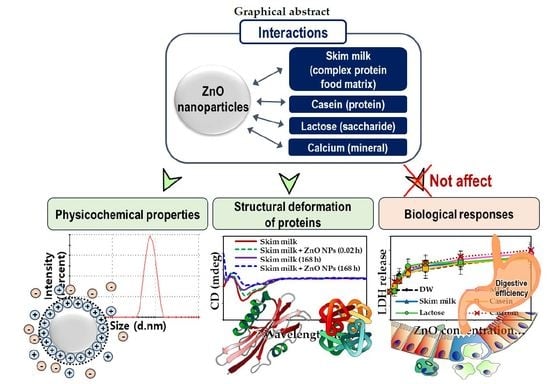
Protein Food Matrix–ZnO Nanoparticle Interactions Affect Protein Conformation, but May not Be Biological Responses
Abstract
:1. Introduction
2. Results
2.1. Characterization of ZnO NPs in Food Matrices
2.2. Interactions between ZnO NPs and Protein Matrices
2.3. Interactions between ZnO NPs and Saccharide in Protein Matrix
2.4. Interactions between ZnO NPs and Mineral in Protein Matrix
2.5. Primary Structural Stability of Proteins
2.6. Conformational Stability of Proteins
2.7. Cytotoxicity
2.8. Cellular Uptake
2.9. Intestinal Transport
2.10. Digestive Efficiency of Proteins
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Characterization of ZnO NPs in Food Matrices
4.3. Solubility of ZnO NPs in Food Matrices
4.4. Interactions between ZnO NPs and Protein Matrices
4.5. Interactions between ZnO NPs and Saccharide in Protein Matrix
4.6. Interactions between ZnO NPs and Mineral in Protein Matrix
4.7. SDS-PAGE Analysis
4.8. CD Analysis
4.9. Cytotoxicity
4.10. Cellular Uptake
4.11. Intestinal Transport
4.12. ICP-AES Analysis
4.13. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mavromichalis, I.; Peter, C.M.; Parr, T.M.; Ganessunker, D.; Baker, D.H. Growth-promoting efficacy in young pigs of two sources of zinc oxide having either a high or a low bioavailability of zinc. J. Anim. Sci. 2000, 78, 2896–2902. [Google Scholar] [CrossRef] [PubMed]
- Espitia, P.J.P.; Soares, N.D.F.; Coimbra, J.S.D.; de Andrade, N.J.; Cruz, R.S.; Medeiros, E.A.A. Zinc oxide nanoparticles: Synthesis, antimicrobial activity and food packaging applications. Food Bioprocess Technol. 2012, 5, 1447–1464. [Google Scholar] [CrossRef]
- Sabir, S.; Arshad, M.; Chaudhari, S.K. Zinc oxide nanoparticles for revolutionizing agriculture: Synthesis and applications. Sci. World J. 2014, 2014, 925494. [Google Scholar] [CrossRef] [PubMed]
- Faiz, H.; Zuberi, A.; Nazir, S.; Rauf, M.; Younus, N. Zinc oxide, zinc sulfate and zinc oxide nanoparticles as source of dietary zinc: Comparative effects on growth and hematological indices of juvenile grass carp (Ctenopharyngodon idella). Int. J. Agric. Biol. 2015, 17, 568–574. [Google Scholar] [CrossRef]
- Zhao, C.Y.; Tan, S.X.; Xiao, X.Y.; Qiu, X.S.; Pan, J.Q.; Tang, Z.X. Effects of dietary zinc oxide nanoparticles on growth performance and antioxidative status in broilers. Biol. Trace Elem. Res. 2014, 160, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Maares, M.; Haase, H. Zinc and immunity: An essential interrelation. Arch. Biochem. Biophys. 2016, 611, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Wessels, I.; Maywald, M.; Rink, L. Zinc as a gatekeeper of immune function. Nutrients 2017, 9, 1286. [Google Scholar] [CrossRef] [PubMed]
- Buracco, S.; Peracino, B.; Andreini, C.; Bracco, E.; Bozzaro, S. Differential effects of iron, zinc, and copper on dictyostelium discoideum cell growth and resistance to Legionella pneumophila. Front. Cell. Infect. Microbiol. 2018, 7, 536. [Google Scholar] [CrossRef] [PubMed]
- FDA. GRAS Notice. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfCFR/CFRSearch.cfm?fr=182.8991 (accessed on 11 October 2018).
