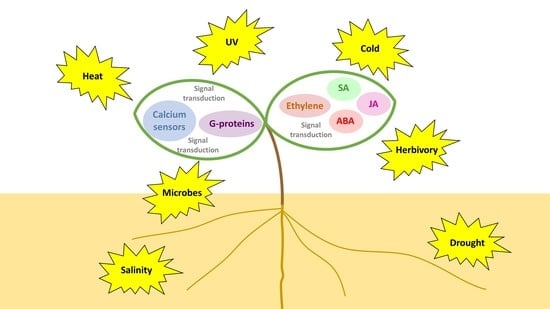
Plant Hormone Signaling Crosstalks between Biotic and Abiotic Stress Responses
Abstract
:1. Introduction
2. Ca2+ Sensors
2.1. CaM/CML
2.2. CBL
2.3. CPK
2.4. CCaMK
2.5. Calcium Sensors Involved in both Abiotic and Biotic Stresses
3. ABA-Mediated Stress Responses
3.1. The Interactions between ABA and JA Pathways in Response to Biotic and Abiotic Stresses
3.2. The Interactions between ABA and Ethylene Pathways under Biotic and Abiotic Stresses
The Antagonistic Relationship between ABA and Ethylene Pathways
3.3. The Interactions between ABA and SA under Biotic and Abiotic stresses
The Crosstalks among ABA, SA, and Phospholipids under Biotic and Abiotic Stresses
4. Roles of G-proteins in Biotic and Abiotic Stress Responses
4.1. Heterotrimeric G-proteins
4.2. Unconventional G-proteins
4.2.1. Obg Superfamily
Obg/Era Family
Drg Family
YchF Family
4.3. Small G-proteins
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ku, Y.; Yung, Y.; Li, M.; Wen, C.; Liu, X. Drought stress and tolerance in soybean. In A Comprehensive Survey of International Soybean Research—Genetics, Physiology, Agronomy and Nitrogen Relationships; Board, J.E., Ed.; IntechOpen: London, UK, 2013; p. 624. ISBN 978-953-51-0876-4. [Google Scholar]
- Phang, T.H.; Shao, G.; Lam, H.M. Salt tolerance in soybean. J. Integr. Plant Biol. 2008, 50, 1196–1212. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Vinocur, B.; Altman, A. Plant responses to drought, salinity and extreme temperatures: Towards genetic engineering for stress tolerance. Planta 2003, 218, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Mauch-Mani, B.; Mauch, F. The role of abscisic acid in plant-pathogen interactions. Curr. Opin. Plant Biol. 2005, 8, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R. Abiotic stress, the field environment and stress combination. Trends Plant Sci. 2006, 11, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, N.; Rivero, R.M.; Shulaev, V.; Blumwald, E.; Mittler, R. Abiotic and biotic stress combinations. New Phytol. 2014, 203, 32–43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trewavas, A. Le calcium, c’est la vie: Calcium makes waves. Plant Physiol. 1999, 120, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Edel, K.H.; Kudla, J. Integration of calcium and ABA signaling. Curr. Opin. Plant Biol. 2016, 33, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Nitta, Y.; Ding, P.; Zhang, Y. Heterotrimeric G proteins in plant defense against pathogens and ABA signaling. Environ. Exp. Bot. 2015, 114, 153–158. [Google Scholar] [CrossRef]
- Ranty, B.; Aldon, D.; Cotelle, V.; Galaud, J.-P.; Thuleau, P.; Mazars, C. Calcium sensors as key hubs in plant responses to biotic and abiotic stresses. Front. Plant Sci. 2016, 7, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Tuteja, N.; Mahajan, S. Calcium signaling network in plants: an overview. Plant Signal. Behav. 2007, 2, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Liese, A.; Romeis, T. Biochemical regulation of in vivo function of plant calcium-dependent protein kinases (CDPK). Biochim. Biophys. Acta—Mol. Cell Res. 2013, 1833, 1582–1589. [Google Scholar] [CrossRef] [PubMed]
- Perochon, A.; Aldon, D.; Galaud, J.P.; Ranty, B. Calmodulin and calmodulin-like proteins in plant calcium signaling. Biochimie 2011, 93, 2048–2053. [Google Scholar] [CrossRef] [PubMed]
- La Verde, V.; Dominici, P.; Astegno, A. Towards understanding plant calcium signaling through calmodulin-like proteins: A biochemical and structural perspective. Int. J. Mol. Sci. 2018, 19, 1331. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Yang, T.; Jurick II, W.M. Calmodulin gene expression in response to mechanical wounding and Botrytis cinerea infection in tomato fruit. Plants 2014, 3, 427–441. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhou, R.-G.; Gao, Y.-J.; Zheng, S.-Z.; Xu, P.; Zhang, S.-Q.; Sun, D.-Y. Molecular and genetic evidence for the key role of AtCaM3 in heat-shock signal transduction in Arabidopsis. Plant Physiol. 2009, 149, 1773–1784. [Google Scholar] [CrossRef] [PubMed]
- Heo, W.D.; Lee, S.H.; Kim, M.C.; Kim, J.C.; Chung, W.S.; Chun, H.J.; Lee, K.J.; Park, C.Y.; Park, H.C.; Choi, J.Y.; et al. Involvement of specific calmodulin isoforms in salicylic acid-independent activation of plant disease resistance responses. Proc. Natl. Acad. Sci. USA 1999, 96, 766–771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Munir, S.; Liu, H.; Xing, Y.; Hussain, S.; Ouyang, B.; Zhang, Y.; Li, H.; Ye, Z. Overexpression of calmodulin-like (ShCML44) stress-responsive gene from Solanum habrochaites enhances tolerance to multiple abiotic stresses. Sci. Rep. 2016, 6, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Magnan, F.; Ranty, B.; Charpenteau, M.; Sotta, B.; Galaud, J.P.; Aldon, D. Mutations in AtCML9, a calmodulin-like protein from Arabidopsis thaliana, alter plant responses to abiotic stress and abscisic acid. Plant J. 2008, 56, 575–589. [Google Scholar] [CrossRef] [PubMed]
- Leba, L.-J.; Cheval, C.; Ortiz-Martín, I.; Ranty, B.; Beuzón, C.R.; Galaud, J.P.; Aldon, D. CML9, an Arabidopsis calmodulin-like protein, contributes to plant innate immunity through a flagellin-dependent signalling pathway. Plant J. 2012, 71, 976–989. [Google Scholar] [CrossRef] [PubMed]
- Scholz, S.S.; Reichelt, M.; Vadassery, J.; Mithöfer, A. Calmodulin-like protein CML37 is a positive regulator of ABA during drought stress in Arabidopsis. Plant Signal. Behav. 2015, 10. [Google Scholar] [CrossRef] [PubMed]
- Scholz, S.S.; Vadassery, J.; Heyer, M.; Reichelt, M.; Bender, K.W.; Snedden, W.A.; Boland, W.; Mithöfer, A. Mutation of the Arabidopsis calmodulin-like protein CML37 deregulates the jasmonate pathway and enhances susceptibility to herbivory. Mol. Plant 2014, 7, 1712–1726. [Google Scholar] [CrossRef] [PubMed]
- Vadassery, J.; Reichelt, M.; Hause, B.; Gershenzon, J.; Boland, W.; Mithofer, A. CML42-mediated calcium signaling coordinates responses to Spodoptera herbivory and abiotic stresses in Arabidopsis. Plant Physiol. 2012, 159, 1159–1175. [Google Scholar] [CrossRef] [PubMed]
- Batistič, O.; Kudla, J. Plant calcineurin B-like proteins and their interacting protein kinases. Biochim. Biophys. Acta—Mol. Cell Res. 2009, 1793, 985–992. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, L.; Ren, F.; Zhou, L.; Wang, Q.Q.; Zhong, H.; Li, X.B. The Brassica napus Calcineurin B-Like 1/CBL-interacting protein kinase 6 (CBL1/CIPK6) component is involved in the plant response to abiotic stress and ABA signalling. J. Exp. Bot. 2012, 63, 6211–6222. [Google Scholar] [CrossRef] [PubMed]
- Cheong, Y.H.; Sung, S.J.; Kim, B.G.; Pandey, G.K.; Cho, J.S.; Kim, K.N.; Luan, S. Constitutive overexpression of the calcium sensor CBL5 confers osmotic or drought stress tolerance in Arabidopsis. Mol. Cells 2010, 29, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.G.; Waadt, R.; Cheong, Y.H.; Pandey, G.K.; Dominguez-Solis, J.R.; Schültke, S.; Lee, S.C.; Kudla, J.; Luan, S. The calcium sensor CBL10 mediates salt tolerance by regulating ion homeostasis in Arabidopsis. Plant J. 2007, 52, 473–484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de la Torre, F.; Gutierrez-Beltran, E.; Pareja-Jaime, Y.; Chakravarthy, S.; Martin, G.B.; del Pozo, O. The tomato calcium sensor Cbl10 and its interacting protein kinase Cipk6 define a signaling pathway in plant immunity. Plant Cell 2013, 25, 2748–2764. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Ji, W.; Zhu, Y.; Gao, P.; Li, Y.; Cai, H.; Bai, X.; Guo, D. GsCBRLK, a calcium/calmodulin-binding receptor-like kinase, is a positive regulator of plant tolerance to salt and ABA stress. J. Exp. Bot. 2010, 61, 2519–2533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valmonte, G.R.; Arthur, K.; Higgins, C.M.; Macdiarmid, R.M. Calcium-dependent protein kinases in plants: Evolution, expression and function. Plant Cell Physiol. 2014, 55, 551–569. [Google Scholar] [CrossRef] [PubMed]
- Simeunovic, A.; Mair, A.; Wurzinger, B.; Teige, M. Know where your clients are: Subcellular localization and targets of calcium-dependent protein kinases. J. Exp. Bot. 2016, 67, 3855–3872. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.-J.; Wei, F.-J.; Wang, C.; Wu, J.-J.; Ratnasekera, D.; Liu, W.-X.; Wu, W.-H. Arabidopsis calcium-dependent protein kinase CPK10 functions in abscisic acid- and Ca2+-mediated stomatal regulation in response to drought stress. Plant Physiol. 2010, 154, 1232–1243. [Google Scholar] [CrossRef] [PubMed]
