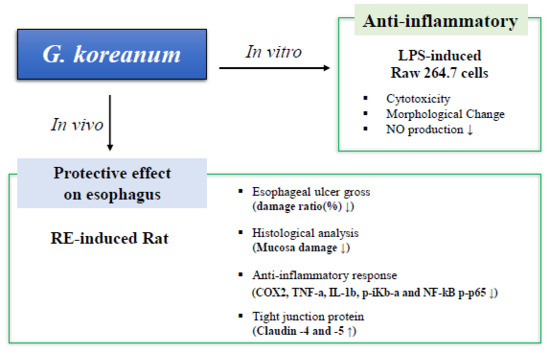
Dichloromethane Extracts of Geranium Koreanum Kom. Alleviates Esophagus Damage in Acute Reflux Esophagitis-Induced Rats by Anti-Inflammatory Activities
Abstract
:1. Introduction
2. Results
2.1. Cell Viability and Optical Morphological Transformation in Raw 264.7 Cells
2.2. Effect of Geranium koreanum on Nitric Oxide (NO) Production and Inducible Nitric Oxide Synthase (iNOS) Expression in Lipopolysaccharide (LPS)-Induced in Raw 264.7 Cells
2.3. Improvement of Esophageal Mucosal Damage in Acute Reflux Esophagitis (RE) Rats
2.4. Histopathology Analysis of Esophagus of RE Rats
2.5. Inflammation-Related Protein Expression in Esophagus
2.6. Claudin-4 and -5 Protein Expression in the Esophagus
2.7. Identifying Polyphenolic Compounds Using Liquid Chromatography-Mass Spectrometry (LC-MS/MS)
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Plant Materials and Extraction
4.3. Cell Culture with Geranium Koreanum Fractions
4.4. Cell Viability and Optical Morphological Transformation in Raw 264.7 Cells
4.5. Nitric Oxide (NO) Production in Raw 264.7 Cells
4.6. Animal Management
4.7. Acute RE Induction
4.8. Animal Management Esophageal Lesion Ratio
4.9. Histopathological Analysis of Esophageal Mucosa
4.10. Preparation of Cytosol and Nuclear Fraction of Esophagus
4.11. Enzyme-Linked Immunosorbent Assay (ELISA)
4.12. Western Blot Analysis in Esophagus
4.13. LC-MS/MS analysis
4.14. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
References
- Mahattanadul, S.; Ridtitid, W.; Nima, S.; Phdoongsombut, N.; Ratanasuwon, P.; Kasiwong, S. Effects of morinda citrifolia aqueous fruit extract and its biomarker scopoletin on reflux esophagitis and gastric ulcer in rats. J. Ethnopharmacol. 2011, 134, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, N. Inflammation and oxidative stress in gastroesophageal reflux disease. J. Clin. Biochem. Nutr. 2007, 40, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.Y.; Shin, Y.O.; Lee, J.Y.; Lee, A.R.; Shin, S.H.; Kwon, O.J.; Seo, B.I.; Roh, S.S. Improving effect of a combined extract of rhei rhizoma and glycyrrhizae rhizoma through anti-oxidative stress in reflux esophagitis rats. Kor. J. Herbol. 2015, 30, 37–44. [Google Scholar] [CrossRef]
- Meining, A.; Classen, M. The role of diet and lifestyle measures in the pathogenesis and treatment of gastroesophageal reflux disease. Am. J. Gastroenterol. 2000, 95, 2692–2697. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, N.; Kamada, K.; Tomasturi, N.; Suzuki, T.; Takagi, T.; Ichikawa, H.; Yoshikawa, T. Management of recurrence of symptoms of gastroesophageal reflux disease: Synergistic effect of rebamipide with 15 mg lansoprazole. Dig. Dis. Sci. 2010, 55, 3393–3398. [Google Scholar] [CrossRef] [PubMed]
- Layli, E.; Nasseri-Moghaddam, S. Meta-analyses: Does long-term PPI use increase the risk of gastric premalignant lesions? Arch. Iran. Med. 2013, 16, 449–458. [Google Scholar]