- Hernández-Sierra, J.F.; Ruiz, F.; Pena, D.C.C.; Martínez-Gutiérrez, F.; Martínez, A.E.; Guillén, A.J.P.; Tapia-Pérez, H.; Castañón, G.M. The antimicrobial sensitivity of Streptococcus mutans to nanoparticles of silver, zinc oxide, and gold. Nanomedicine 2008, 4, 237–240. [Google Scholar] [CrossRef]
- Shi, X.; Nguyen, T.A.; Suo, Z.; Liu, Y.; Avci, R. Effect of nanoparticles on the anticorrosion and mechanical properties of epoxy coating. Surf. Coat. Technol. 2009, 204, 237–245. [Google Scholar] [CrossRef]
- Sambandan, D.R.; Ratner, D. Sunscreens: An overview and update. J. Am. Acad. Dermatol. 2011, 64, 748–758. [Google Scholar] [CrossRef] [PubMed]
- Sahu, D.; Kannan, G.M.; Vijayaraghavan, R. Size-dependent effect of zinc oxide on toxicity and inflammatory potential of human monocytes. J. Toxicol. Environ. Health A 2014, 77, 177–191. [Google Scholar] [CrossRef] [PubMed]
- Katsumiti, A.; Arostegui, I.; Oron, M.; Gilliland, D.; Valsami-Jones, E.; Cajaraville, M.P. Cytotoxicity of Au, ZnO and SiO2 NPs using in vitro assays with mussel hemocytes and gill cells: Relevance of size, shape and additives. Nanotoxicology 2016, 10, 185–193. [Google Scholar] [PubMed]
- Kuang, H.J.; Yang, P.F.; Yang, L.; Aguilar, Z.P.; Xu, H.Y. Size dependent effect of ZnO nanoparticles on endoplasmic reticulum stress signaling pathway in murine liver. J. Hazard. Mater. 2016, 317, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Jo, M.R.; Yu, J.; Kim, H.J.; Song, J.H.; Kim, K.M.; Oh, J.M.; Choi, S.J. Titanium dioxide nanoparticle-biomolecule interactions influence oral absorption. Nanomaterials 2016, 6, 225. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.A.; Kim, M.K.; Song, J.H.; Jo, M.R.; Yu, J.; Kim, K.M.; Kim, Y.R.; Oh, J.M.; Choi, S.J. Biokinetics of food additive silica nanoparticles and their interactions with food components. Colloids Surf. B Biointerfaces 2017, 150, 384–392. [Google Scholar] [CrossRef] [PubMed]
- Go, M.R.; Bae, S.H.; Kim, H.J.; Yu, J.; Choi, S.J. Interactions between food additive silica nanoparticles and food matrices. Front. Microbiol. 2017, 8, 1013. [Google Scholar] [CrossRef] [PubMed]
- Go, M.R.; Yu, J.; Bae, S.H.; Kim, H.J.; Choi, S.J. Effects of interactions between ZnO nanoparticles and saccharides on biological responses. Int. J. Mol. Sci. 2018, 19, 486. [Google Scholar]
- Aubin-Tam, M.E.; Hamad-Schifferli, K. Gold nanoparticle-cytochrome C complexes: The effect of nanoparticle ligand charge on protein structure. Langmuir 2005, 21, 12080–12084. [Google Scholar] [CrossRef]
- Huang, R.X.; Carney, R.R.; Ikuma, K.; Stellacci, F.; Lau, B.L.T. Effects of surface compositional and structural heterogeneity on nanoparticle-protein interactions: Different protein configurations. ACS Nano 2014, 8, 5402–5412. [Google Scholar] [CrossRef]