- Mehlmer, N.; Wurzinger, B.; Stael, S.; Hofmann-Rodrigues, D.; Csaszar, E.; Pfister, B.; Bayer, R.; Teige, M. The Ca2+-dependent protein kinase CPK3 is required for MAPK-independent salt-stress acclimation in Arabidopsis. Plant J. 2010, 63, 484–498. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Tian, Y.S.; Peng, R.H.; Xiong, A.S.; Zhu, B.; Jin, X.F.; Gao, F.; Fu, X.Y.; Hou, X.L.; Yao, Q.H. AtCPK6, a functionally redundant and positive regulator involved in salt/drought stress tolerance in Arabidopsis. Planta 2010, 231, 1251–1260. [Google Scholar] [CrossRef] [PubMed]
- Mori, I.C.; Murata, Y.; Yang, Y.; Munemasa, S.; Wang, Y.F.; Andreoli, S.; Tiriac, H.; Alonso, J.M.; Harper, J.F.; Ecker, J.R.; et al. CDPKs CPK6 and CPK3 function in ABA regulation of guard cell S-type anion- and Ca2+-permeable channels and stomatal closure. PLoS Biol. 2006, 4, 1749–1762. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Zhang, D.; Wang, L.; Pan, J.; Liu, Y.; Kong, X.; Zhou, Y.; Li, D. A maize calcium-dependent protein kinase gene, ZmCPK4, positively regulated abscisic acid signaling and enhanced drought stress tolerance in transgenic Arabidopsis. Plant Physiol. Biochem. 2013, 71, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; Hu, W.; Deng, X.; Zhang, Y.; Liu, X.; Zhao, X.; Luo, Q.; Jin, Z.; Li, Y.; Zhou, S.; et al. A rice calcium-dependent protein kinase OsCPK9 positively regulates drought stress tolerance and spikelet fertility. BMC Plant Biol. 2014, 14, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Asano, T.; Hayashi, N.; Kobayashi, M.; Aoki, N.; Miyao, A.; Mitsuhara, I.; Ichikawa, H.; Komatsu, S.; Hirochika, H.; Kikuchi, S.; et al. A rice calcium-dependent protein kinase OsCPK12 oppositely modulates salt-stress tolerance and blast disease resistance. Plant J. 2012, 69, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Sathyanarayanan, P.V.; Cremo, C.R.; Poovaiah, B.W. Plant chimeric Ca2+/calmodulin-dependent protein kinase. Role of the neural visinin-like domain in regulating autophosphorylation and calmodulin affinity. J. Biol. Chem. 2000, 275, 30417–30422. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-P.; Munyampundu, J.-P.; Xu, Y.-P.; Cai, X.-Z. Phylogeny of plant calcium and calmodulin-dependent protein kinases (CCaMKs) and functional analyses of tomato CCaMK in disease resistance. Front. Plant Sci. 2015, 6, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Yan, J.; Liu, W.; Liu, L.; Sheng, Y.; Sun, Y.; Li, Y.; Scheller, H.V.; Jiang, M.; Hou, X.; et al. Phosphorylation of a NAC transcription factor by a calcium/calmodulin-dependent protein kinase regulates abscisic acid-induced antioxidant defense in maize. Plant Physiol. 2016, 171, 1651–1664. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Katzer, K.; Lambert, J.; Cerri, M.; Parniske, M. CYCLOPS, a DNA-binding transcriptional activator, orchestrates symbiotic root nodule development. Cell Host Microbe 2014, 15, 139–152. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Li, A.; Zhao, Y.; Zhang, Z.; Zhu, Y.; Tan, X.; Geng, S.; Guo, H.; Zhang, X.; Kang, Z.; et al. Overexpression of a wheat CCaMK gene reduces ABA sensitivity of Arabidopsis thaliana during seed germination and seedling growth. Plant Mol. Biol. Report. 2011, 29, 681–692. [Google Scholar] [CrossRef]
- Ahmad, P.; Rasool, S.; Gul, A.; Sheikh, S.A.; Akram, N.A.; Ashraf, M.; Kazi, A.M.; Gucel, S. Jasmonates: Multifunctional roles in stress tolerance. Front. Plant Sci. 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Kazan, K. Diverse roles of jasmonates and ethylene in abiotic stress tolerance. Trends Plant Sci. 2015, 20, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Xie, D. Jasmonate in plant defence: Sentinel or double agent? Plant Biotechnol. J. 2015, 13, 1233–1240. [Google Scholar] [CrossRef] [PubMed]
- Schulze-Lefert, P.; Robatzek, S. Plant pathogens trick guard cells into opening the gates. Cell 2006, 126, 831–834. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Rojas, C.M.; Oh, S.; Kang, M.; Choudhury, S.R.; Lee, H.K.; Allen, R.D.; Pandey, S.; Mysore, K.S. Nucleolar GTP-binding protein 1-2 (NOG1-2) interacts with jasmonate-ZIMDomain protein 9 (JAZ9) to regulate stomatal aperture during plant immunity. Int. J. Mol. Sci. 2018, 19. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Thilmony, R.; Bender, C.L.; Schaller, A.; He, S.Y.; Howe, G.A. Virulence systems of Pseudomonas syringae pv. tomato promote bacterial speck disease in tomato by targeting the jasmonate signaling pathway. Plant J. 2003, 36, 485–499. [Google Scholar] [CrossRef] [PubMed]
- Boter, M.; Ruíz-Rivero, O.; Abdeen, A.; Prat, S. Conserved MYC transcription factors play a key role in jasmonate signaling both in tomato and Arabidopsis. Genes Dev. 2004, 18, 1577–1591. [Google Scholar] [CrossRef] [PubMed]
- Pauwels, L.; Barbero, G.F.; Geerinck, J.; Tilleman, S.; Grunewald, W.; Pérez, A.C.; Chico, J.M.; Bossche, R.V.; Sewell, J.; Gil, E.; et al. NINJA connects the co-repressor TOPLESS to jasmonate signalling. Nature 2010, 464, 788–791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thines, B.; Katsir, L.; Melotto, M.; Niu, Y.; Mandaokar, A.; Liu, G.; Nomura, K.; He, S.Y.; Howe, G.A.; Browse, J. JAZ repressor proteins are targets of the SCFCOI1complex during jasmonate signalling. Nature 2007, 448, 661–665. [Google Scholar] [CrossRef] [PubMed]
- Chini, A.; Fonseca, S.; Fernández, G.; Adie, B.; Chico, J.M.; Lorenzo, O.; García-Casado, G.; López-Vidriero, I.; Lozano, F.M.; Ponce, M.R.; et al. The JAZ family of repressors is the missing link in jasmonate signalling. Nature 2007, 448, 666–671. [Google Scholar] [CrossRef] [PubMed]
- Sheard, L.B.; Tan, X.; Mao, H.; Withers, J.; Ben-Nissan, G.; Hinds, T.R.; Kobayashi, Y.; Hsu, F.F.; Sharon, M.; Browse, J.; et al. Jasmonate perception by inositol-phosphate-potentiated COI1-JAZ co-receptor. Nature 2010, 468, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Senthil-Kumar, M.; Kang, M.; Rojas, C.M.; Tang, Y.; Oh, S.; Choudhury, S.R.; Lee, H.K.; Ishiga, Y.; Allen, R.D.; et al. The small GTPase, nucleolar GTP-binding protein 1 (NOG1), has a novel role in plant innate immunity. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.S.; Howe, G.A. A critical role for the TIFY motif in repression of jasmonate signaling by a stabilized splice variant of the JASMONATE ZIM-Domain protein JAZ10 in Arabidopsis. Plant Cell 2009, 21, 131–145. [Google Scholar] [CrossRef] [PubMed]
- Manners, J.M.; Penninckx, I.A.M.A.; Vermaere, K.; Kazan, K.; Brown, R.L.; Morgan, A.; Maclean, D.J.; Curtis, M.D.; Cammue, B.P.A.; Broekaert, W.F. The promoter of the plant defensin gene PDF1.2 from Arabidopsis is systemically activated by fungal pathogens and responds to methyl jasmonate but not to salicylic acid. Plant Mol. Biol. 1998, 38, 1071–1080. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo, O. ETHYLENE RESPONSE FACTOR1 integrates signals from ethylene and jasmonate pathways in plant defense. Plant Cell 2003, 15, 165–178. [Google Scholar] [CrossRef] [PubMed]
- Shakeel, S.N.; Gao, Z.; Amir, M.; Chen, Y.F.; Rai, M.I.; Haq, N.U.; Schaller, G.E. Ethylene regulates levels of ethylene receptor/CTR1 signaling complexes in Arabidopsis thaliana. J. Biol. Chem. 2015, 290, 12415–12424. [Google Scholar] [CrossRef] [PubMed]
- Yasumura, Y.; Pierik, R.; Kelly, S.; Sakuta, M.; Voesenek, L.A.C.J.; Harberd, N.P. An ancestral role for CONSTITUTIVE TRIPLE RESPONSE1 proteins in both ethylene and abscisic acid signaling. Plant Physiol. 2015, 169, 283–298. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Chen, Y.F.; Randlett, M.D.; Zhao, X.C.; Findell, J.L.; Kieber, J.J.; Schaller, G.E. Localization of the Raf-like kinase CTR1 to the endoplasmic reticulum of Arabidopsis through participation in ethylene receptor signaling complexes. J. Biol. Chem. 2003, 278, 34725–34732. [Google Scholar] [CrossRef] [PubMed]
- Ju, C.; Yoon, G.M.; Shemansky, J.M.; Lin, D.Y.; Ying, Z.I.; Chang, J.; Garrett, W.M.; Kessenbrock, M.; Groth, G.; Tucker, M.L.; et al. CTR1 phosphorylates the central regulator EIN2 to control ethylene hormone signaling from the ER membrane to the nucleus in Arabidopsis. Proc. Natl. Acad. Sci. USA 2012, 109, 19486–19491. [Google Scholar] [CrossRef] [PubMed]
- An, F.; Zhao, Q.; Ji, Y.; Li, W.; Jiang, Z.; Yu, X.; Zhang, C.; Han, Y.; He, W.; Liu, Y.; et al. Ethylene-induced stabilization of ETHYLENE INSENSITIVE3 and EIN3-LIKE1 is mediated by proteasomal degradation of EIN3 binding F-box 1 and 2 that requires EIN2 in Arabidopsis. Plant Cell 2010, 22, 2384–2401. [Google Scholar] [CrossRef] [PubMed]