- Jabri, M.A.; Tounsi, H.; Abdellaoui, A.; Marzouki, L.; Sebai, H. Protective effects of Artemisia campestris extract against gastric acid reflux-induced esophageal mucosa injuries. Pathophysiology 2018, 25, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Lian, B.; Liu, Z.H.; Wei, H.T.; Zhang, Z.; Shi, G.N. Protection effect Xuanfudaizhetang on reflux esophagitis in rats. Asian Pac. J. Trop. Med. 2014, 7, 267–270. [Google Scholar] [CrossRef]
- Shin, M.R.; Seo, B.I.; Son, C.G.; Roh, S.S.; An, H.J. Banhasasim-tang treatment reduces the severity of esophageal mucosal ulcer on chronic acid reflux esophagitis in rats. BioMed Res. Int. 2017, 7157212. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Qiao, Q.; Wang, J.L.; Yao, L.P.; Fu, Y.J. Inhibitory effects of geraniin on LPS-induced inflammation via regulating NF-κB and Nrf2 pathways in RAW 264.7 Cells. Chem. Biol. Interact. 2016, 253, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Hossen, M.J.; Cho, J.Y.; Kim, D.W. PDK1 in NF-κB signaling is a target of Xanthium strumarium methanolic extract-mediated anti-inflammatory activities. J. Ethnopharmacol. 2016, 190, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.P.; Lin, C.H.; Chen, Y.C.; Kao, S.H. Anti-inflammatory effects of Perilla frutescens leaf extract on lipopolysaccaharide-stimulated RAW 264.7 cells. Mol. Med. Rep. 2014, 10, 1077–1083. [Google Scholar] [CrossRef] [PubMed]
- Souza, R.F.; Huo, X.; Mittal, V.; Schuler, C.M.; Carmack, S.W.; Zhang, H.Y.; Zhang, X.; Yu, C.; Hormi-Carver, K.; Genta, R.M.; et al. Gastroesophageal reflux might cause esophagitis through a cytokine-mediated mechanism rather than caustic acid injury. Gastroenterology 2009, 137, 1776–1784. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.W.; Lee, S.M. Protective effects of chlorogenic acid against experimental reflux esophagitis in rats. Biomol. Ther. 2014, 22, 420–425. [Google Scholar] [CrossRef] [PubMed]
- Klein, A.; Kluge, S.; Peskar, B.M. Stimulation of prostaglandin biosynthesis mediates gastroprotective effect of rebamipide in rats. Dig. Dis. Sci. 1933, 38, 1441–1449. [Google Scholar] [CrossRef]
- Katada, D.; Yoschida, N.; Isozaki, Y.; Tomatsuri, N.; Ichikawa, H.; Naito, Y.; Okanoue, T.; Yoshikawa, T. Prevention by rebamipide of acute reflux esophagitis in rats. Dig. Dis. Sci. 2005, 50, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, R.C.; Onwuegbusi, B.A.; Majaj-Elliott, M.; Saeed, I.T.; Burnham, W.R.; Farthing, M.J.G. Diversity in the oesophageal phenotypic response to gastro-oesophageal reflux: Immunological determinants. Gut 2002, 50, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Miwa, H.; Koseki, J.; Oshima, T.; Kondo, T.; Tomita, T.; Watari, J.; Matsumoto, T.; Hattori, T.; Kubota, K.; Iizuka, S. Rikkunshito, a traditional Japanese medicine, may relieve abdominal symptoms in rats with experimental esophagitis by improving the barrier function of epithelial cells in esophageal mucosa. J. Gastroenterol. 2010, 45, 478–487. [Google Scholar] [CrossRef] [PubMed]
