Effect of Roasting Levels and Drying Process of Coffea canephora on the Quality of Bioactive Compounds and Cytotoxicity
Abstract
:1. Introduction
2. Results and Discussion
2.1. Physicochemical Results
2.1.1. Colorimetry
2.1.2. Bioactive Properties of Aqueous Coffee Extract
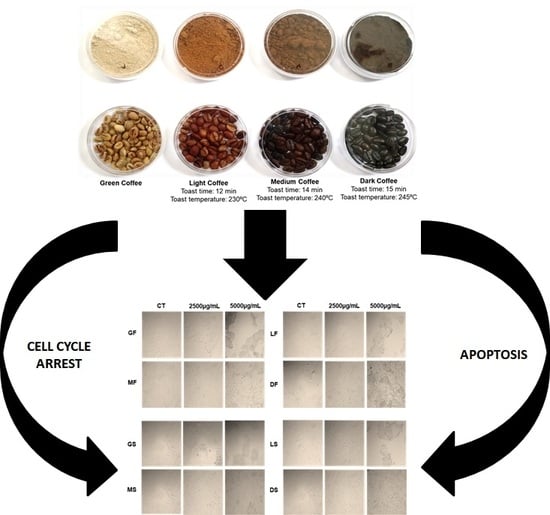
2.2. Cell Results
3. Materials and Methods
3.1. Samples
3.2. Bean Processing and Production of Extracts
3.3. Colorimetric Analysis
3.4. Antioxidant Activity Analyses
3.4.1. Trolox Equivalent Antioxidant Capacity (ABTS/TEAC)
3.4.2. DPPH Assay
3.4.3. Ferric Reducing Ability (FRAP)
3.4.4. ORAC
3.5. Total Phenolic Assay
3.6. High Performance Liquid Chromatography(HPLC) Analyses
3.6.1. Amino Acids
3.6.2. Reducing Sugars
3.6.3. Caffeine
3.6.4. Chlorogenic Acids
3.7. Cell Culture and Treatment Protocol
3.7.1. Cell Viability
3.7.2. Cell Cycle Analysis
3.7.3. Detection of Apoptosis by Annexin V-FITC
3.8. Statistical Analyses
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Esquivel, P.; Jiménez, V.M. Functional properties of coffee and coffee by-products. Food Res. Int. 2012, 46, 488–495. [Google Scholar] [CrossRef]
- Xie, C.; Cui, L.; Zhu, J.; Wang, K.; Sun, N.; Sun, C. Coffee consumption and risk of hypertension: A systematic review and dose–response meta-analysis of cohort studies. J. Hum. Hypertens. 2018. [Google Scholar] [CrossRef] [PubMed]
- Bizzo, M.L.G.; Farah, A.; Kemp, J.A.; Scancetti, L.B. Highlights in the History of Coffee Science Related to Health. In Coffee in Health and Disease Prevention; Elsevier: Amsterdam, The Netherlands, 2015; pp. 11–17. ISBN 9780124095175. [Google Scholar]
- Riedel, A.; Hochkogler, C.M.; Lang, R.; Bytof, G.; Lantz, I.; Hofmann, T.; Somoza, V. N-Methylpyridinium, a degradation product of trigonelline upon coffee roasting, stimulates respiratory activity and promotes glucose utilization in HepG2 cells. Food Funct. 2014, 5, 454. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.A.; Maalik, A.; Murtaza, G. Inhibitory mechanism against oxidative stress of caffeic acid. J. Food Drug Anal. 2016, 24, 695–702. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.; Ahn, H.R.; Jo, H.; Kim, K.-A.; Lee, E.H.; Lee, K.W.; Jung, S.H.; Lee, C.Y. Chlorogenic Acid and Coffee Prevent Hypoxia-Induced Retinal Degeneration. J. Agric. Food Chem. 2014, 62, 182–191. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.; Tan, L.; Zhao, J.; Hu, R.; Lu, M. Characterization of Fatty Acid, Amino Acid and Volatile Compound Compositions and Bioactive Components of Seven Coffee (Coffea robusta) Cultivars Grown in Hainan Province, China. Molecules 2015, 20, 16687–16708. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naveed, M.; Hejazi, V.; Abbas, M.; Kamboh, A.A.; Khan, G.J.; Shumzaid, M.; Ahmad, F.; Babazadeh, D.; FangFang, X.; Modarresi-Ghazani, F.; et al. Chlorogenic acid (CGA): A pharmacological review and call for further research. Biomed. Pharmacother. 2018, 97, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Farah, A. Coffee Constituents. In Coffee: Emerging Health Effects and Disease Prevention; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2012; pp. 21–58. ISBN 978-1-84569-556-9. [Google Scholar]