- Solano, R.; Stepanova, A.; Chao, Q.; Ecker, J.R. Nuclear events in ethylene signaling: A transcriptional cascade mediated by ETHYLENE-INSENSITIVE3 and ETHYLENE-RESPONSE-FACTOR1. Genes Dev. 1998, 12, 3703–3714. [Google Scholar] [CrossRef] [PubMed]
- Nakano, T.; Suzuki, K.; Fujimura, T.; Shinshi, H. Genome-wide analysis of the ERF gene family. Plant Physiol. 2006, 140, 411–432. [Google Scholar] [CrossRef] [PubMed]
- Ohmetakagi, M.; Shinshi, H. Ethylene-inducible DNA-bidning proteins that interact with an ethylene-responsive element. Plant Cell 1995, 7, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Sessa, G.; Meller, Y.; Fluhr, R. A GCC element and a G-box motif participate in ethylene-induced expression of the PRB-1b gene. Plant Mol. Biol. 1995, 28, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, S.Y. Arabidopsis ethylene-responsive element binding factors act as transcriptional activators or repressors of GCC box-mediated gene expression. Plant Cell 2000, 12, 393–404. [Google Scholar] [CrossRef] [PubMed]
- Shinshi, H.; Usami, S.; Ohme-Takagi, M. Identification of an ethylene-responsive region in the promoter of a tobacco class I chitinase gene. Plant Mol. Biol. 1995, 27, 923–932. [Google Scholar] [CrossRef] [PubMed]
- Sakuma, Y.; Liu, Q.; Dubouzet, J.G.; Abe, H.; Yamaguchi-Shinozaki, K.; Shinozaki, K. DNA-binding specificity of the ERF/AP2 domain of Arabidopsis DREBs, transcription factors involved in dehydration- and cold-inducible gene expression. Biochem. Biophys. Res. Commun. 2002, 290, 998–1009. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.-C.; Liao, P.-M.; Kuo, W.-W.; Lin, T.-P. The Arabidopsis ETHYLENE RESPONSE FACTOR1 regulates abiotic stress-responsive gene expression by binding to different cis-acting elements in response to different stress signals. Plant Physiol. 2013, 162, 1566–1582. [Google Scholar] [CrossRef] [PubMed]
- Dubois, M.; Skirycz, A.; Claeys, H.; Maleux, K.; Dhondt, S.; De Bodt, S.; Vanden Bossche, R.; De Milde, L.; Yoshizumi, T.; Matsui, M.; et al. ETHYLENE RESPONSE FACTOR6 acts as a central regulator of leaf growth under water-limiting conditions in Arabidopsis. Plant Physiol. 2013, 162, 319–332. [Google Scholar] [CrossRef] [PubMed]
- Seo, Y.J.; Park, J.B.; Cho, Y.J.; Jung, C.; Seo, H.S.; Park, S.K.; Nahm, B.H.; Song, J.T. Overexpression of the ethylene-responsive factor gene BrERF4 from Brassica rapa increases tolerance to salt and drought in Arabidopsis plants. Mol. Cells 2010, 30, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Sewelam, N.; Kazan, K.; Thomas-Hall, S.R.; Kidd, B.N.; Manners, J.M.; Schenk, P.M. Ethylene response factor 6 is a regulator of reactive oxygen species signaling in Arabidopsis. PLoS ONE 2013, 8, e70289. [Google Scholar] [CrossRef] [PubMed]
- Jisha, V.; Dampanaboina, L.; Vadassery, J.; Mithöfer, A.; Kappara, S.; Ramanan, R. Overexpression of an AP2/ERF type transcription factor OsEREBP1 confers biotic and abiotic stress tolerance in rice. PLoS ONE 2015, 10. [Google Scholar] [CrossRef] [PubMed]
- Park, J.M.; Park, C.J.; Lee, S.B.; Ham, B.K.; Shin, R.; Paek, K.H. Overexpression of the tobacco Tsi1 gene encoding an EREBP/AP2-type transcription factor enhances resistance against pathogen attack and osmotic stress in tobacco. Plant Cell 2001, 13, 1035–1046. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Huang, Z.; Chen, Q.; Zhang, Z.; Zhang, H.; Wu, Y.; Huang, D.; Huang, R. Ectopic overexpression of tomato JERF3 in tobacco activates downstream gene expression and enhances salt tolerance. Plant Mol. Biol. 2004, 55, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Chen, M.; Li, L.; Xu, Z.; Chen, X.; Guo, J.; Ma, Y. Overexpression of the soybean GmERF3 gene, an AP2/ERF type transcription factor for increased tolerances to salt, drought, and diseases in transgenic tobacco. J. Exp. Bot. 2009, 60, 3781–3796. [Google Scholar] [CrossRef] [PubMed]
- Rong, W.; Qi, L.; Wang, A.; Ye, X.; Du, L.; Liang, H.; Xin, Z.; Zhang, Z. The ERF transcription factor TaERF3 promotes tolerance to salt and drought stresses in wheat. Plant Biotechnol. J. 2014, 12, 468–479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, Y.; Seymour, G.B.; Lu, C.; Hu, Z.; Chen, X.; Chen, G. An ethylene response factor (ERF5) promoting adaptation to drought and salt tolerance in tomato. Plant Cell Rep. 2012, 31, 349–360. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Chen, X.; Ren, H.; Zhang, Z.; Zhang, H.; Wang, J.; Wang, X.C.; Huang, R. ERF protein JERF1 that transcriptionally modulates the expression of abscisic acid biosynthesis-related gene enhances the tolerance under salinity and cold in tobacco. Planta 2007, 226, 815–825. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wan, L.; Zhang, L.; Zhang, Z.; Zhang, H.; Quan, R.; Zhou, S.; Huang, R. An ethylene response factor OsWR1 responsive to drought stress transcriptionally activates wax synthesis related genes and increases wax production in rice. Plant Mol. Biol. 2012, 78, 275–288. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Tian, L.; Latoszek-Green, M.; Brown, D.; Wu, K. Arabidopsis ERF4 is a transcriptional repressor capable of modulating ethylene and abscisic acid responses. Plant Mol. Biol. 2005, 58, 585–596. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Zhang, Z.; Zhang, H.; Wang, X.-C.; Huang, R. Transcriptional modulation of ethylene response factor protein JERF3 in the oxidative stress response enhances tolerance of tobacco seedlings to salt, drought, and freezing. Plant Physiol. 2008, 148, 1953–1963. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, Z.; Quan, R.; Li, G.; Wang, R.; Huang, R. An AP2 domain-containing gene, ESE1, targeted by the ethylene signaling component EIN3 is important for the salt response in Arabidopsis. Plant Physiol. 2011, 157, 854–865. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Huang, R. Enhanced tolerance to freezing in tobacco and tomato overexpressing transcription factor TERF2/LeERF2 is modulated by ethylene biosynthesis. Plant Mol. Biol. 2010, 73, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Qi, L.; Liu, X.; Cai, S.; Xu, H.; Huang, R.; Li, J.; Wei, X.; Zhang, Z. The wheat ethylene response factor transcription factor PATHOGEN-INDUCED ERF1 mediates host responses to both the necrotrophic pathogen Rhizoctonia cerealis and freezing stresses. Plant Physiol. 2014, 164, 1499–1514. [Google Scholar] [CrossRef] [PubMed]
- Yi, S.Y.; Kim, J.-H.; Joung, Y.; Lee, S.; Kim, W.; Yu, S.H.; Choi, D. The pepper transcription factor CaPF1 confers pathogen and freezing tolerance in Arabidopsis. Plant Physiol. 2004, 136, 2862–2874. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Y.; Wang, Y.; Li, Y.; Lei, T.; Yan, F.; Su, L.; Li, X.; Zhao, Y.; Sun, X.; Li, J.; et al. Isolation and molecular characterization of GmERF7, a soybean ethylene-response factor that increases salt stress tolerance in tobacco. Gene 2013, 513, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.-G.; Li, H.; Liu, J.-Y. Molecular characterization of three ethylene responsive element binding factor genes from cotton. J. Integr. Plant Biol. 2010. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Qin, L.; Liu, W.; Zhang, D.; Wang, Y. A novel ethylene-responsive factor from Tamarix hispida, ThERF1, is a GCC-box- and DRE-motif binding protein that negatively modulates abiotic stress tolerance in Arabidopsis. Physiol. Plant. 2014, 152, 84–97. [Google Scholar] [CrossRef] [PubMed]
- Serra, T.S.; Figueiredo, D.D.; Cordeiro, A.M.; Almeida, D.M.; Lourenço, T.; Abreu, I.A.; Sebastián, A.; Fernandes, L.; Contreras-Moreira, B.; Oliveira, M.M.; et al. OsRMC, a negative regulator of salt stress response in rice, is regulated by two AP2/ERF transcription factors. Plant Mol. Biol. 2013, 82, 439–455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, Z.J.; Chen, X.J.; Wu, X.L.; Ling, J.Q.; Xu, P. Overexpression of the AP2/EREBP transcription factor OPBP1 enhances disease resistance and salt tolerance in tobacco. Plant Mol. Biol. 2004, 55, 607–618. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, C.; Qin, L.; Liu, W.; Wang, Y. ThERF1 regulates its target genes via binding to a novel cis-acting element in response to salt stress. J. Integr. Plant Biol. 2015, 57, 838–847. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Yu, Y.; Li, S.; Wang, J.; Tang, S.; Huang, R. Abscisic acid antagonizes ethylene production through the ABI4-mediated transcriptional repression of ACS4 and ACS8 in Arabidopsis. Mol. Plant 2016, 9, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Ghassemian, M.; Nambara, E.; Cutler, S.; Kawaide, H.; Kamiya, Y.; McCourt, P. Regulation of abscisic acid signaling by the ethylene response pathway in Arabidopsis. Plant Cell 2000, 12, 1117–1126. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, N.J.; Urwin, P.E. The interaction of plant biotic and abiotic stresses: From genes to the field. J. Exp. Bot. 2012, 63, 3523–3544. [Google Scholar] [CrossRef] [PubMed]