- Burek, M.; Aras-Loza, P.A.; Roewer, N.; Forster, C.Y. Claudin-5 as a novel estrogen target in vascular endothelium. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Poliak, S.; Matlis, S.; Ullmer, C.; Scherer, S.S.; Peles, E. Distinct claudins and associated PDZ proteins form different autotypic tight junctions in myelinating Schwann cells. J. Cell Ciol. 2002, 159, 361–372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amasheh, S.; Dullat, S.; Fromm, M.; Schulzke, J.D.; Buhr, H.J.; Kroesen, A.J. Inflamed pouch mucosa possesses altered tight junctions indicating recurrence of inflammatory bowel disease. Int. J. Colorectal. Dis. 2009, 24, 1149–1156. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.; Jun, K.H.; Jung, J.H.; Chin, H.M.; Park, W.B. The expression of claudin-1, claudin-2, claudin-3, and claudin-4 in gastric cancer tissue. J. Surg. Res. 2011, 167, e185–e191. [Google Scholar] [CrossRef] [PubMed]
- Sanford, J.L.; Edwards, J.D.; Mays, T.A.; Gong, B.; Merriam, A.P.; Rafael-Fortney, J.A. Claudin-5 localizes to the lateral membranes of cardiomyocytes and is altered in utrophin/dystrophin-deficient cardiomyophathic mice. J. Mol. Cell Cardiol. 2005, 38, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Kupeli, E.; Tatli, I.I.; Akdemir, Z.S.; Yesilada, E. Estimation of antinociceptive and anti-inflammatory activity on Geranium pretense subp. Finitimum and its phenolic compounds. J. Ethopharmacol. 2007, 114, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.Y.; Lee, K.J.; Wei, B.; Roh, J.H.; Kang, M.; Cha, S.Y.; Jang, H.K. Antibacterial activities of bark extracts from Fraxinus rhynchophylla Hance and Geranium koreanum Kom. against clinical strains of Clostridium perfringens in chickens. Korean J. Vet. Res. 2015, 55, 117–123. [Google Scholar] [CrossRef] [Green Version]
- Xiao, F.; Zhai, Z.; Jiang, C.; Liu, X.; Li, H.; Qu, X.; Quyang, Z.; Fan, Q.; Tang, T.; Qin, A.; et al. Geraniin suppresses RANKL-induced osteoclastogenesis in vitro and ameliorates wear particle-induced osteolysis in mouse model. Exp. Cell Res. 2015, 330, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Shim, J.U.; Oh, P.S.; Lim, K.T. Anti-inflammatory activity of ethanol extract from Geranium sibiricum Linne. J. Ethnopharmacol. 2009, 126, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.H.; Li, Y.Y.; Li, L.J.; Liang, L.Y.; Shen, Y.M. Anti-inflammatory activities fraction from Geranium nepalense and related polyphenols. Drug Discov. Ther. 2013, 6, 194–196. [Google Scholar] [CrossRef]
- Choo, B.K.; Roh, S.S. Berberine protects against esophageal mucosal damage in reflux esophagitis by suppressing proinflammatory cytokines. Exp. Ther. Med. 2013, 6, 663–670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gweon, T.G.; Park, J.H.; Kim, B.W.; Choi, Y.K.; Kim, J.S.; Park, S.M.; Kim, C.W.; Kim, H.G.; Chung, J.W.; et al. Additive effects of rebamipide plus protone pump inhibitors on the expression of tight junction proteins in a rat model of gastro-esophageal reflux disease. Gut. Liver 2017, 12, 46–50. [Google Scholar] [CrossRef] [PubMed]