- Rodrigues, N.P. Café: Compostos Bioativos e Capacidade Desativadora de Espécies Reativas de Oxigênio e de Nitrogênio In Vitro; UNICAMP: São Paulo, Braizl, 2013. [Google Scholar]
- Rincon, M.A.D. Fracionamento do Óleo de Café Verde por Destilação Molecular; UNICAMP: São Paulo, Braizl, 2011. [Google Scholar]
- Yılmaz, B.; Acar-Tek, N. Turkish cultural heritage: A cup of coffee. J. Ethn. Foods 2017, 4, 213–220. [Google Scholar] [CrossRef]
- do Livramento, K.G.; Borém, F.M.; José, A.C.; Santos, A.V.; do Livramento, D.E.; Alves, J.D.; Paiva, L.V. Proteomic analysis of coffee grains exposed to different drying process. Food Chem. 2017, 221, 1874–1882. [Google Scholar] [CrossRef] [PubMed]
- Benetou, V.; Orfanos, P.; Lagiou, P.; Trichopoulos, D.; Boffetta, P.; Trichopoulou, A. Vegetables and fruits in relation to cancer risk: Evidence from the Greek EPIC cohort study. Cancer Epidemiol. Biomark. Prev. Publ. Am. Assoc. Cancer Res. Cosponsored Am. Soc. Prev. Oncol. 2008, 17, 387–392. [Google Scholar] [CrossRef] [PubMed]
- INCA. Estimativa 2018 de Incidência de Câncer no Brasil, 2018th ed.; INCA: Rio de Janeiro, Braizl, 2018. [Google Scholar]
- Murad, L.D. Efeito dos Ácidos Fenólicos e 5-Cafeoilquínico Sobre o Crescimento de Adenocarcinoma de Cólon Humano; Universidade Federal do Estado do Rio de Janeiro—Unirio: Rio de Janeiro, Brazil, 2013. [Google Scholar]
- Magnani, C.; Isaac, V.L.B.; Correa, M.A.; Salgado, H.R.N. Caffeic acid: A review of its potential use in medications and cosmetics. Anal. Methods 2014, 6, 3203–3210. [Google Scholar] [CrossRef]
- Fraser, B.; Murphy, C.; Bunting, F. Real World Color Management, 2nd ed.; Pearson Education: London, UK, 2005; ISBN 0-13-277795-9. [Google Scholar]
- Bicho, N.C.; Leitão, A.E.; Ramalho, J.C.; Lidon, F.C. Utilização dos parâmetros de cor para avaliação do café torrado. Food Sci. Technol. 2012, 32, 436–442. [Google Scholar] [CrossRef]
- Schmidt, C.A.P.; Miglioranza, É.; Prudêncio, S.H. Influence of roasting and milling on consumers coffee preference at Paraná west-Brazil. Ciênc. Rural 2008, 38, 1111–1117. [Google Scholar] [CrossRef]
- Knopp, S.; Bytof, G.; Selmar, D. Influence of processing on the content of sugars in green Arabica coffee beans. Eur. Food Res. Technol. 2006, 223, 195–201. [Google Scholar] [CrossRef]
- Preedy, V.R. Coffee in Health and Disease Prevention; Academic Press: London, UK, 2015; ISBN 9780124095175. [Google Scholar]