- Di Silvio, D.; Rigby, N.; Bajka, B.; Mackie, A.; Bombelli, F.B. Effect of protein corona magnetite nanoparticles derived from bread in vitro digestion on CaCO2 cells morphology and uptake. Int. J. Biochem. Cell B 2016, 75, 212–222. [Google Scholar] [CrossRef] [PubMed]
- Zhu, R.R.; Wang, W.R.; Sun, X.Y.; Liu, H.; Wang, S.L. Enzyme activity inhibition and secondary structure disruption of nano-TiO2 on pepsin. Toxicol. In Vitro 2010, 24, 1639–1647. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.J.; Leonard, J.P.; Nettleship, I.; Lee, J.K.; Soong, Y.; Martello, D.V.; Chyu, M.K. Characterization of ZnO nanoparticle suspension in water: Effectiveness of ultrasonic dispersion. Powder Technol. 2009, 194, 75–80. [Google Scholar] [CrossRef]
- Churchman, A.H.; Wallace, R.; Milne, S.J.; Brown, A.P.; Brydson, R.; Beales, P.A. Serum albumin enhances the membrane activity of ZnO nanoparticles. Chem. Commun. 2013, 49, 4172–4174. [Google Scholar] [CrossRef] [PubMed]
- Anders, C.B.; Chess, J.J.; Wingett, D.G.; Punnoose, A. Serum proteins enhance dispersion stability and influence the cytotoxicity and dosimetry of ZnO nanoparticles in suspension and adherent cancer cell models. Nanoscale Res. Lett. 2015, 10, 1–22. [Google Scholar] [CrossRef]
- Jo, M.R.; Chung, H.E.; Kim, H.J.; Bae, S.H.; Go, M.R.; Yu, J.; Choi, S.J. Effects of zinc oxide nanoparticle dispersants on cytotoxicity and cellular uptake. Mol. Cell. Toxicol. 2016, 12, 281–288. [Google Scholar] [CrossRef]
- Peng, Y.H.; Tsai, Y.C.; Hsiung, C.E.; Lin, Y.H.; Shih, Y.H. Influence of water chemistry on the environmental behaviors of commercial ZnO nanoparticles in various water and wastewater samples. J. Hazard. Mater. 2017, 322, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Seo, G.Y.; Lee, J.H.; Kim, J.H.; Shim, J.B.; Hong, H.J.; Kim, E.; Kwon, O.W.; Lee, J.; Kim, Y.J. Effects of emulsifying agents on the safety of titanium dioxide and zinc oxide nanoparticles in sunscreens. J. Disper. Sci. Technol. 2018, 39, 1544–1549. [Google Scholar] [CrossRef]
- Schilling, C.H.; Sikora, M.; Tomasik, P.; Li, C.; Garcia, V. Rheology of alumina–nanoparticle suspensions: Effects of lower saccharides and sugar alcohols. J. Eur. Ceram. Soc. 2002, 22, 917–921. [Google Scholar] [CrossRef]
- Li, C.; Akinc, M. Role of bound water on the viscosity of nanometric alumina suspensions. J. Am. Ceram. Soc. 2005, 88, 1448–1454. [Google Scholar] [CrossRef]
- Falkowski, P.; Bednarek, P.; Danelska, A.; Mizerski, T.; Szafran, M. Application of monosaccharides derivatives in colloidal processing of aluminum oxide. J. Eur. Ceram. Soc. 2010, 30, 2805–2811. [Google Scholar] [CrossRef]
- Maldiney, T.; Bessiere, A.; Seguin, J.; Teston, E.; Sharma, S.K.; Viana, B.; Bos, A.J.J.; Dorenbos, P.; Bessodes, M.; Gourier, D.; et al. The in vivo activation of persistent nanophosphors for optical imaging of vascularization, tumours and grafted cells. Nat. Mater. 2014, 13, 418–426. [Google Scholar] [CrossRef] [PubMed]
