajec, L.; Rojek, M.; Nowak, K.; Kwasniewska, J.; Wolny, E.; Kurczynska, E.; Hasterok, R. 5-Azacitidine Induces Cell Death in a Tissue Culture of Brachypodium distachyon. Int. J. Mol. Sci. 2018, 19, 1806. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Wen, X.; Deng, X. Genetic and epigenetic evaluations of citrus calluses recovered from slow-growth culture. J. Plant Physiol. 2004, 161, 479. [Google Scholar] [CrossRef] [PubMed]
- Landey, R.B.; Cenci, A.; Guyot, R.; Bertrand, B.; Georget, F.; Dechamp, E.; Herrera, J.-C.; Aribi, J.; Lashermes, P.; Etienne, H. Assessment of genetic and epigenetic changes during cell culture ageing and relations with somaclonal variation in Coffea arabica. Plant Cell Tissue Organ Cult. 2015, 122, 517–531. [Google Scholar] [CrossRef] [Green Version]
- Leljak-Levanić, D.; Bauer, N.; Mihaljević, S.; Jelaska, S. Changes in DNA methylation during somatic embryogenesis in Cucurbita pepo L. Plant Cell Rep. 2004, 23, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Leljak-Levanić, D.; Mrvková, M.; Turečková, V.; Pěnčík, A.; Rolčík, J.; Strnad, M.; Mihaljević, S. Hormonal and epigenetic regulation during embryogenic tissue habituation in Cucurbita pepo L. Plant Cell Rep. 2016, 35, 77–89. [Google Scholar] [CrossRef] [PubMed]
- Pedrali-Noy, G.; Bernacchia, G.; do Rosario Alvelos, M.; Cella, R. Daucus carota cells contain specific DNA methyltransferase inhibitors that interfere with somatic embryogenesis. Plant Biol. 2003, 5, 383–392. [Google Scholar] [CrossRef]
- Yamamoto, N.; Kobayashi, H.; Togashi, T.; Mori, Y.; Kikuchi, K.; Kuriyama, K.; Tokuji, Y. Formation of embryogenic cell clumps from carrot epidermal cells is suppressed by 5-azacytidine, a DNA methylation inhibitor. J. Plant Physiol. 2005, 162, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Jaligot, E.; Rival, A.; Beulé, T.; Dussert, S.; Verdeil, J. Somaclonal variation in oil palm (Elaeis guineensis Jacq.): The DNA methylation hypothesis. Plant Cell Rep. 2000, 19, 684–690. [Google Scholar] [CrossRef]
- Rival, A.; Ilbert, P.; Labeyrie, A.; Torres, E.; Doulbeau, S.; Personne, A.; Dussert, S.; Beulé, T.; Durand-Gasselin, T.; Tregear, J.W. Variations in genomic DNA methylation during the long-term in vitro proliferation of oil palm embryogenic suspension cultures. Plant Cell Rep. 2013, 32, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarty, D.; Yu, K.-W.; Paek, K.Y. Detection of DNA methylation changes during somatic embryogenesis of Siberian ginseng (Eleuterococcus senticosus). Plant Sci. 2003, 165, 61–68. [Google Scholar] [CrossRef]
- Gao, X.; Yang, D.; Cao, D.; Ao, M.; Sui, X.; Wang, Q.; Kimatu, J.; Wang, L. In vitro micropropagation of Freesia hybrida and the assessment of genetic and epigenetic stability in regenerated plantlets. J. Plant Growth Regul. 2010, 29, 257–267. [Google Scholar] [CrossRef]
- Fiuk, A.; Bednarek, P.T.; Rybczyński, J.J. Flow cytometry, HPLC-RP, and metAFLP analyses to assess genetic variability in somatic embryo-derived plantlets of Gentiana pannonica Scop. Plant Mol. Biol. Rep. 2010, 28, 413–420. [Google Scholar] [CrossRef]
- Bednarek, P.T.; Orłowska, R.; Koebner, R.M.; Zimny, J. Quantification of the tissue-culture induced variation in barley (Hordeum vulgare L.). BMC Plant Biol. 2007, 7, 10. [Google Scholar] [CrossRef] [PubMed]
- Orłowska, R.; Machczyńska, J.; Oleszczuk, S.; Zimny, J.; Bednarek, P.T. DNA methylation changes and TE activity induced in tissue cultures of barley (Hordeum vulgare L.). J. Biol. Res. (Thessalon) 2016, 23, 19. [Google Scholar] [CrossRef] [PubMed]