- Kang, K.A.; Lee, K.H.; Zhang, R.; Piao, M.; Chae, S.; Kim, K.N.; Jeon, Y.J.; Park, D.B.; You, H.J.; Kim, J.S.; et al. Caffeic acid protects hydrogen peroxide induced cell damage in WI-38 human lung fibroblast cells. Biol. Pharm. Bull. 2006, 29, 1820–1824. [Google Scholar] [CrossRef] [PubMed]
- Nursten, H.E. The Maillard Reaction: Chemistry, Biochemistry and Implications; Royal Society of Chemistry: Cambridge, UK, 2005; ISBN 978-0-85404-964-6. [Google Scholar]
- Murkovic, M.; Derler, K. Analysis of amino acids and carbohydrates in green coffee. J. Biochem. Biophys. Methods 2006, 69, 25–32. [Google Scholar] [CrossRef] [PubMed]
- López-Galilea, I.; De Peña, M.P.; Cid, C. Correlation of selected constituents with the total antioxidant capacity of coffee beverages: Influence of the brewing procedure. J. Agric. Food Chem. 2007, 55, 6110–6117. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Hernández, L.M.; Chávez-Quiroz, K.; Medina-Juárez, L.Á.; Gámez Meza, N. Phenolic characterization, melanoidins, and antioxidant activity of some commercial coffees from Coffea arabica and Coffea canephora. J. Mex. Chem. Soc. 2012, 56, 430–435. [Google Scholar]
- Liu, Y.; Kitts, D.D. Confirmation that the Maillard reaction is the principle contributor to the antioxidant capacity of coffee brews. Food Res. Int. 2011, 44, 2418–2424. [Google Scholar] [CrossRef]
- Yashin, A.; Yashin, Y.; Wang, J.Y.; Nemzer, B. Antioxidant and Antiradical Activity of Coffee. Antioxidants 2013, 2, 230–245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kulisic, T.; Radonic, A.; Katalinic, V.; Milos, M. Use of different methods for testing antioxidative activity of oregano essential oil. Food Chem. 2004, 85, 633–640. [Google Scholar] [CrossRef]
- Pokorná, J.; Venskutonis, P.R.; Kraujalyte, V.; Kraujalis, P.; Dvořák, P.; Tremlová, B.; Kopřiva, V.; Ošťádalová, M. Comparison of different methods of antioxidant activity evaluation of green and roast C. Arabica and C. Robusta coffee beans. Acta Aliment. 2015, 44, 454–460. [Google Scholar] [CrossRef] [Green Version]
- Nebesny, E.; Budryn, G. Antioxidative activity of green and roasted coffee beans as influenced by convection and microwave roasting methods and content of certain compounds. Eur. Food Res. Technol. 2003, 217, 157–163. [Google Scholar] [CrossRef]
- Perrone, D.; Farah, A.; Donangelo, C.M.; de Paulis, T.; Martin, P.R. Comprehensive analysis of major and minor chlorogenic acids and lactones in economically relevant Brazilian coffee cultivars. Food Chem. 2008, 106, 859–867. [Google Scholar] [CrossRef]
- Crozier, A.; Ashihara, H.; Tomás-Barbéran, F. Teas, Cocoa and Coffee; Blackwell Publishing Ltd.: Hoboken, NJ, USA, 2007; Volume 21, ISBN 978-1-4443-4707-4. [Google Scholar]
- Halal, S.L. El Composição, Processamento E Qualidade Do Café; Universidade Federal de Pelotas: Rio Grande do Sul, Brazil, 2008. [Google Scholar]