- Seo, P.J.; Xiang, F.; Qiao, M.; Park, J.-Y.; Lee, Y.N.; Kim, S.-G.; Lee, Y.-H.; Park, W.J.; Park, C.-M. The MYB96 transcription factor mediates abscisic acid signaling during drought stress response in Arabidopsis. Plant Physiol. 2009, 151, 275–289. [Google Scholar] [CrossRef] [PubMed]
- Wildermuth, M.C.; Dewdney, J.; Wu, G.; Ausubel, F.M. Isochorismate synthase is required to synthesize salicylic acid for plant defence. Nature 2001, 414, 562–565. [Google Scholar] [CrossRef] [PubMed]
- Seo, P.J.; Park, C.M. MYB96-mediated abscisic acid signals induce pathogen resistance response by promoting salicylic acid biosynthesis in Arabidopsis. New Phytol. 2010, 186, 471–483. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, L.; Vernooij, B.; Gaffney, T.; Morse, A.; Ryals, J. Characterization of tobacco plants expressing a bacterial salicylate hydroxylase gene. Plant Mol. Biol. 1995, 29, 959–968. [Google Scholar] [CrossRef] [PubMed]
- Leckie, C.P.; McAinsh, M.R.; Allen, G.J.; Sanders, D.; Hetherington, A.M. Abscisic acid-induced stomatal closure mediated by cyclic ADP-ribose. Proc. Natl. Acad. Sci. USA 1998, 95, 15837–15842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Munemasa, S.; Hauser, F.; Park, J.; Waadt, R.; Brandt, B.; Schroeder, J.I. Mechanisms of abscisic acid-mediated control of stomatal aperture. Curr. Opin. Plant Biol. 2015, 28, 154–162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hao, J.; Wang, X.; Dong, C.; Zhang, Z.; Shang, Q. Salicylic acid induces stomatal closure by modulating endogenous hormone levels in cucumber cotyledons. Russ. J. Plant Physiol. 2011, 58, 906–913. [Google Scholar] [CrossRef]
- Khokon, M.A.R.; Okuma, E.; Hossain, M.A.; Munemasa, S.; Uraji, M.; Nakamura, Y.; Mori, I.C.; Murata, Y. Involvement of extracellular oxidative burst in salicylic acid-induced stomatal closure in Arabidopsis. Plant Cell Environ. 2011, 34, 434–443. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Zeevaart, J.A.D. Overexpression of a 9-cis-epoxycarotenoid dioxygenase gene in Nicotiana plumbaginifolia increases abscisic acid and phaseic acid levels and enhances drought tolerance. Plant Physiol. 2002, 128, 544–551. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.I.; Kwak, J.S.; Song, J.T.; Seo, H.S. The E3 SUMO ligase AtSIZ1 functions in seed germination in Arabidopsis. Physiol. Plant. 2016, 158, 256–271. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Nam, J.; Park, H.C.; Na, G.; Miura, K.; Jin, J.B.; Yoo, C.Y.; Baek, D.; Kim, D.H.; Jeong, J.C.; et al. Salicylic acid-mediated innate immunity in Arabidopsis is regulated by SIZ1 SUMO E3 ligase. Plant J. 2007, 49, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Miura, K.; Okamoto, H.; Okuma, E.; Shiba, H.; Kamada, H.; Hasegawa, P.M.; Murata, Y. SIZ1 deficiency causes reduced stomatal aperture and enhanced drought tolerance via controlling salicylic acid-induced accumulation of reactive oxygen species in Arabidopsis. Plant J. 2013, 73, 91–104. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Devaiah, S.P.; Zhang, W.; Welti, R. Signaling functions of phosphatidic acid. Prog. Lipid Res. 2006, 45, 250–278. [Google Scholar] [CrossRef] [PubMed]
- Kotel’nikova, I.M.; Nekrasov, E.V.; Krylov, A.V. Effect of tobacco mosaic virus on phospholipid content and phospholipase D activity in tobacco leaves. Russ. J. Plant Physiol. 2004, 51, 63–69. [Google Scholar] [CrossRef]
- Hyodo, K.; Taniguchi, T.; Manabe, Y.; Kaido, M.; Mise, K.; Sugawara, T.; Taniguchi, H.; Okuno, T. Phosphatidic acid produced by phospholipase D promotes RNA replication of a plant RNA virus. PLoS Pathog. 2015, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinosa, F.; Buhot, N.; Kwaaitaal, M.; Fahlberg, P.; Thordal-Christensen, H.; Ellerstrom, M.; Andersson, M.X. Arabidopsis phospholipase D is involved in basal defense and nonhost resistance to powdery mildew fungi. Plant Physiol. 2013, 163, 896–906. [Google Scholar] [CrossRef] [PubMed]
- Johansson, O.N.; Fahlberg, P.; Karimi, E.; Nilsson, A.K.; Ellerström, M.; Andersson, M.X. Redundancy among phospholipase D isoforms in resistance triggered by recognition of the Pseudomonas syringae effector AvrRpm1 in Arabidopsis thaliana. Front. Plant Sci. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Bargmann, B.O.R.; Laxalt, A.M.; Riet, B.T.; van Schooten, B.; Merquiol, E.; Testerink, C.; Haring, M.A.; Bartels, D.; Munnik, T. Multiple PLDs required for high salinity and water deficit tolerance in plants. Plant Cell Physiol. 2009, 50, 78–89. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Lin, F.; Mao, T.; Nie, J.; Yan, M.; Yuan, M.; Zhang, W. Phosphatidic acid regulates microtubule organization by interacting with MAP65-1 in response to salt stress in Arabidopsis. Plant Cell 2012, 24, 4555–4576. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Nie, J.; Cao, C.; Jin, Y.; Yan, M.; Wang, F.; Liu, J.; Xiao, Y.; Liang, Y.; Zhang, W. Phosphatidic acid mediates salt stress response by regulation of MPK6 in Arabidopsis thaliana. New Phytol. 2010, 188, 762–773. [Google Scholar] [CrossRef] [PubMed]
- Katagiri, T.; Takahashi, S.; Shinozaki, K. Involvement of a novel Arabidopsis phospholipase D, AtPLD, in dehydration-inducible accumulation of phosphatidic acid in stress signalling. Plant J. 2001, 26, 595–605. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Li, M.; Zhang, W.; Welti, R.; Wang, X. The plasma membrane-bound phospholipase Ddelta enhances freezing tolerance in Arabidopsis thaliana. Nat. Biotechnol. 2004, 22, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Marcus, A.I.; Moore, R.C.; Cyr, R.J. The role of microtubules in guard cell function. Plant Physiol. 2001, 125, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Wang, X. Regulatory functions of phospholipase D and phosphatidic acid in plant growth, development, and stress responses. Plant Physiol. 2005, 139, 566–573. [Google Scholar] [CrossRef] [PubMed]
- Wang, X. Multiple forms of phospholipase D in plants: The gene family, catalytic and regulatory properties, and cellular functions. Prog. Lipid Res. 2000, 39, 109–149. [Google Scholar] [CrossRef]
- Jiang, Y.; Wu, K.; Lin, F.; Qu, Y.; Liu, X.; Zhang, Q. Phosphatidic acid integrates calcium signaling and microtubule dynamics into regulating ABA-induced stomatal closure in Arabidopsis. Planta 2014, 239, 565–575. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Qin, C.; Zhao, J.; Wang, X. Phospholipase D 1-derived phosphatidic acid interacts with ABI1 phosphatase 2C and regulates abscisic acid signaling. Proc. Natl. Acad. Sci. USA 2004, 101, 9508–9513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, L.M.; Zhao, Z.; Assmann, S.M. Guard cells: A dynamic signaling model. Curr. Opin. Plant Biol. 2004, 7, 537–546. [Google Scholar] [CrossRef] [PubMed]
- Pappan, K.; Zheng, L.; Krishnamoorthi, R.; Wang, X. Evidence for and characterization of Ca2+ binding to the catalytic region of Arabidopsis thaliana phospholipase Dβ. J. Biol. Chem. 2004, 279, 47833–47839. [Google Scholar] [CrossRef] [PubMed]
- Sang, Y. Phospholipase D and phosphatidic acid-mediated generation of superoxide in Arabidopsis. Plant Physiol. 2001, 126, 1449–1458. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Gu, Y.; Lee, Y.Y.; Yang, Z.; Lee, Y.Y. Phosphatidic acid induces leaf cell death in Arabidopsis by activating the Rho-related small G protein GTPase-mediated pathway of reactive oxygen species generation. Plant Physiol. 2004, 134, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.D.G.; Dangl, J.L. The plant immune system. Nature 2006, 444, 323–329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Q.; Xiao, S. Lipids in salicylic acid-mediated defense in plants: focusing on the roles of phosphatidic acid and phosphatidylinositol 4-phosphate. Front. Plant Sci. 2015, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Ding, P.; Sun, T.; Nitta, Y.; Dong, O.; Huang, X.; Yang, W.; Li, X.; Botella, J.R.; Zhang, Y. Heterotrimeric G proteins serve as a converging point in plant defense signaling activated by multiple receptor-like kinases. Plant Physiol. 2013, 161, 2146–2158. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, S.; Yang, G.; Hayashi, N.; Kaku, H.; Umemura, K.; Iwasaki, Y. Alterations by a defect in a rice G protein α subunit in probenazole and pathogen-induced responses. Plant Cell Environ. 2004, 27, 947–957. [Google Scholar] [CrossRef]