- Zong, Y.; Sun, L.; Liu, B.; Deng, Y.S.; Zhan, D.; Chen, Y.L.; He, Y.; Liu, J.; Zhang, Z.J.; Sun, J.; et al. Resveratrol inhibits LPS-induced MAPKs activation via activation of the phosphatidylinositol 3-kinase pathway murine Raw 264.7 macrophage cells. PLoS ONE 2012, 7, e44107. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.W.; Sifrim, D.; Xie, C.; Chen, M.; Xiao, Y.L. Relationship between salivary pepsin concentration and esophageal mucosal integrity in patients with gastroesophageal reflux disease. J. Neurogastroenterol. Motil. 2017, 23, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Hatware, K.V.; Sharma, S.; Patil, K.; Shete, M.; Karri, S.; Gupta, G. Evidence for gastroprotective, anti-infalmmatory and antioxidant potential of methanolic extract of Cordia dichotoma leaves on indomethacin and stress induced gastric lesions in Wistar rats. BioMed Pharmacother. 2018, 103, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Ma, Y.; Wang, Y.; Su, Z.; Gu, M.; Janson, J.C. One-step separation and purification of hydrolysable tannis from Geranium wilfordii Maxim by adsorption Chromatography on cross-linked 12% agarose gel. J. Sep. Sci. 2011, 34, 995–998. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Xin, X.; Liu, Y.; Huang, Y.; Li, K.; Wu, C. Geraniin attenuates LPS-induced acute lung injury via inhibiting NF-κB and activating Nrf2 Signaling pathway. Oncotarget 2017, 8, 22835–22841. [Google Scholar] [PubMed]
- Wang, X.; Chen, Z.; Li, X.; Jiang, Z.K.; Zhao, Y.Q.; Ping, F.F. Geraniin suppresses ovarian cancer growth through inhibition of NF-κB activation and downregulation of Mcl-1 expression. J. Biochem. Mol. Toxicol. 2017, 31. [Google Scholar] [CrossRef] [PubMed]
- Aayadi, H.; Mittal, S.P.K.; Deshpande, A.; Gore, M.; Ghaskadbi, S.S. Cytoprotective effect exerted by geraniin in HepG2 cells is through microRNA mediated regulation of BACH-1 and HO-1. BMB Rep. 2017, 50, 560–565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Graca, V.C.; Barros, L.; Calhelha, R.C.; Dias, M.I.; Carvalho, A.M.; Santos-Buelga, C.; Santos, P.F.; Ferreira, I.C. Cheminal characterization and bioactive properties of aqueous and organic extracts of Geranium robertianum L. Food Funct. 2016, 7, 3807–3814. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.J.; Choi, H.J.; Park, M.J.; Lee, J.Y.; Jeong, S.I.; Lee, S.G.; Kim, K.H.; Joo, M.S.; Jeong, H.S.; Kim, J.E.; et al. The inhibitory effects of Geranium thunbergii on imterferom-γ- and LPS-induced inflammatory responses are mediated by Nrf2 activation. Int. J. Mol. Med. 2015, 35, 1237–1245. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.; Zu, Y.; Fu, Y.; Kong, Y.; Zhao, J.; Li, X.; Li, J.; Wink, M.; Efferth, T. Antioxidant activities and xanthine oxidase inhibitory effects of extracts and main polyphenolic compounds obtained from Geranium Sibiricum L. J. Agric. Food Chem. 2010, 58, 4737–4743. [Google Scholar] [CrossRef] [PubMed]
- Ctarino, M.D.; Silva, A.M.S.; Cruz, M.T.; Cardoso, S.M. Antioxidant and anti-inflammatory activities of Geranium robertianum L. decoctions. Food Funct. 2017, 8, 3355–3365. [Google Scholar] [CrossRef] [PubMed]









| No. | Component Name | Neutral Nass (Da) | Observed Neutral Mass (Da) | Detector Counts | Adducts | Group |
|---|---|---|---|---|---|---|