- Nogueira, M.; Trugo, L.C. Distribuição de isômeros de ácido clorogênico e teores de cafeína e trigonelina em cafés solúveis brasileiros. Ciênc. E Tecnol. Aliment. 2003, 23, 296–299. [Google Scholar] [CrossRef] [Green Version]
- Perrone, D.; Farah, A.; Donangelo, C.M. Influence of coffee roasting on the incorporation of phenolic compounds into melanoidins and their relationship with antioxidant activity of the brew. J. Agric. Food Chem. 2012, 60, 4265–4275. [Google Scholar] [CrossRef] [PubMed]
- Murthy, P.S.; Naidu, M.M. Recovery of Phenolic Antioxidants and Functional Compounds from Coffee Industry By-Products. Food Bioprocess Technol. 2012, 897–903. [Google Scholar] [CrossRef]
- Ramalakshmi, K.; Mohan, L.J.; Takano-ishikawa, Y.; Goto, M. Bioactivities of low-grade green coffee and spent coffee in different in vitro model systems. Food Chem. 2009, 115, 79–85. [Google Scholar] [CrossRef]
- Del Castollo, M.D.; Ames, J.M.; Gordon, M.H. Effect of Roasting on the Antioxidant Activity of Coffee Brews. J. Agric. Food Chem. 2002, 50, 3698–3703. [Google Scholar] [CrossRef]
- Camacho-cristóbal, J.J.; Anzellotti, D.; González-fontes, A. Changes in phenolic metabolism of tobacco plants during short-term boron deficiency. Plant Physiol. Biochem. 2002, 40, 997–1002. [Google Scholar] [CrossRef]
- Farah, A.; Donangelo, C.M. Phenolic compounds in coffee. Braz. J. Plant Physiol. 2006, 18, 23–36. [Google Scholar] [CrossRef]
- Guillot, F.; Malnoe, A.; Standler, R. Antioxidant properties of novel tetraoxygenated phenylindan isomers fomred during thermal decomposition of caffeic acid. Agric. Food Chem. 1996, 44, 2503–2510. [Google Scholar] [CrossRef]
- Clifford, M.N.; Knight, S.; Surucu, B.; Kuhnert, N. Characterization by LC-MS n of Four New Classes of Chlorogenic Acids in Green Coffee Beans: Dimethoxycinnamoylquinic Acids, Diferuloylquinic Acids, Caffeoyl-dimethoxycinnamoylquinic Acids, and Feruloyl-dimethoxycinnamoylquinic Acids. J. Agric. Food Chem. 2006, 54, 1957–1969. [Google Scholar] [CrossRef] [PubMed]
- Andueza, S.; Manzocco, L.; Paz de Peña, M.; Cid, C.; Nicoli, C. Caffeic acid decomposition products: Antioxidants or pro-oxidants? Food Res. Int. 2009, 42, 51–55. [Google Scholar] [CrossRef] [Green Version]
- Russo, G.; Campisi, D.; Di Mauro, M.; Regis, F.; Reale, G.; Marranzano, M.; Ragusa, R.; Solinas, T.; Madonia, M.; Cimino, S.; et al. Dietary Consumption of Phenolic Acids and Prostate Cancer: A Case-Control Study in Sicily, Southern Italy. Molecules 2017, 22, 2159. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.; Kim, M.H.; Park, J.H.; Jeong, Y.; Ko, K.S. Cellular Antioxidant and Anti-Inflammatory Effects of Coffee Extracts with Different Roasting Levels. J. Med. Food 2017, 20, 626–635. [Google Scholar] [CrossRef] [PubMed]