- Teyssier, C.; Maury, S.; Beaufour, M.; Grondin, C.; Delaunay, A.; Le Metté, C.; Ader, K.; Cadene, M.; Label, P.; Lelu-Walter, M.A. In search of markers for somatic embryo maturation in hybrid larch (Larix × eurolepis): Global DNA methylation and proteomic analyses. Physiol. Plant. 2014, 150, 271–291. [Google Scholar] [CrossRef] [PubMed]
- Santos, D.; Fevereiro, P. Loss of DNA methylation affects somatic embryogenesis in Medicago truncatula. Plant Cell Tissue Organ Cult. 2002, 70, 155–161. [Google Scholar] [CrossRef]
- Hanai, L.R.; Floh, E.I.S.; Fungaro, M.H.P.; Santa-Catarina, C.; de Paula, F.M.; Viana, A.M.; Vieira, M.L.C. Methylation patterns revealed by MSAP profiling in genetically stable somatic embryogenic cultures of Ocotea catharinensis (Lauraceae). In Vitro Cell. Dev. Biol. Plant 2010, 46, 368–377. [Google Scholar] [CrossRef]
- Morrish, F.M.; Vasil, I.K. DNA methylation and embryogenic competence in leaves and callus of napiergrass (Pennisetum purpureum Schum.). Plant Physiol. 1989, 90, 37–40. [Google Scholar] [CrossRef] [PubMed]
- Leljak-Levanic, D.; Mihaljevic, S.; Jelaska, S. Variations in DNA methylation in Picea omorika (Panc) purk. Embryogenic tissue and the ability for embryo maturation. Propag. Ornam. Plants 2009, 9, 3–9. [Google Scholar]
- Pérez, M.; Viejo, M.; LaCuesta, M.; Toorop, P.; Cañal, M.J. Epigenetic and hormonal profile during maturation of Quercus suber L. somatic embryos. J. Plant Physiol. 2015, 173, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Li, X.; Korban, S.S. DNA-methylation alterations and exchanges during in vitro cellular differentiation in rose (Rosa hybrida L.). Theor. Appl. Genet. 2004, 109, 899–910. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.K.; Bryan, G.J.; Winfield, M.O.; Millam, S. Stability of potato (Solanum tuberosum L.) plants regenerated via somatic embryos, axillary bud proliferated shoots, microtubers and true potato seeds: A comparative phenotypic, cytogenetic and molecular assessment. Planta 2007, 226, 1449–1458. [Google Scholar] [CrossRef] [PubMed]
- Adu-Gyamfi, R.; Wetten, A.; Lopez, C.M.R. Effect of cryopreservation and post-cryopreservation somatic embryogenesis on the epigenetic fidelity of cocoa (Theobroma cacao L.). PLoS ONE 2016, 11, e0158857. [Google Scholar] [CrossRef] [PubMed]
- Machczyńska, J.; Orłowska, R.; Mańkowski, D.R.; Zimny, J.; Bednarek, P.T. DNA methylation changes in triticale due to in vitro culture plant regeneration and consecutive reproduction. Plant Cell Tissue Organ Cult. 2014, 119, 289–299. [Google Scholar] [CrossRef] [Green Version]
- Schellenbaum, P.; Mohler, V.; Wenzel, G.; Walter, B. Variation in DNA methylation patterns of grapevine somaclones (Vitis vinifera L.). BMC Plant Biol. 2008, 8, 78. [Google Scholar] [CrossRef] [PubMed]
- Kaeppler, S.; Phillips, R. Tissue culture-induced DNA methylation variation in maize. Proc. Natl. Acad. Sci. USA 1993, 90, 8773–8776. [Google Scholar] [CrossRef] [PubMed]
- Stelpflug, S.C.; Eichten, S.R.; Hermanson, P.J.; Springer, N.M.; Kaeppler, S.M. Consistent and heritable alterations of DNA methylation are induced by tissue culture in maize. Genetics 2014, 198, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Sáenz, L.; Herrera-Herrera, G.; Uicab-Ballote, F.; Chan, J.L.; Oropeza, C. Influence of form of activated charcoal on embryogenic callus formation in coconut (Cocos nucifera). Plant Cell Tissue Organ Cult. 2010, 100, 301–308. [Google Scholar] [CrossRef]
- Argemí, A.; Saurina, J. Study of the degradation of 5-azacytidine as a model of unstable drugs using a stopped-flow method and further data analysis with multivariate curve resolution. Talanta 2007, 74, 176–182. [Google Scholar] [CrossRef] [PubMed]




| Species | Family | Detection of DNA Methylation (Method) | DNA Methylation Inhibitor Used | Effects of Inhibitor | References |