| 1 | 6′-O-Galloyl-homoarbutin | 438.1192 | 438.1162 | 149,619 | −H | Ellagitannin |
| 2 | Chlorogenin | 432.324 | 432.3236 | 470,753 | −H | Glycoside |
| 3 | Markogenin | 432.324 | 432.3236 | 470,753 | −H | Glycoside |
| 4 | 23-Acetate alisol E | 532.3764 | 532.3758 | 35,139 | +HCOO | Triterpenoid |
| 5 | 9,16-Dioxyhydroxy-10,12,14-triene-18 carbonic acid | 310.2144 | 310.214 | 33,679 | −H | Terpene |
| 6 | Castalagin | 934.0712 | 934.0725 | 11,056 | −H | Ellagitannin |
| 7 | Euphormisin M2 | 924.0869 | 924.0882 | 19,280 | +HCOO, −H | tannin |
| 8 | Geraniin | 952.0818 | 952.0834 | 18,307 | −H | Ellagitannin |
| 9 | Koryoginsenoside R1 | 868.5184 | 868.5195 | 14,293 | −H | Glycoside |
| 10 | Koryoginsenoside R1 | 868.5184 | 868.5194 | 10,068 | −H | Glycoside |
| 11 | Sanguiin H-4 | 634.0806 | 634.0809 | 12,355 | −H | Ellagitannin |
| Group | Body Weight (g) | |
|---|---|---|
| 1 | Normal (n = 6) | 225.0 ± 4.328 |
| 2 | Control (n = 8) | 226.6 ± 2.171 |
| 3 | DICHO 100 mg/kg (n = 8) | 224.6 ± 2.104 |
| 4 | DICHO 200 mg/kg (n = 8) | 220.7 ± 2.179 |
| Parameter | Condition | |||
|---|---|---|---|---|
| Ultra-performance liquid chromatography (UPLC) | ACQUITY UPLC HSS T3 | |||
| Column | 100 mm × 2.1 mm, 1.8 µm, Waters | |||
| Column temperature | 40 °C | |||
| Flow rate | 0.5 mL/min | |||
| mobile phase | A (water + 0.1% formic acid) | |||
| B (acetonitrile + 0.1% formic acid) | ||||
| Time | A (%) | B (%) | ||
| 0 | 97 | 3 | ||
| 5 | 97 | 3 | ||
| 16 | 0 | 100 | ||
| 17 | 0 | 100 | ||
| 19 | 97 | 3 | ||
| 25 | 97 | 3 | ||
| injection volume | 5 µL | |||
| metabolite eluted | SYNAPT G2 Si HDMS QTOF (Waters), positive and negative | |||
| positive | capillary voltage (kV) | 2 | ||
| cone voltage (V) | 40 | |||
| negative | capillary voltage (kV) | 1 | ||
| cone voltage (V) | 40 | |||
| scan range (Da) | 50 to 1200 | |||
| scan time (s) | 0.2 | |||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nam, H.H.; Nan, L.; Choo, B.K. Dichloromethane Extracts of Geranium Koreanum Kom. Alleviates Esophagus Damage in Acute Reflux Esophagitis-Induced Rats by Anti-Inflammatory Activities. Int. J. Mol. Sci. 2018, 19, 3622. https://doi.org/10.3390/ijms19113622
Nam HH, Nan L, Choo BK. Dichloromethane Extracts of Geranium Koreanum Kom. Alleviates Esophagus Damage in Acute Reflux Esophagitis-Induced Rats by Anti-Inflammatory Activities. International Journal of Molecular Sciences. 2018; 19(11):3622. https://doi.org/10.3390/ijms19113622
Chicago/Turabian StyleNam, Hyeon Hwa, Li Nan, and Byung Kil Choo. 2018. "Dichloromethane Extracts of Geranium Koreanum Kom. Alleviates Esophagus Damage in Acute Reflux Esophagitis-Induced Rats by Anti-Inflammatory Activities" International Journal of Molecular Sciences 19, no. 11: 3622. https://doi.org/10.3390/ijms19113622
APA StyleNam, H. H., Nan, L., & Choo, B. K. (2018). Dichloromethane Extracts of Geranium Koreanum Kom. Alleviates Esophagus Damage in Acute Reflux Esophagitis-Induced Rats by Anti-Inflammatory Activities. International Journal of Molecular Sciences, 19(11), 3622. https://doi.org/10.3390/ijms19113622