- Trusov, Y.; Sewelam, N.; Rookes, J.E.; Kunkel, M.; Nowak, E.; Schenk, P.M.; Botella, J.R. Heterotrimeric G proteins-mediated resistance to necrotrophic pathogens includes mechanisms independent of salicylic acid-, jasmonic acid/ethylene- and abscisic acid-mediated defense signaling. Plant J. 2009, 58, 69–81. [Google Scholar] [CrossRef] [PubMed]
- Asakura, Y.; Kurosaki, F. Cloning and expression of Dcga gene encoding alpha subunit of GTP-binding protein in carrot seedlings. Biol. Pharm. Bull. 2007, 30, 1800–1804. [Google Scholar] [CrossRef] [PubMed]
- Misra, S.; Wu, Y.; Venkataraman, G.; Sopory, S.K.; Tuteja, N. Heterotrimeric G-protein complex and G-protein-coupled receptor from a legume (Pisum sativum): Role in salinity and heat stress and cross-talk with phospholipase C. Plant J. 2007, 51, 656–669. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.R.J.; Assmann, S.M. Arabidopsis thaliana “extra-large GTP-binding protein” (AtXLG1): A new class of G-protein. Plant Mol. Biol. 1999, 40, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Li, G.J.; Ding, L.; Cui, X.; Berg, H.; Assmann, S.M.; Xia, Y. Arabidopsis extra large G-protein 2 (XLG2) interacts with the Gβ subunit of heterotrimeric G protein and functions in disease resistance. Mol. Plant 2009, 2, 513–525. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.; Sharma, M.; Pandey, G.K. Elucidation of Abiotic Stress Signaling in Plants; Springer: New York, NY, USA, 2015; ISBN 978-1-4939-2539-1. [Google Scholar]
- Lin, D.; Jiang, Q.; Ma, X.; Zheng, K.; Gong, X.; Teng, S.; Xu, J.; Dong, Y. Rice TSV3 encoding Obg-like GTPase protein is essential for chloroplast development during the early leaf stage under cold stress. G3 Genes Genomes Genet. 2017, 8, 253–263. [Google Scholar] [CrossRef]
- Suwastika, I.N.; Ohniwa, R.L.; Takeyasu, K.; Shiina, T. Plant Drg proteins are cytoplasmic small GTPase-Obg homologue. Procedia Environ. Sci. 2014, 20, 357–364. [Google Scholar] [CrossRef]
- Cheung, M.Y.; Xue, Y.; Zhou, L.; Li, M.W.; Sun, S.S.M.; Lam, H.M. An ancient P-loop GTPase in rice is regulated by a higher plant-specific regulatory protein. J. Biol. Chem. 2010, 285, 37359–37369. [Google Scholar] [CrossRef] [PubMed]
- Cheung, M.Y.; Li, M.W.; Yung, Y.L.; Wen, C.Q.; Lam, H.M. The unconventional P-loop NTPase OsYchF1 and its regulator OsGAP1 play opposite roles in salinity stress tolerance. Plant Cell Environ. 2013, 36, 2008–2020. [Google Scholar] [CrossRef] [PubMed]
- Cheung, M.-Y.; Zeng, N.-Y.; Tong, S.-W.; Li, W.-Y.F.; Xue, Y.; Zhao, K.-J.; Wang, C.; Zhang, Q.; Fu, Y.; Sun, Z.; et al. Constitutive expression of a rice GTPase-activating protein induces defense responses. New Phytol. 2008, 179, 530–545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheung, M.-Y.; Li, X.; Miao, R.; Fong, Y.-H.; Li, K.-P.; Yung, Y.-L.; Yu, M.-H.; Wong, K.-B.; Chen, Z.; Lam, H.-M. ATP binding by the P-loop NTPase OsYchF1 (an unconventional G protein) contributes to biotic but not abiotic stress responses. Proc. Natl. Acad. Sci. USA 2016, 113, 2648–2653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nibau, C.; Wu, H.M.; Cheung, A.Y. RAC/ROP GTPases: “hubs” for signal integration and diversification in plants. Trends Plant Sci. 2006, 11, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Schultheiss, H.; Preuss, J.; Pircher, T.; Eichmann, R.; Hückelhoven, R. Barley RIC171 interacts with RACB in planta and supports entry of the powdery mildew fungus. Cell. Microbiol. 2008, 10, 1815–1826. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, G.K.; Iwahashi, H.; Rakwal, R. Small GTPase “Rop”: Molecular switch for plant defense responses. FEBS Lett. 2003, 546, 173–180. [Google Scholar] [CrossRef]
- Sano, H.; Seo, S.; Orudgev, E.; Youssefian, S.; Ishizuka, K.; Ohashi, Y. Expression of the gene for a small GTP binding protein in transgenic tobacco elevates endogenous cytokinin levels, abnormally induces salicylic acid in response to wounding, and increases resistance to tobacco mosaic virus infection. Proc. Natl. Acad. Sci. USA 1994, 91, 10556–10560. [Google Scholar] [CrossRef] [PubMed]
- Lemichez, E.; Wu, Y.; Sanchez, J.P.; Mettouchi, A.; Mathur, J.; Chua, N.H. Inactivation of AtRac1 by abscisic acid is essential for stomatal closure. Genes Dev. 2001, 15, 1808–1816. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zang, A.; Xu, X.; Neill, S.; Cai, W. Overexpression of OsRAN2 in rice and Arabidopsis renders transgenic plants hypersensitive to salinity and osmotic stress. J. Exp. Bot. 2010, 61, 777–789. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Xu, Y.; Wang, X.; Du, C.; Du, J.; Yuan, M.; Xu, Z.; Chong, K. OsRAN2, essential for mitosis, enhances cold tolerance in rice by promoting export of intranuclear tubulin and maintaining cell division under cold stress. Plant Cell Environ. 2011, 34, 52–64. [Google Scholar] [CrossRef] [PubMed]
- Zeng, W.; He, S.Y. A prominent role of the flagellin receptor FLAGELLIN-SENSING2 in mediating stomatal response to Pseudomonas syringae pv tomato DC3000 in Arabidopsis. Plant Physiol. 2010, 153, 1188–1198. [Google Scholar] [CrossRef] [PubMed]
- Ono, E.; Wong, H.L.; Kawasaki, T.; Hasegawa, M.; Kodama, O.; Shimamoto, K. Essential role of the small GTPase Rac in disease resistance of rice. Proc. Natl. Acad. Sci. USA 2001, 98, 759–764. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Ca2+ Sensor | Plant Species | Gene | Stress | Response | Treatment Description | Positive/Negative Regulator | Reference |
|---|---|---|---|---|---|---|---|
| Calmodulin (CaM) | Solanum lycopersicum | SlCaM1 to SlCaM6 | Mechanical wounding, Botrytis cinerea infection | The expressions of all six SlCaM genes were induced by mechanical wounding and Botrytis cinerea infection. Transgenic tomato overexpressing SlCaM2 was more resistant to Botrytis cinerea infection. | Mechanical wounding: the tomato fruit pericarp was manually cut into one inch-pieces using a sharp knife. Botrytis cinerea infection: mechanically injured tomato fruit was inoculated with Botrytis cinerea strain 22B conidial suspension. | SlCaM2 was a positive regulator. The other five genes were not tested. | [15] |
| Arabidopsis thaliana | AtCaM3 | Heat | Arabidopsis thaliana overexpressing AtCaM3 had improved tolerance to heat shock; cam3 mutants were less tolerant to heat shock. | Six-day-old seedlings on phytagel plates supplemented with Murashige and Skoog medium and sucrose were exposed to 45°C for 50 min or 70 min before the recovery at 22°C for six days. | Positive regulator of heat stress. | [16] | |
| Soybean | SCaM-4 | Non-specific fungal elicitor prepared from Fuasrium solani, Phytophthora parasitica pv. nicotianae infection. | The expression of SCaM-4 was induced by a non-specific fungal elicitor prepared from Fuasrium solani to soybean suspension cell culture (SB-P). Overexpression of SCaM-4 in Nicotiana tabacum conferred enhanced resistance to Phytophthora parasitica pv. nicotianae infection. | Soybean suspension cell culture (SB-P) was treated with a non-specific fungal elicitor prepared from Fuasrium solani. Transgenic Nicotiana tabacum overexpressing SCaM-4 was inoculated with Phytophthora parasitica pv. nicotianae by syringe infiltration into leaves. | Positive regulator of Fuasrium solani, Phytophthora parasitica pv. nicotianae infection. | [17] | |
| SCaM-5 | Non-specific fungal elicitor prepared from Fuasrium solani, Phytophthora parasitica pv. nicotianae infection. | The expression of SCaM-5 was induced by a non-specific fungal elicitor prepared from Fuasrium solani to soybean suspension cell culture (SB-P). Overexpression of SCaM-4 in Nicotiana tabacum conferred enhanced resistance to Phytophthora parasitica pv. nicotianae infection. | Soybean suspension cell culture (SB-P) was treated with a non-specific fungal elicitor prepared from Fuasrium solani. Transgenic Nicotiana tabacum overexpressing SCaM-5 was inoculated with Phytophthora parasitica pv. nicotianae by syringe infiltration into leaves. | Positive regulator of Fuasrium solani, Phytophthora parasitica pv. nicotianae infection. | [17] | ||