- Affonso, R.C.L.; Voytena, A.P.L.; Fanan, S.; Pitz, H.; Coelho, D.S.; Horstmann, A.L.; Pereira, A.; Uarrota, V.G.; Hillmann, M.C.; Varela, L.A.C.; et al. Phytochemical Composition, Antioxidant Activity, and the Effect of the Aqueous Extract of Coffee (Coffea arabica L.) Bean Residual Press Cake on the Skin Wound Healing. Available online: https://www.hindawi.com/journals/omcl/2016/1923754/ (accessed on 27 September 2018).
- Tverdal, A. Boiled coffee consumption and the risk of prostate cancer: Follow-up of 224,234 Norwegian men 20–69 years. Br. J. Cancer 2015, 112, 576–579. [Google Scholar] [CrossRef] [PubMed]
- Kolberg, M.; Pedersen, S.; Mitake, M.; Holm, K.L.; Bøhn, S.K.; Blomhoff, H.K.; Carlsen, H.; Blomhoff, R.; Paur, I. Coffee inhibits nuclear factor-kappa B in prostate cancer cells and xenografts. J. Nutr. Biochem. 2016, 27, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Abraham, S.K.; Vukicevic, V.; Stopper, H. Coffee-mediated protective effects against directly acting genotoxins and gamma-radiation in mouse lymphoma cells. Cell Biol. Toxicol. 2004, 20, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Abraham, S.K.; Stopper, H. Anti-genotoxicity of coffee against N-methyl-N-nitro-N-nitrosoguanidine in mouse lymphoma cells. Mutat. Res. Toxicol. Environ. Mutagen. 2004, 561, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Loftfield, E.; Shiels, M.S.; Graubard, B.I.; Katki, H.A.; Chaturvedi, A.K.; Trabert, B.; Pinto, L.A.; Kemp, T.J.; Shebl, F.M.; Mayne, S.T.; et al. Associations of Coffee Drinking with Systemic Immune and Inflammatory Markers. Cancer Epidemiol. Biomarkers Prev. 2015, 24, 1052–1060. [Google Scholar] [CrossRef] [PubMed]
- Ullrich, K.; Blumenthal-Barby, F.; Lamprecht, B.; Köchert, K.; Lenze, D.; Hummel, M.; Mathas, S.; Dörken, B.; Janz, M. The IL-15 cytokine system provides growth and survival signals in Hodgkin lymphoma and enhances the inflammatory phenotype of HRS cells. Leukemia 2015, 29, 1213–1218. [Google Scholar] [CrossRef] [PubMed]
- Porto, A.C.V. Utilização de Asparaginase na Prevenção da Formação de Acrilamida em Café, Programa de Pós-Graduação em Alimentos e Nutrição; Universidade Federal do Estado do Rio de Janeiro: Rio de Janeiro, Brazil, 2015. [Google Scholar]
- de Lima, J.P.; Rodrigues, L.F.; Monteiro, A.G.D.P.; Boas, E.V.D.B. Climacteric pattern of mangaba fruit (Hancornia speciosa Gomes) and its responses to temperature. Sci. Hortic. 2015, 197, 399–403. [Google Scholar] [CrossRef]
- Maria do Socorro, M.R.; Alves, R.E.; de Brito, E.S.; Pérez-Jiménez, J.; Saura-Calixto, F.; Mancini-Filho, J. Bioactive compounds and antioxidant capacities of 18 non-traditional tropical fruits from Brazil. Food Chem. 2010, 121, 996–1002. [Google Scholar] [CrossRef] [Green Version]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Prior, R.L.; Hoang, H.; Gu, L.; Wu, X.; Bacchiocca, M.; Howard, L.; Hampsch-Woodill, M.; Huang, D.; Ou, B.; Jacob, R. Assays for Hydrophilic and Lipophilic Antioxidant Capacity (oxygen radical absorbance capacity (ORAC FL)) of Plasma and Other Biological and Food Samples. J. Agric. Food Chem. 2003, 51, 3273–3279. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Liu, H.J.; Chang, B.Y.; Yan, H.W.; Yu, F.H.; Liu, X.X. Determination of amino acids in food and feed by derivatization with 6-aminoquinolyl-N-hydroxysuccinimidyl carbamate and reversed-phase liquid chromatographic separation. J. AOAC Int. 1995, 78, 736–744. [Google Scholar]
- AOAC Official Method 994.12, Amino Acids in Feeds. Official Methods of Analysis of AOAC Int., 18th ed.; Rev. 4; AOAC Intl.: Gaithers-burg, MD, USA, 2011; Chapter 4; pp. 9–19. [Google Scholar]
- Macrae, R. HPLC in Food Analysis; Academic Press: Cambridge, MA, USA, 1988; ISBN 0124647812. [Google Scholar]
- Perrone, D.; Donangelo, C.M.; Farah, A. Fast simultaneous analysis of caffeine, trigonelline, nicotinic acid and sucrose in coffee by liquid chromatography-mass spectrometry. Food Chem. 2008, 110, 1030–1035. [Google Scholar] [CrossRef] [PubMed]
- Trugo, L.C.; Macrae, R. A Study of the Effect of Roasting on the Chlorogenic Acid Composition of Coffee Using HPLC. Food Chem. 1984, 15, 219–227. [Google Scholar] [CrossRef]
- Vindelov, L.L. Flow microfluorometric analysis of nuclear DNA in cells from solid tumors and cell suspensions. A new method for rapid isolation and straining of nuclei. Virchows Arch. B Cell Pathol. 1977, 24, 227–242. [Google Scholar] [PubMed]





| GF | LF | MF | DF | GS | LS | MS | DS | |
|---|---|---|---|---|---|---|---|---|
| Coordinate | ||||||||
| L* | 58.9 ± 0.1 a | 28.6 ± 0.0 b | 27.2 ± 0.0 c | 27.4 ± 0.0 d | 73.5 ± 0.0 e | 54.2 ± 0.0 f | 51.1 ± 0.0 g | 57.5 ± 0.0 h |
| a* | 2.6 ± 0.0 a | 9.1 ± 0.0 b | 7.5 ± 0.0 c | 7.4 ± 0.0 d | 0.4 ± 0.0 e | 8.9 ± 0.0 f | 8.2 ± 0.0 g | 8.7 ± 0.0 h |
| b* | 17.8 ± 0.0 a | 11.0 ± 0.0 b | 9.9 ± 0.0 c | 9.8 ± 0.0 d | 15.9 ± 0.0 e | 20.9 ± 0.0 f | 19.1 ± 0.0 g | 18.8 ± 0.0 h |
| GF | LF | MF | DF | GS | LS | MS | DS | |
|---|---|---|---|---|---|---|---|---|
| Sugars (g/100 g) | ||||||||
| Sucrose | 11.6 ± 0.1 a | ND | ND | ND | 11.8 ± 0.2 a | ND | ND | ND |
| Fructose | 1.4 ± 0.1 a | ND | ND | ND | 1.3 ± 0.0 a | ND | ND | ND |
| Amino Acids (g/100 g) | ||||||||
| Asparagine | 1.5 ± 0.1 a | 0.6 ± 0.0 b | 0.6 ± 0.0 b | 0.4 ± 0.1 c | 1.5 ± 0.1 a | 0.4 ± 0.1 b,c | 0.6 ± 0.0 b,c | 0.4 ± 0.1 c |
| Glutamine | 3.4 ± 0.2 a | 2.2 ± 0.0 b | 2.8 ± 0.1 a,b | 2.5 ± 0.4 a,b | 3.4 ± 0.2 a | 1.5 ± 0.2 c | 2.8 ± 0.3 d | 2.6 ± 0.4 e |
| Histidine | 0.3 ± 0.0 a | 0.2 ± 0.0 b | 0.2 ± 0.0 b | 0.1 ± 0.0 c | 0.4 ± 0.1 a | 0.1 ± 0.0 b,c | 0.2 ± 0.01 b,c | 0.1 ± 0.0 c |
| Arginine | 1.0 ± 0.0 a | 0.2 ± 0.0 b | 0.1 ± 0.0 b | 0.1 ± 0.0 b | 1.0 ± 0.0 a | 0.1 ± 0.0 b | 0.1 ± 0.0 b | 0.1 ± 0.0 b |
| Proline | 1.1 ± 0.0 a | 0.6 ± 0.0 b | 0.6 ± 0.0 b | 0.5 ± 0.1 b | 1.2 ± 0.0 a | 0.4 ± 0.0 b,c | 0.6 ± 0.7 b | 0.5 ± 0.1 b |