- Buford, M.C.; Hamilton, R.F.; Holian, A. A comparison of dispersing media for various engineered carbon nanoparticles. Part. Fibre Toxicol. 2007, 4, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bihari, P.; Vippola, M.; Schultes, S.; Praetner, M.; Khandoga, A.G.; Reichel, C.A.; Coester, C.; Tuomi, T.; Rehberg, M.; Krombach, F. Optimized dispersion of nanoparticles for biological in vitro and in vivo studies. Part. Fibre Toxicol. 2008, 5, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montero, M.; Molina, T.; Szafran, M.; Moreno, R.; Nieto, M.I. Alumina porous nanomaterials obtained by colloidal processing using d-fructose as dispersant and porosity promoter. Ceram. Int. 2012, 38, 2779–2784. [Google Scholar] [CrossRef]
- Valenti, L.E.; Giacomelli, C.E. Unaffected features of BSA stabilized Ag nanoparticles after storage and reconstitution in biological relevant media. Colloids Surf. B 2015, 132, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Des Rieux, A.; Fievez, V.; Théate, I.; Mast, J.; Préat, V.; Schneider, Y.J. An improved in vitro model of human intestinal follicle-associated epithelium to study nanoparticle transport by M cells. Eur. J. Pharm. Sci. 2007, 30, 380–391. [Google Scholar] [CrossRef]









| Matrix | Hydrodynamic Radius (nm) | Zeta Potential (mV) | ||
|---|---|---|---|---|
| DW | MEM | DW | MEM | |
| DW | 1957.0 ± 113.3 a | 2347.7 ± 347.9 a | 14.8 ± 0.7 a | −8.4 ± 1.2 a |
| MEM | 585.6 ± 34.5 c | 574.0 ± 25.7 b | −24.2 ± 1.4 d | −9.2 ± 1.0 a |
| Skim milk | 1123.0 ± 266.1 ac | 1176.7 ± 120.8 ab | −18.0 ± 0.1 c | −9.5 ± 1.4 a |
| Casein | 2417.0 ± 744.1 b | 2667.0 ± 1094.1 a | −15.1 ± 0.3 c | −10.0 ± 0.5 a |
| Lactose | 1509.0 ± 462.1 a | 1800.3 ± 573.5 ab | 15.6 ± 0.3 a | −9.6 ± 0.5 a |
| Calcium | 3186.4 ± 319.6 d | 2301.8 ± 477.5 a | 9.3 ± 3.4 b | −9.5 ± 2.6 a |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bae, S.-H.; Yu, J.; Lee, T.G.; Choi, S.-J. Protein Food Matrix–ZnO Nanoparticle Interactions Affect Protein Conformation, but May not Be Biological Responses. Int. J. Mol. Sci. 2018, 19, 3926. https://doi.org/10.3390/ijms19123926
Bae S-H, Yu J, Lee TG, Choi S-J. Protein Food Matrix–ZnO Nanoparticle Interactions Affect Protein Conformation, but May not Be Biological Responses. International Journal of Molecular Sciences. 2018; 19(12):3926. https://doi.org/10.3390/ijms19123926
Chicago/Turabian StyleBae, Song-Hwa, Jin Yu, Tae Geol Lee, and Soo-Jin Choi. 2018. "Protein Food Matrix–ZnO Nanoparticle Interactions Affect Protein Conformation, but May not Be Biological Responses" International Journal of Molecular Sciences 19, no. 12: 3926. https://doi.org/10.3390/ijms19123926
APA StyleBae, S. -H., Yu, J., Lee, T. G., & Choi, S. -J. (2018). Protein Food Matrix–ZnO Nanoparticle Interactions Affect Protein Conformation, but May not Be Biological Responses. International Journal of Molecular Sciences, 19(12), 3926. https://doi.org/10.3390/ijms19123926