| Calmodulin-like protein (CML) | Arabidopsis thaliana | AtCML9 | Salt, cold, dehydration, ABA treatment | The expression of AtCML9 was induced by NaCl, cold, and ABA treatments. The expression of AtCML9 was induced by dehydration in the first 10 min, but the expression level decreased from 10 min to 40 min after the treatment. The cml9 mutant had increased sensitivity to ABA and enhanced tolerance to salt and dehydration. | Salt: 10-day-old Arabidopsis thaliana seedlings were transferred to agar plates supplemented with 150 mM NaCl for expression study. Arabidopsis thaliana seeds were sown onto filter paper saturated with 200mM NaCl or 400mM mannitol before imbibition and germination assay. Three-week-old Arabidopsis thaliana was grown on soil and irrigated with 150 mM NaCl every three days, with the phenotype monitored for two weeks. Cold: 10-day old Arabidopsis thaliana seedlings on agar plates were exposed at 4°C under light. ABA: 10-day-old Arabidopsis thaliana seedlings were sprayed with 100 µM ABA for expression study. Arabidopsis thaliana seeds were sown on Murashige and Skoog (MS) medium supplemented with 0.2 µM ABA for cotyledon opening and greening assay. Dehydration: excised leaves of Arabidopsis thaliana were desiccated in growth chamber for expression study. Watered 3-week-old Arabidopsis thaliana grown on soil had the irrigation withheld for 11 days before re-watering. | Negative regulator of salt stress and dehydration | [19] |
| Pseudomonas syringae pv. tomato (Pto) strain DC3000 infection | The expression of AtCML9 was induced by P. syringae pv. tomato (Pto) strain DC3000 infection half an hour and one hour after infection, but repressed three hours after the infection. The expression response of AtCML9 to flagellin application was similar to that after Pto strain DC3000 infection. Arabidopsis thaliana overexpressing AtCLM9 was more resistant to Pto strain DC3000 infection, while cml9 mutant was more sensitive to the infection. | Flagellin application: Arabidopsis thaliana seedlings were grown for 11 days on MS medium. 1 µM flg22 was applied to the fresh MS medium on the ninth day for gene expression study. P. syringae pv. tomato (Pto) strain DC3000 infection: 4-week-old Arabidopsis thaliana was inoculated with Pto strain DC3000 in suspension culture by syringe infiltration on the abaxial side of the leaves. | Positive regulator of P. syringae pv. tomato (Pto) strain DC3000 infection. | [20] | |||
| Arabidopsis thaliana | AtCML37 | Drought | cml37 mutant was highly susceptible to drought stress. | Four-week-old Arabidopsis thaliana were not watered for one week, then re-watered for one week before being not watered for another one week. | Positive regulator of drought stress | [21] | |
| Herbivory | cml37 mutant was more susceptible to herbivory. CML37 deregulates the JA pathway. | Five-week-old Arabidopsis thaliana were subject to feeding by Spodoptera littoralis larvae for 24 or 48 h. | Positive regulator of herbivory | [22] | |||
| AtCML42 | Herbivory, UV-B, drought | cml42 mutant was more resistant to herbivory but less tolerant to UV-B. cml42 mutants had higher levels of ABA under drought stress. | Herbivory: Five-week-old Arabidopsis thaliana was subjected to feeding by Spodoptera littoralis larvae for 24 h. UV-B: Arabidopsis thaliana grown on MS plates for eight days were exposed to UV-B for one hour at the intensity of 100 μW∙cm−2 and then allowed to grow for five weeks. Drought: Three-week-old Arabidopsis thaliana were unwatered for 16 days for survival study, and were unwatered for eight days, rewatered, and then unwatered for eight days for measuring ABA level. | Positive regulator of UV-B stress, negative regulator of herbivory. The drought-resistant phenotype was uncertain. | [23] | ||
| Solanum habrochaites | ShCML44 | Cold, drought, osmotic stress, salt, ABA and JA treatments | The expression of ShCML44 was induced by cold, drought, osmotic stress, salt, and ABA treatments. Transgenic tomato plants overexpressing ShCML44 were more tolerant to cold, drought, and salinity stresses. | Six-week-old seedlings were put into a growth chamber for five days as an adaptation period before treatment. Cold: seedlings were transferred to a growth chamber at 4 °C. Drought: seedlings were uprooted, washed, and dehydrated on filter paper. Salt: seedlings were irrigated with 200 mM NaCl. ABA treatment: seedlings were sprayed with 100 µM ABA. JA: seedlings were sprayed with 100 µM MeJA. | Positive regulator of cold, drought, and salinity stresses. | [18] | |
| Calcineurin-B-like protein (CBL) | Arabidopsis thaliana | AtCBL5 | Drought, salt | Overexpression of AtCBL5 in Arabidopsis thaliana improved tolerance to drought and salt stresses. | Drought: 4-week-old Arabidopsis thaliana grown on potting soil were unwatered for three weeks. Salt stress: 4-week-old Arabidopsis thaliana grown on potting soil were treated with 300 mM NaCl once every three days for two weeks. | Positive regulator of drought and salt stresses. | [26] |
| Brassica napus | BnCBL1 | Salt stress, osmotic stress, low inorganic phosphate (Pi), ABA treatment. | The expression of BnCBL1 was induced by salt stress, osmotic stress, low Pi, and ABA treatment. Overexpression of BnCBL1 conferred improved tolerance to salt stress and low Pi. | Salt stress: 1-week-old seedlings of Brassica napus were transferred to MS medium containing 150 mM NaCl for expression study. Transgenic Arabidopsis thaliana seedlings were transferred to MS medium containing 0 to 250 mM NaCl for stress tolerance study. Osmotic stress: 1-week-old seedlings of Brassica napus were transferred to MS medium containing 200 mM mannitol for expression study. ABA treatment: 1-week-old seedlings of Brassica napus were transferred to MS medium containing 100 μM ABA for expression study. Low Pi: 1-week-old seedlings of Brassica napus were transferred to MS medium containing 10 μM phosphate for expression study. Six-day-old transgenic Arabidopsis thaliana seedlings were transferred to 50 μM low phosphate (LP) medium for a few days. | Positive regulator of salt stress and low Pi. | [25] | |
| Arabidopsis thaliana | AtCBL10 | Salt | cbl10 mutant was more sensitive to salt stress. | Four-week-old Arabidopsis thaliana plants were treated with 300 mM NaCl once every three days for two weeks. | Positive regulator of salt stress. | [27] | |
| Solanum lycopersicum | SlCBL10 | Pseudomonas syringae pv tomato (Pto) strain DC3000 infection | Silencing of SlCBL10 led to improved resistance to Pto strain DC3000 infection. | Solanum lycopersicum plants were infected with Pto strain DC3000. | Negative regulator of Pto strain DC3000 infection. | [28] | |
| Calcium-dependent protein kinase (CPK) | Arabidopsis thaliana | AtCPK10 | Drought | cpk10 mutant plants were more sensitive to drought stress. Overexpression of AtCPK10 conferred improved tolerance to drought stress. | One-week-old seedlings were grown for 20 days with or without watering. | Positive regulator of drought stress. | [32] |
| AtCPK6 | PEG-induced drought stress, salt | Overexpression of AtCPK6 in Arabidopsis thaliana conferred tolerance to drought and salt stress. | Drought stress: 3-week-old plants grown in potting soil were watered with 15% polyethylene glycol (PEG) for two weeks. Salt stress: 3-week-old plants grown in potting soil were watered with 250 mM NaCl for two weeks. | Positive regulator of drought stress and salt stress. | [34] | ||
| Zea mays | ZmCPK4 | Drought, ABA | Overexpression of ZmCPK4 in Arabidopsis thaliana conferred tolerance to drought stress and increased sensitivity to ABA. | ABA treatment: Arabidopsis thaliana seeds were planted on MS medium supplemented with 0, 1, 2, or 5 µM ABA for germination study. Four-day-old Arabidopsis thaliana seedlings were transferred to MS medium with 50 µM ABA for phenotypic study. Rosette leaves of Arabidopsis thaliana were treated with 10 µM ABA under light for two hours for stomatal aperture study. Drought: 4-week-old Arabidopsis thaliana plants were subject to drought stress by withholding water for 25 days. | Positive regulator of drought stress and ABA sensitivity. | [36] | |
| Oryza sativa | OsCPK9 | Drought, ABA, salt, osmotic stress. | The expression of OsCPK9 was induced by ABA, NaCl and osmotic stress. Overexpression of OsCPK9 in Oryza sativa conferred increased tolerance to drought stress. Silencing of OsCPK9 led to reduced tolerance to drought stress. | ABA: 2-week-old Oryza sativa seedlings were transferred to plastic boxes containing 100 μM ABA for 24 h for expression study. Salt: 2-week-old Oryza sativa seedlings were transferred to plastic boxes containing 200 mM NaCl for 24 h for expression study. Osmotic stress: 2-week-old Oryza sativa seedlings were transferred to plastic boxes containing 20% PEG-6000 for 24 h for expression study. Drought: 3-week-old Oryza sativa seedlings were deprived of water for 20 or 27 days before recovery with watering for three days for tolerance study. | Positive regulator of drought stress and ABA sensitivity. | [37] | |