| Leucine | 1.2 ± 0.1 a | 0.6 ± 0.0 b | 0.7 ± 0.0 b | 0.6 ± 0.1 b | 1.3 ± 0.0 a | 0.4 ± 0.1 b,c | 0.8 ± 0.1 b | 0.6 ± 0.1 b |
| Phenylalanine | 0.8 ± 0.0 a | 0.4 ± 0.0 b | 0.4 ± 0.0 b | 0.3 ± 0.0 b | 0.9 ± 0.0 a | 0.3 ± 0.4 b | 0.4 ± 0.1 b | 0.3 ± 0.0 b |
| GF | LF | MF | DF | GS | LS | MS | DS | |
|---|---|---|---|---|---|---|---|---|
| Caffeine (g/100 g) | 0.3 ± 0.0 a | 0.2 ± 0.1 b | 0.2 ± 0.0 b | 0.1 ± 0.0 c | 0.4 ± 0.1 a | 0.2 ± 0.0 b,c | 0.2 ± 0.0 b,c | 0.1 ± 0.0 c |
| Total Phenolic compounds (mg of gallic ac./100 g) | 3051.1 ± 33.7 a | 3792.0 ± 13.4 b | 2177.2 ± 70.8 c | 2198.7 ± 71.0 c | 2816.6 ± 19.2 a | 3046.4 ± 64.0 a | 1889.0 ± 24.6 d | 1437.1 ± 17.5 e |
| Sample | Cell Cycle Phase | Control | 2500 µg/mL | 5000 µg/mL |
|---|---|---|---|---|
| GF | G0/G1 | 50.8 ± 3.1 a | 64.8 ± 2.1 b | 75.6 ± 0.5 c |
| S | 6.1 ± 2.7 a | 6.6 ± 1.0 a | 8.4 ± 2.9 a | |
| G2/M | 44.3 ± 1.0 a | 30.2 ± 3.1 b | 13.5 ± 2.8 c | |
| LF | G0/G1 | 53.9 ± 3.3 a | 54.1 ± 2.9 a | 63.4 ± 4.6 b |
| S | 8.8 ± 1.4 a | 9.8 ± 2.1 a | 4.6 ± 1.0 b | |
| G2/M | 39.8 ± 3.4 a | 36.4 ± 4.2 a,b | 30.1 ± 1.3 b | |
| MF | G0/G1 | 59.3 ± 2.0 a | 71.8 ± 1.4 b | 85.2 ± 3.2 c |
| S | 5.6 ± 1.3 a | 4.7 ± 1.0 a | 2.9 ± 0.5 a | |
| G2/M | 34.2 ± 1.0 a | 22.4 ± 2.1 b | 11.0 ± 3.1 c | |
| DF | G0/G1 | 51.1 ± 0.1 a | 51.0 ± 8.0 a | 39.7 ± 3.1 a,b |
| S | 6.2 ± 0.1 a | 5.6 ± 0.4 a | 11.0 ± 0.4 b | |
| G2/M | 41.8 ± 0.1 a | 42.6 ± 8.0 a | 48.3 ± 2.6 a | |
| GS | G0/G1 | 50.0 ± 3.3 a | 45.1 ± 4.2 a | 34.4 ± 4.6 b |
| S | 7.56 ± 1.3 a | 9.7 ± 2.2 b | 8.3 ± 3.2 c | |
| G2/M | 44.6 ± 3.0 a | 46.0 ± 3.4 a | 58.9 ± 3.0 b | |
| LS | G0/G1 | 61.5 ± 11.0 a | 65.1 ± 1.1 a | 52.1 ± 3.7 a |
| S | 12.4 ± 4.9 a | 8.0 ± 1.2 a | 10.5 ± 0.6 a | |
| G2/M | 25.0 ± 7.0 a | 25.8 ± 2.4 a | 36.4 ± 4.3 a,b | |
| MS | G0/G1 | 54.3 ± 0.2 a | 22.3 ± 3.7 b | 70.4 ± 7.1 c |
| S | 7.0 ± 0.3 a | 3.8 ± 0.4 a | 11.5 ± 1.9 b | |
| G2/M | 37.5 ± 0.4 a | 72.6 ± 2.6 b | 18.9 ± 3.1 c | |
| DS | G0/G1 | 56.2 ± 2.8 a | 65.1 ± 1.0 b | 83.0 ± 0.7 c |
| S | 4.0 ± 0.2 a | 7.2 ± 1.7 b | 3.7 ± 0.2 a | |
| G2/M | 38.3 ± 2.9 a | 27.0 ± 1.2 b | 12.9 ± 0.5 c |
| Sample | Phases of Cell Death Process | CT | 2500 µg/mL | 5000 µg/mL | Sample | Phases of Cell Death Process | CT | 2500 µg/mL | 5000 µg/mL |
|---|---|---|---|---|---|---|---|---|---|
| GF | Viable cells | 86.4 ± 0.6 a | 85.3 ± 0.9 a | 20.3 ± 16.1 b | GS | Viable cells | 90.0 ± 1.8 a | 87.2 ± 0.6 a | 15.7 ± 0.8 a |
| Early apoptosis | 1.9 ± 0.3 a | 1.8 ± 0.4 a | 55.3 ± 5.0 b | Early apoptosis | 5.7 ± 0.5 a | 5.9 ± 1. 5 a | 30.8 ± 10.8 a | ||
| Late apoptosis/Necrosis | 11.2 ± 1.0 a | 12.4 ± 1.4 a | 23.6 ± 11.5 a | Late apoptosis/Necrosis | 3.9 ± 1.4 a | 6.7 ± 2.2 a | 52.0 ± 10.2 b | ||
| Non-apoptotic cells | 0.5 ± 0.1 a | 0.4 ± 0.1 a | 0.8 ± 0.3 a | Non-apoptotic cells | 0.3 ± 0.1 a | 0.2 ± 0.1 a | 1.4 ± 0.3 b | ||