|---|---|---|---|---|---|
| Acca sellowiana | Myrtaceae | CRED-RA | NA | NA | [80] |
| HPLC | 5-AzaC | 5-AzaC (50 μM) induced an increase in GDM and improved the induction of SE. However, in the conversion phase, somatic embryos had a deregulatory effect during the formation of autotrophic plants, resulting in significantly lower conversion rates | [81] | ||
| Arabidopsis thaliana | Brassicaceae | ELISA | 5-AzaC | Explants treated with 5-AzaC (10 μM) showed a drastic inhibition of SE and the explants produced massive non-embryogenic callus, whereas in non-treated-explants they formed somatic embryos quickly and efficiently | [34] |
| Araucaria angustifolia | Araucariaceae | HPLC | NA | NA | [82] |
| Bactris gasipaes | Arecaceae | HPLC | NA | NA | [83] |
| Brachypodium distachyon | Poaceae | TUNEL | 5-AzaC | At a concentration of 50 µM of 5-AzaC, induction of embryogenic masses (EM) was totally inhibited, while in 5 µM of 5-AzaC 10% of explants (zygotic embryos) developed callus with EM. | [84] |
| Brassica napus | Brassicaceae | ELISA | 5-AzaC | Induction of embryos increased when explants were treated four days in 5-AzaC (2.5 μM). In longer treatments with 5-AzaC the formation of somatic embryos decreased | [78] |
| Castanea sativa | Fagaceae | HPCE | NA | NA | [66] |
| Citrus paradise | Rutaceae | MSAP | NA | NA | [85] |
| Coffea canephora | Rubiaceae | HPLC | 5-AzaC | Embryogenic process was strongly inhibited when 5-AzaC was added earlier. However, this negative effect was not observed when added to the 35 days post induction (dpi). The effect of 5-AzaC (20 μM) added at day 21 dpi not only synchronized the embryogenic process but also reduced the maturation of somatic embryos | [60] |
| MSAP | NA | NA | [86] | ||
| Cucurbita pepo | Cucurbitaceae | MSAP | 5-AzaC | Addition of 5-AzaC (12.3 μM) to the basal medium (MSC) with or without 2,4-D did not significantly alter the proportion of embryos in different stages compared to that found in the same medium without 5-AzaC. In the MSC medium with 2,4-D and 5-AzaC, most embryos remained in the early stages of development; however, some developed to more mature stages | [87] |
| CRED-RA/MSAP | 5-AzaC | 5-AzaC had no effects (global DNA methylation or capacities for the development and regeneration) on embryogenic cultures | [88] | ||
| Daucus carota | Apiaceae | HPLC | 5-AzaC/ECP | When ECP is added, SE is immediately blocked. Isolated mutant line that is resistant to the hypomethylating activity of ECP and 5-AzaC shows a higher level of endogenous indole acetic acid (IAA) and a different metabolism of IAA, suggesting the endogenous synthesis of IAA in the habituated tissue could be the reason for its low sensitivity to methylation inhibitors | [65] |
| Immunodetection | 5-AzaC | 5-AzaC suppresses embryogenesis but does not prevent the proliferation of dedifferentiated cells from cells in suspension. | [89] | ||
| 5-AzaC | When 5-AzaC (0.41 μM) was added to the medium, somatic embryos were formed to the same extent as in the control without 5-AzaC. When 5-AzaC (20.5 μM) was supplemented for 3 days after the 24-hour treatment with 2,4-D, the formation of somatic embryos was severely inhibited | [90] | |||
| HPLC | NA | NA | [62] | ||
| Elaeis guineensis | Arecaceae | HPLC/MSAP | NA | NA | [91] |
| HPLC | NA | NA | [92] | ||
| Eleuterococcus senticosus | Araliaceae | HPLC/MSAP | NA | NA | [93] |
| Freesia hybrida | Iridaceae | MSAP | NA | NA | [94] |
| Gentiana pannonica | Gentianaceae | HPLC | NA | NA | [95] |
| Hordeum vulgare | Poaceae | MS-AFLP | NA | NA | [96] |
| ELISA | NA | NA | [67] | ||