| OsCPK12 | Salt, Magnaporthe grisea infection | Overexpression of OsCPK12 in Oryza sativa conferred increased tolerance to salt stress and increased sensitivity to ABA. Silencing and mutation of OsCPK12 led to increased sensitivity to salt stress. Overexpression of OsCPK12 in Oryza sativa conferred increased sensitivity to blast fungus, Ina86-137. | Salt stress: 2-week-old seedlings were exposed to 200 mM NaCl solution for five days. ABA treatment: 5-day-old seedlings were transferred to Yoshida’s nutrient solution supplemented with 0.5 µM ABA for two weeks. Magnaporthe grisea infection: agar slice with Magnaporthe grisea was attached to wounded leaves of 2–4-week-old Oryza sativa seedlings. | Positive regulator of salt stress, negative regulator of M. grisea infection. | [38] | ||
| Calcium/calmodulin-dependent protein kinase (CCaMK) | Glycine soja | GsCBRLK | Cold, ABA, salt, osmotic stress | The expression of GsCBRLK was induced in leaf by cold, ABA, NaCl, and PEG treatments. The expression of GsCBRLK in root had diverse responses to cold, ABA, NaCl and PEG. Overexpression of GsCBRLK in Arabidopsis thaliana led to improved tolerance to NaCl and reduced sensitivity to ABA. | Cold: 1-month-old soybean seedlings were incubated at 4°C for 0.5, 1, 3, or 6 h for expression study. ABA treatment: 1-month-old soybean seedlings were treated with 100 µM ABA for 0.5, 1, 3, or 6 h for expression study. Twenty-one-day-old transgenic Arabidopsis thaliana plants were treated with 100 µM ABA for stress response study. Salt: 1-month-old soybean seedlings were treated with 200 mM NaCl for 0.5, 1, 3, or 6 h for expression study. 21-day-old transgenic Arabidopsis thaliana plants were treated with 200 mM NaCl for stress response study. Osmotic stress: 1-month-old soybean seedlings were treated with 30% PEG 6000 for 0.5, 1, 3, or 6 h for expression study. | Positive regulator of salt stress but negative regulator of ABA sensitivity | [29] |
| Solanum lycopersicum | SlCCaMK | Sclerotinia sclerotiorum infection, Pseudomonas syringae pv. tomato (Pto) DC3000 infection, Xanthomonas oryzae pv. oryzae (Xoo) infection | The expression of SlCCaMK was induced in leaf by S. sclerotiorum infection but repressed in leaf by Pto DC3000 /Xoo infection. Knock-down of SlCCaMK led to reduced resistance to S. sclerotiorum and Pto DC3000 infections. | Sclerotinia sclerotiorum infection: S. sclerotiorum was inoculated into leaves of 7–8-week-old Solanum lycopersicum for expression study. Pto DC3000 infection: bacterial suspension culture was infiltrated into leaves for stress response study. Xoo infection: bacterial suspension was infiltrated into leaves for stress response study. | Positive regulator of S. sclerotiorum and Pto DC3000 infections. | [40] | |
| Triticum aestivum | TaCCaMK | Salt, PEG-induced drought stress, ABA treatment | The expression of TaCCaMK was reduced by NaCl, PEG, and ABA treatments. Overexpression of TaCCaMK in Arabidopsis thaliana led to decreased sensitivity to ABA and improved tolerance to NaCl. | Salt: 7-day-old Triticum aestivum seedlings were treated with 200 mM NaCl in Hoagland’s solution for expression study. Seeds of transgenic Arabidopsis thaliana was germinated on MS agar supplemented with 0, 50, 100, 150 or 200 mM NaCl for a germination assay. Four-day-old seedlings of transgenic Arabidopsis thaliana grown on MS agar were transferred to MS agar supplemented with 0, 100, 170, or 200 mM NaCl for 10 days for phenotypic study. PEG-induced drought stress: 7-day-old Triticum aestivum seedlings were treated with 16% PEG in Hoagland solution for expression study. ABA treatment: 7-day-old Triticum aestivum seedlings were treated with 5 µM ABA in Hoagland’s solution for expression study. Seeds of transgenic Arabidopsis thaliana were germinated on MS agar supplemented with 0, 1, or 3 µM ABA for germination assay. Four-day-old seedlings of transgenic Arabidopsis thaliana grown on MS agar were transferred to MS agar supplemented with 0, 5, 10, 20, 40, or 80 µM ABA for 10 days for phenotypic study. | Positive regulator of salt stress, negative regulator of ABA sensitivity. | [43] |
| Plant | ERF | Induction by | Target Gene Promoter Sequence | Results of Overexpression | References |
|---|---|---|---|---|---|
| Pepper | CaPF1 | Xanthomonas axonopodis | GCC box/DRE sequence | Resistance to disease and cold | [88] |
| Cotton | GhERF6 | Ethylene, ABA, salt, cold, and drought | GCC box | Resistance to salt, cold & drought | [90] |
| Soybean | GmERF3 | Ethylene, ABA, SA, JA, Soybean Mosaic Virus, dehydration, salt | GCC box/DRE sequence | Resistance to disease, drought and high salt, induction of PR genes | [78] |
| Tomato | JERF1 | Ethylene, MeJA, ABA, and salt | GCC box/DRE sequence | Resistance to salt and cold, induction of the ABA biosynthesis-related gene NtSDR, accumulation of ABA | [81] |
| JERF3 | Ethylene, JA, ABA, cold, salt | GCC box/DRE sequence | Resistance to salt, induction of PR genes | [77] | |
| LeERF3b (class II) | Ethylene, cold, drought | GCC box/DRE sequence/C-repeat | Cold tolerance | [86] | |
| SlERF5 | High salinity, drought, flooding, wounding and cold | GCC box | Resistance to drought and salt | [80] | |
| Wheat | TaERF3 | Salt, polyethylene glycol (PEG) | GCC box | Resistance to drought and salt | [79] |
| TaERF7 | Drought, salt, MeJA, ethylene and ABA. Depressed by cold | GCC box | Resistance to salt, accumulation of soluble carbohydrates and decreased concentration of malondialdehyde, susceptibility to cold | [89] | |
| TaPIE1 | Ethylene, Rhizoctonia cerealis and freezing stresses | GCC box | Resistance to R. cerealis and freezing stress, higher accumulation of soluble sugars and proline | [87] | |
| Rice | OsWR1 | Drought, ABA and salt | GCC box/DRE sequence | Induction of wax/cutin synthesis genes | [91] |
| OsEREBP1 | Xanthomonas oryzae | GCC box | Resistance to cold, salinity, drought & submergence, induction of genes for JA and ABA biosynthesis, lipid metabolism, alcohol dehydrogenases (related to submergence), and PR genes | [75,92] | |
| Tobacco | OPBP1 | Cryptogein, salt, ethephon, MeJA, cycloheximide. | GCC box | Resistance to pathogen and salt stress, induction of PR genes | [93] |
| Tsi1 | Salt, ethephon, SA | GCC box/DRE sequence | Resistance to pathogen and salt, induction of PR genes | [76] |
| Class of G-Protein | Plant Species | Gene | Stress | Response | Treatment Description | Positive/Negative Regulator | Reference |
|---|---|---|---|---|---|---|---|
| Heterotrimeric G-protein α subunit | Arabidopsis thaliana | AGA1 | Pseudomonas syringae pv. tomato (Pto) DC3000 infection | aga1 mutant failed to close stomata after coronatine treatment, and thus exhibited higher susceptibility to Pto DC3000 infection | Five-week-old seedlings were dipped upside down in coronatine (COR)-deficient mutant Pto DC3000 bacterial suspension for a few seconds | Positive regulator of coronatine-induced stomatal closure, and in turn, stomatal defense against Pto DC3000 infection. | [152] |
| Oryza sativa | D1 | Xanthomonas oryzae pv. oryzae infection | d1 mutant, which is deficient in G-protein α subunit failed to defend against X. oryzae pv. oryzae infection and exhibited delayed induction of probenazole-inducible protein (PBZ1) | At four weeks after sowing, the uppermost fully opened leaves were inoculated by the double-needle pricking and cutting method | Positive regulator of bacterial blight resistance. Acts through the phosphorylation of both the 48-kDa putative MAPK and the 55-kDa putative CDPK, and induces PBZ1 production | [132] | |
| Heterotrimeric G-protein β subunit | Arabidopsis thaliana | AGB1 | Hyaloperono-spora arabidopsidis Noco2 and Pseudomonas syringae pv. tomato (Pto) DC3000 infections | agb1 mutant is identified to suppress cell death and defense response phenotypes of bir1-1 mutant | H. arabidopsidis infection: 2-week-old seedlings were sprayed with spore suspensions of H. arabidopsidis Noco2 at a concentration of 50,000 spores per mL water Pto DC3000 infection: 5- to 6-week-old seedlings pre-infiltrated with 1 µM flg22, 1 µM elf18, or 200 µg∙mL−1 chitin (PAMPs) followed by infiltration with Pto DC3000 suspension | Positive regulator of PAMP-trigged responses. Functions downstream of BIR1-1 and plays positive role in salicylic acid (SA) level | [131] |