| LF | Viable cells | 92.3 ± 0.8 a | 92.9 ± 0.6 a | 14.8 ± 4.7 b | LS | Viable cells | 91.9 ± 2.2 a | 92.2 ± 0.4 a | 9.7 ± 1.4 b |
| Early apoptosis | 1.4 ± 0.3 a | 4.3 ± 0.1 a | 39.4 ± 1.4 b | Early apoptosis | 3.3 ± 1.7 a | 4.2 ± 0.5 a | 26.9 ± 4.4 b | ||
| Late apoptosis/Necrosis | 5.3 ± 1.0 a | 2.9 ± 0.7 a | 44.4 ± 3.6 b | Late apoptosis/Necrosis | 4.6 ± 0.5 a | 3.4 ± 0.1 a | 62.2 ± 3.2 b | ||
| Non-apoptotic cells | 0.9 ± 0.5 a | 0.1 ± 0.0 a | 1.4 ± 0.4 a | Non-apoptotic cells | 0.3 ± 0.0 a | 0.1 ± 0.0 a | 1.0 ± 0.1 a | ||
| MF | Viable cells | 95.2 ± 1.0 a | 90.4 ± 1.2 a | 69.3 ± 5.6 b | MS | Viable cells | 89.5 ± 1.1 a | 92.1 ± 0.2 b | 72.7 ± 1.7 c |
| Early apoptosis | 0.6 ± 0.1 a | 3.2 ± 0.5 a | 18.4 ± 5.3 b | Early apoptosis | 2.2 ± 0.2 a | 3.2 ± 0.4 a | 14.5 ± 0.4 b | ||
| Late apoptosis/Necrosis | 3.1 ± 1.0 a | 5.3 ± 1.1 b | 8.6 ± 1.2 c | Late apoptosis/Necrosis | 7.3 ± 1.0 a | 2.0 ± 0.2 b | 10.6 ± 0.2 c | ||
| Non-apoptotic cells | 1.2 ± 0.5 a | 1.0 ± 0.3 a | 3.6 ± 0.8 b | Non-apoptotic cells | 1.0 ± 0.1 a | 2.6 ± 0.0 b | 2.2 ± 0.4 b | ||
| DF | Viable cells | 90.4 ± 4.4 a | 89.6 ± 0.4 a | 74.3 ± 3.5 b | DS | Viable cells | 93.6 ± 0.4 a | 90.5 ± 0.9 a | 96.6 ± 0.1 a |
| Early apoptosis | 1.6 ± 1.0 a | 3.7 ± 0.2 a | 18.4 ± 2.2 b | Early apoptosis | 2.7 ± 0.2 a | 3.9 ± 0.5 b | 0.1 ± 0.0 c | ||
| Late apoptosis/Necrosis | 6.7 ± 4.4 a | 5.6 ± 0.2 a | 4.5 ± 1.2 a | Late apoptosis/Necrosis | 3.1 ± 0.1 a | 4.2 ± 0.8 a | 0.1 ± 0.0 b | ||
| Non-apoptotic cells | 1.3 ± 0.9 a | 1.1 ± 0.0 a | 2.6 ± 0.2 b | Non-apoptotic cells | 0.7 ± 0.0 a | 1.4 ± 0.1 b | 3.3 ± 0.1 c |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bauer, D.; Abreu, J.; Jordão, N.; Rosa, J.S.d.; Freitas-Silva, O.; Teodoro, A. Effect of Roasting Levels and Drying Process of Coffea canephora on the Quality of Bioactive Compounds and Cytotoxicity. Int. J. Mol. Sci. 2018, 19, 3407. https://doi.org/10.3390/ijms19113407
Bauer D, Abreu J, Jordão N, Rosa JSd, Freitas-Silva O, Teodoro A. Effect of Roasting Levels and Drying Process of Coffea canephora on the Quality of Bioactive Compounds and Cytotoxicity. International Journal of Molecular Sciences. 2018; 19(11):3407. https://doi.org/10.3390/ijms19113407
Chicago/Turabian StyleBauer, Deborah, Joel Abreu, Nathállia Jordão, Jeane Santos da Rosa, Otniel Freitas-Silva, and Anderson Teodoro. 2018. "Effect of Roasting Levels and Drying Process of Coffea canephora on the Quality of Bioactive Compounds and Cytotoxicity" International Journal of Molecular Sciences 19, no. 11: 3407. https://doi.org/10.3390/ijms19113407
APA StyleBauer, D., Abreu, J., Jordão, N., Rosa, J. S. d., Freitas-Silva, O., & Teodoro, A. (2018). Effect of Roasting Levels and Drying Process of Coffea canephora on the Quality of Bioactive Compounds and Cytotoxicity. International Journal of Molecular Sciences, 19(11), 3407. https://doi.org/10.3390/ijms19113407