| ELISA | 5-AzaC | Induction of embryos increased with four days of treatment with 5-AzaC (2.5 μM), the response was associated with a decrease in DNA methylation. In contrast, longer 5-AzaC treatments decreased embryo generation | [78] | ||
| HPLC/MS-AFLP | NA | NA | [97] | ||
| Laris x eurolepis | Pinaceae | HPLC | 5-AzaC/Hydroxy-urea | 5-AzaC (100 μM) altered the overall DNA methylation status of embryogenic cultures and significantly reduced their relative growth rate and embryogenic potential | [98] |
| Medicago truncatula | Fabaceae | MSAP | 5-AzaC | 5-AzaC (100 μM) stopped the generation of somatic embryos in the embryogenic line and the proliferation of callus in the non-embryogenic line. Analysis with restriction enzymes sensitive to total DNA methylation extracted from the untreated 5-AzaC-treated callus showed a decrease in DNA methylation levels | [99] |
| Ocotea catharinensis | Lauraceae | MSAP | NA | NA | [100] |
| Pennisetum purpureum | Poaceae | HPLC/MSAP | NA | NA | [101] |
| Picea omorika | Pinaceae | MS-RAPD | 5-AzaC | DNA methylation decreased by 19% compared to the same medium without 5-AzaC (12.3 μM). However, the total number of embryos developed in the subsequent transfer to the maturation medium was not significantly different (182 and 190 somatic embryos, respectively) | [102] |
| Pinus nigra | Pinaceae | CRED-RA | NA | NA | [63] |
| Pinus pinaster | Pinaceae | HPLC | 5-AzaC | Embryonal masses grew when they were exposed 9 days to 5-AzaC. Growth was inversely proportional to the increase in drug concentration. The highest amounts of somatic embryos were obtained at the 10 and 15 μm concentrations of 5-AzaC, the treatments with the highest levels of methylation (19.5% and 21.3%, respectively) | [77] |
| Quercus alba | Fagaceae | ELISA | NA | NA | [64] |
| Quercus suber | Fagaceae | HPCE/Immunolocalization | NA | NA | [103] |
| Rosa hybrida | Rosaceae | MS-AFLP | NA | NA | [104] |
| Solanum tuberosum | Solanaceae | MS-AFLP | NA | NA | [105] |
| Theobroma cacao | Malvaceae | MSAP | NA | NA | [61] |
| MSAP | NA | NA | [106] | ||
| HPLC | 5-AzaC | GDM increased as SE proceeded and during the extended SE the aged somatic embryos could recover embryogenic potential when treated with 5-AzaC (20 μM). The results of this study suggested that long-term SE in cocoa induced a decrease in embryogenic potential, but that it could be reversed by 5-AzaC supplementation | [79] | ||
| Triticosecale | Poaceae | HPLC | NA | NA | [107] |
| Vitis vinifera | Vitaceae | MSAP | NA | NA | [108] |
| Zea mays | Poaceae | MSAP | NA | NA | [109] |
| meDIP | NA | NA | [110] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Osorio-Montalvo, P.; Sáenz-Carbonell, L.; De-la-Peña, C. 5-Azacytidine: A Promoter of Epigenetic Changes in the Quest to Improve Plant Somatic Embryogenesis. Int. J. Mol. Sci. 2018, 19, 3182. https://doi.org/10.3390/ijms19103182
Osorio-Montalvo P, Sáenz-Carbonell L, De-la-Peña C. 5-Azacytidine: A Promoter of Epigenetic Changes in the Quest to Improve Plant Somatic Embryogenesis. International Journal of Molecular Sciences. 2018; 19(10):3182. https://doi.org/10.3390/ijms19103182
Chicago/Turabian StyleOsorio-Montalvo, Pedro, Luis Sáenz-Carbonell, and Clelia De-la-Peña. 2018. "5-Azacytidine: A Promoter of Epigenetic Changes in the Quest to Improve Plant Somatic Embryogenesis" International Journal of Molecular Sciences 19, no. 10: 3182. https://doi.org/10.3390/ijms19103182
APA StyleOsorio-Montalvo, P., Sáenz-Carbonell, L., & De-la-Peña, C. (2018). 5-Azacytidine: A Promoter of Epigenetic Changes in the Quest to Improve Plant Somatic Embryogenesis. International Journal of Molecular Sciences, 19(10), 3182. https://doi.org/10.3390/ijms19103182