| Fusarium oxysporum f. sp. conglutinans and Alternaria brassicicola (isolate UQ4273) infections | agb1 mutant is more susceptible to necrotrophic pathogens (F. oxysporum and A. brassicicola). At the initial phase of infection, AGB1 works in a pathway independent of SA, JA/ethylene and ABA signalling. | F. oxysporum f. sp. conglutinans infection: 2-week-old plants removed from soil were immersed in F. oxysporum spore solution (106 spores∙mL−1) for 30–60 s, and then replanted in fresh autoclaved soil. Phenotype was recorded by counting the number of yellow-veined leaves. A plant was considered dead when all leaves had turned yellow. A. brassicicola infection: 3-week-old Arabidopsis thaliana seedlings were grown in 100% humidity chambers for five days before inoculation with A. brassicicola (isolate UQ4273) spore suspension. Number of leaves with spreading lesions was recorded at five days post-inoculation. | Positive regulator against F. oxysporum and A. brassicicola infections through pathways both dependent on and independent of SA, JA and ethylene. | [133] | |||
| Heterotrimeric G-protein γ subunit | Arabidopsis thaliana | AGG1 and AGG2 | Pseudomonas syringae pv. tomato (Pto) DC3000 | agg1 agg2 double mutant fails to exhibit PAMP-triggered responses | Five- to 6-week-old seedlings were pre-infiltrated with 1 µM flg22, 1 µM elf18, or 200 µg∙mL−1 chitin (PAMPs), and then infiltrated with Pto DC3000 suspension | Positive regulator of PAMP-triggered responses | [131] |
| Extra-large G-protein | Arabidopsis thaliana | XLG2 | Infection by virulent and avirulent Pseudomonas strains | xlg2 mutant exhibits higher susceptibility towards both virulent and avirulent Pseudomonas strains | Leaves of 5-week-old seedlings were infiltrated with 3 × 104 cfu/mL (for virulent Pseudomonas syringae pv. tomato DC3000) or 1 × 105 cfu/mL (for avirulent Pto avrRpm1 and P. syringae pv. phaseolicola strains) bacterial suspension | Positive regulator of non-host basal resistance | [137] |
| Obg protein | Oryza sativa | TSV3 | Cold stress | tsv3 mutant exhibits albino phenotype under cold stress at early-leaf stages | Seedlings were grown in growth chamber with 12 h light and 12 h dark and at constant temperature of either 20 °C (cold treatment) or 30 °C. Phenotypes were observed at 3- and 4-leaf stages. | Positive regulator of cold stress, associated with biogenesis of chloroplast ribosome 50S subunit at 3-leaf stage under cold stress | [139] |
| DRG protein | Arabidopsis thaliana | DRG1-3 | Heat | DRG1-3 expression is induced by heat stress after 1-4 h | Arabidopsis wild type seeds were germinated and grown on Jiffy medium under continuous light at 23 °C constant temperature, and heat shock was performed for up to four hours | Positive regulator of heat stress response | [140] |
| YchF protein | Oryza sativa and Arabidopsis thaliana | OsYchF1 and AtYchF1 | Xanthomonas oryzae pv. oryzae (Xoo) and Pseudomonas syringae pv. tomato (Pto) DC3000 infections | Ectopic expression and over-expression of OsYchF1 and AtYchF1 in Arabidopsis enhanced susceptibilities to bacterial infection. Arabidopsis AtYchF1- knockdown mutant exhibits enhanced resistance. | Eight-week-old seedlings were inoculated with Xoo and Pto DC3000 via syringe infiltration on abaxial surface of leaves. Disease lesions, pathogen titers and expressions of PR genes were examined three days after inoculation. | Negative regulator of resistance against Pto DC3000 | [141] |
| Salt stress | Ectopic expression and over-expression of OsYchF1 and AtYchF1 in Arabidopsis enhances sensitivity to salt stress. Arabidopsis AtYchF1-knockdown mutant is more salt-tolerant | Ten-day-old seedlings grown on MS medium were transferred onto MS medium supplemented with 150 mM NaCl. Chlorosis phenotype, chlorophyll content, lipid peroxidation, and salt-responsive gene expressions were recorded after 10 days of salt treatment | Negative regulator of salt stress | [142] | |||
| Small G-protein | Oryza sativa | OsRAC1 | Magnaporthe grisea and Xanthomonas oryzae pv. oryzae infections | Over-expression of OsRac1 shows hypersensitive responses and increases resistance against a virulent race of rice blast fungus (M. grisea, race 007) and bacterial blight (X. oryzae pv. oryzae, race 1) | Magnaporthe grisea inoculation was performed via press-injured spots of 2.0 mm diameter made with a specially designed pressing machine (Fujihara co.) on leaf blades of 60-day-old seedlings. A piece of agar covered with spores was then placed on the injured spots. Xanthomonas oryzae pv. oryzae inoculation was done at panicle initiation to bolting stage by clipping off the leaves at 2–3 cm from leaf tip with sterilized scissors and dipping the clipped edge of leaves in the bacterial suspension (approximately 109 cfu/mL). | Positive regulator of resistance to both necrotrophic and biotrophic pathogens | [153] |
| Nicotiana tabacum | RGP1 | Tobacco mosaic virus (TMV) infection | Over-expression of a Ras-related small G-protein, RGP1, shows higher production of salicylic acid and PR proteins and increased resistance towards TMV | Wounding was made on fully expanded fifth leaves (leaf 5) by punching out leaf discs from seedlings at 16-leaves stage. For quantitation of SA and SAG, the target leaves were wounded by gentle rubbing of the upper epidermis with wet carborundum. For TMV inoculation, upper fully expanded leaves were detached and inoculated with TMV (10 viral particles /g/mL) by using carborundum (Mesh 600) and incubated at 20 °C under continuous illumination. | Positive regulator of resistance against tobacco mosaic virus infection | [148] | |
| Arabidopsis thaliana | AtRac1 | Drought and ABA treatments | rac1 mutant fails to interrupt the ABA-mediated actin skeleton in guard cells during drought condition and thus blocks stomatal closure | 10–50 μM ABA was applied to 2-week-old seedlings for 30 min after 48 h induction with 10 μM DEX in white-light condition. Widths and lengths of stomatal opening were measured using a LSM410 inverted confocal microscope. | Positive regulator of stomatal closure during drought condition or ABA treatment | [149] | |
| Oryza sativa and Arabidopsis thaliana | OsRAN2 | Salt, osmotic stress, and ABA treatments | Over-expression of OsRAN2 in both rice and Arabidopsis lead to shorter and fewer roots and smaller leaves under salt and osmotic stress. | Transgenic Arabidopsis plants with ectopically expressed OsRAN2 were grown on MS agar plates supplemented with 100 mM NaCl, 100 mM KCl. Phenotypes were recorded after two weeks. | Negative regulator of salt and osmotic stress, likely acting through ABA signalling pathway | [150] | |
| Cold stress | OsRAN2 expression is induced during cold stress. OsRAN2-overexpressing lines show higher survival rates (seedlings being able to grow and stay green) under cold treatment compared to wild type | Transgenic rice lines over-expressing OsRAN2 were germinated in water (as control) or in water containing 100 mM NaCl, 100 mM KCl, 10% PEG 6000, or 10 μM ABA. Phenotypes were observed after five and 10 days. Two-week-old OsRAN2-overexpressing rice lines at the tetraphyllous leaf stage were treated at 4 °C for 72 h. The seedlings were allowed to recover in normal greenhouse conditions for two weeks. | Positive regulator of cold tolerance, by maintaining cell division via promoting the export of intra-nuclear tubulin at the end of mitosis, thus maintaining normal nuclear envelope under cold stress | [151] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ku, Y.-S.; Sintaha, M.; Cheung, M.-Y.; Lam, H.-M. Plant Hormone Signaling Crosstalks between Biotic and Abiotic Stress Responses. Int. J. Mol. Sci. 2018, 19, 3206. https://doi.org/10.3390/ijms19103206
Ku Y-S, Sintaha M, Cheung M-Y, Lam H-M. Plant Hormone Signaling Crosstalks between Biotic and Abiotic Stress Responses. International Journal of Molecular Sciences. 2018; 19(10):3206. https://doi.org/10.3390/ijms19103206
Chicago/Turabian StyleKu, Yee-Shan, Mariz Sintaha, Ming-Yan Cheung, and Hon-Ming Lam. 2018. "Plant Hormone Signaling Crosstalks between Biotic and Abiotic Stress Responses" International Journal of Molecular Sciences 19, no. 10: 3206. https://doi.org/10.3390/ijms19103206
APA StyleKu, Y. -S., Sintaha, M., Cheung, M. -Y., & Lam, H. -M. (2018). Plant Hormone Signaling Crosstalks between Biotic and Abiotic Stress Responses. International Journal of Molecular Sciences, 19(10), 3206. https://doi.org/10.3390/ijms19103206