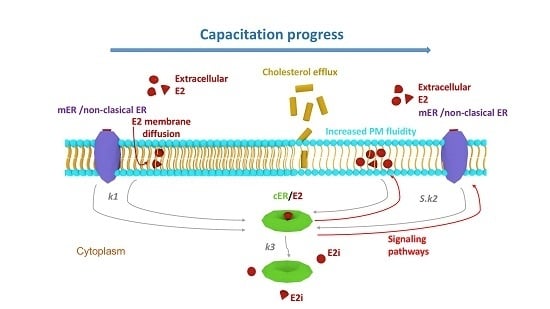
New Insight into Sperm Capacitation: A Novel Mechanism of 17β-Estradiol Signalling
Abstract
:1. Introduction
2. Results
2.1. Monitoring of Estradiol Concentration during Capacitation In Vitro by HPLC
2.2. Kinetic Analysis
2.3. Capacitation Status Monitored by TyrP
2.4. Sperm Motility and Hyperactivation Measured by CASA
2.5. Acrosome Reaction
3. Discussion
4. Materials and Methods
4.1. Chemicals, Reagents, and Animals
4.2. Instrumentation and Chromatographic Conditions
4.3. Capacitation of Mouse Sperm In Vitro and Sample Preparation for HPLC-MS/MS Analysis
4.4. SDS-PAGE with Immunoblotting
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yanagimachi, R. Mammalian fertilization. In The Physiology of Reproduction; Knobil, E., Neill, J.D., Eds.; Raven Press: New York, NY, USA, 1994; pp. 189–317. [Google Scholar]
- Naz, R.K.; Rajesh, P.B. Role of tyrosine phosphorylation in sperm capacitation/acrosome reaction. Reprod. Biol. Endocrinol. 2004, 2, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Visconti, P.E.; Bailey, J.L.; Moore, G.D.; Pan, D.; Olds-Clarke, P.; Kopf, G.S. Capacitation of mouse spermatozoa. I. Correlation between the capacitation state and protein tyrosine phosphorylation. Development 1995, 121, 1129–1137. [Google Scholar] [PubMed]
- Wang, J.; Qi, L.; Huang, S.; Zhou, T.; Guo, Y.; Wang, G.; Guo, X.; Zhou, Z.; Sha, J. Quantitative Phosphoproteomics Analysis Reveals a Key Role of IGF1R Tyrosine Kinase in Human Sperm Capacitation. Mol. Cell. Proteomics 2015, 14, 1104–1112. [Google Scholar] [CrossRef] [PubMed]
- Alvau, A.; Battistone, M.A.; Gervasi, M.G.; Navarrete, F.A.; Xu, X.; Sánchez-Cárdenas, C.; De la Vega-Beltran, J.L.; Da Ros, V.G.; Greer, P.A.; Darszon, A.; et al. The tyrosine kinase FER is responsible for the capacitation-associated increase in tyrosine phosphorylation in murine sperm. Development 2016, 143, 2325–2333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Itach, S.B.; Finkelstein, M.; Etkovitz, N.; Breitbart, H. Hyper-activated motility in sperm capacitation is mediated by phospholipase D-dependent actin polymerization. Dev. Biol. 2012, 362, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Suarez, S.S.; Pacey, A.A. Sperm transport in the female reproductive tract. Hum. Reprod. Update 2006, 12, 23–37. [Google Scholar] [CrossRef] [PubMed]
- Peknicová, J.; Kyselová, V.; Boubelík, M.; Buckiová, D. Effect of an endocrine disruptor on mammalian fertility. Application of monoclonal antibodies against sperm proteins as markers for testing sperm damage. Am. J. Reprod. Immunol. 2002, 47, 311–318. [Google Scholar] [CrossRef]
- Baldi, E.; Luconi, M.; Muratori, M.; Marchiani, S.; Tamburrino, L.; Forti, G. Nongenomic activation of spermatozoa by steroid hormones: Facts and fictions. Mol. Cell. Endocrinol. 2009, 308, 39–46. [Google Scholar] [CrossRef] [Green Version]
- Ded, L.; Dostalova, P.; Dorosh, A.; Dvorakova-Hortova, K.; Peknicova, J. Effect of estrogens on boar sperm capacitation in vitro. Reprod. Biol. Endocrinol. 2010, 8, 87. [Google Scholar] [CrossRef] [Green Version]
- Sebkova, N.; Cerna, M.; Ded, L.; Peknicova, J.; Dvorakova-Hortova, K. The slower the better: How sperm capacitation and acrosome reaction is modified in the presence of estrogens. Reproduction 2012, 143, 297–307. [Google Scholar] [CrossRef]
- Ded, L.; Sebkova, N.; Cerna, M.; Elzeinova, F.; Dostalova, P.; Peknicova, J.; Dvorakova-Hortova, K. In vivo exposure to 17β-estradiol triggers premature sperm capacitation in cauda epididymis. Reproduction 2013, 145, 255–263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luconi, M.; Muratori, M.; Forti, G.; Baldi, E. Identification and characterization of a novel functional estrogen receptor on human sperm membrane that interferes with progesterone effects. J. Clin. Endocrinol. Metabol. 1999, 84, 1670–1678. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, T.; Fujinoki, M.; Kitazawa, M.; Inaba, N. Regulation of hyperactivation of hamster spermatozoa by progesterone. Reprod. Med. Biol. 2008, 7, 63–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujinoki, M. Suppression of progesterone-enhanced hyperactivation in hamster spermatozoa by estrogen. Reproduction 2010, 140, 453–464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujinoki, M. Regulation and disruption of hamster sperm hyperactivation by progesterone, 17β-estradiol and diethylstilbestrol. Reprod. Med. Biol. 2014, 13, 143–152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aquila, S.; De Amicis, F. Steroid receptors and their ligands: Effects on male gamete functions. Exp. Cell Res. 2014, 328, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Dostalova, P.; Zatecka, E.; Dvorakova-Hortova, K. Of Oestrogens and Sperm: A Review of the Roles of Oestrogens and Oestrogen Receptors in Male Reproduction. Int. J. Mol. Sci. 2017, 18, 904. [Google Scholar] [CrossRef]
- Marino, M.; Acconcia, F.; Bresciani, F.; Weisz, A.; Trentalance, A. Distinct nongenomic signal transduction pathways controlled by 17beta-estradiol regulate DNA synthesis and cyclin D(1) gene transcription in HepG2 cells. Mol. Biol. Cell 2002, 13, 3720–3729. [Google Scholar] [CrossRef]
- Acconcia, F.; Ascenzi, P.; Bocedi, A.; Spisni, E.; Tomasi, V.; Trentalance, A.; Visca, P.; Marino, M. Palmitoylation-dependent estrogen receptor alpha membrane localization: Regulation by 17beta-estradiol. Mol. Biol. Cell 2005, 16, 231–237. [Google Scholar] [CrossRef]
- Ahola, T.M.; Manninen, T.; Alkio, N.; Ylikomi, T. G protein-coupled receptor 30 is critical for a progestin-induced growth inhibition in MCF-7 breast cancer cells. Endocrinology 2002, 143, 3376–3384. [Google Scholar] [CrossRef]
- Thomas, P.; Pang, Y.; Filardo, E.J.; Dong, J. Identity of an estrogen membrane receptor coupled to a G protein in human breast cancer cells. Endocrinology 2005, 146, 624–632. [Google Scholar] [CrossRef] [PubMed]
- Vivacqua, A.; Bonofiglio, D.; Recchia, A.G.; Musti, A.M.; Picard, D.; Andò, S.; Maggiolini, M. The G protein-coupled receptor GPR30 mediates the proliferative effects induced by 17beta-estradiol and hydroxytamoxifen in endometrial cancer cells. Mol. Endocrinol. 2006, 20, 631–646. [Google Scholar] [CrossRef] [PubMed]
- Hewitt, S.C.; Deroo, B.J.; Korach, K.S. Signal transduction. A new mediator for an old hormone? Science 2005, 307, 1572–1573. [Google Scholar] [CrossRef] [PubMed]
- Manavathi, B.; Kumar, R. Steering estrogen signals from the plasma membrane to the nucleus: Two sides of the coin. J. Cell. Physiol. 2006, 207, 594–604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marino, M.; Galluzzo, P.; Ascenzi, P. Estrogen signaling multiple pathways to impact gene transcription. Curr. Genomics 2006, 7, 497–508. [Google Scholar] [CrossRef] [PubMed]
- Klinge, C.M. Estrogen receptor interaction with estrogen response elements. Nucleic Acids Res. 2001, 29, 2905–2919. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, C.; Briggs, M.R.; Ahlborn, T.E.; Kraemer, F.B.; Liu, J. Requirement of Sp1 and estrogen receptor alpha interaction in 17beta-estradiol-mediated transcriptional activation of the low density lipoprotein receptor gene expression. Endocrinology 2001, 142, 1546–1553. [Google Scholar] [CrossRef] [PubMed]
- Safe, S.H.; Pallaroni, L.; Yoon, K.; Gaido, K.; Ross, S.; Saville, B.; McDonnell, D. Toxicology of environmental estrogens. Reprod. Fertil. Dev. 2001, 13, 307–315. [Google Scholar] [CrossRef]
- Stossi, F.; Likhite, V.S.; Katzenellenbogen, J.A.; Katzenellenbogen, B.S. Estrogen-occupied estrogen receptor represses cyclin G2 gene expression and recruits a repressor complex at the cyclin G2 promoter. J. Biol. Chem. 2006, 281, 16272–16278. [Google Scholar] [CrossRef] [PubMed]
- Glass, C.K.; Rosenfeld, M.G. The coregulator exchange in transcriptional functions of nuclear receptors. Genes Dev. 2000, 14, 121–141. [Google Scholar] [CrossRef]
- McKenna, N.J.; O’Malley, B.W. Combinatorial control of gene expression by nuclear receptors and coregulators. Cell 2002, 108, 465–474. [Google Scholar] [CrossRef]
- Sztein, J.M.; Farley, J.S.; Mobraaten, L.E. In vitro fertilization with cryopreserved inbred mouse sperm. Biol. Reprod. 2000, 63, 1774–1780. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Nutter, L.M.; Law, N.; McKerlie, C. Sperm freezing and in vitro fertilization in three substrains of C57BL/6 mice. J. Am. Assoc. Lab. Anim. Sci. 2009, 48, 39–43. [Google Scholar] [PubMed]
- Goodson, S.G.; Zhang, Z.; Tsuruta, J.K.; Wang, W.; O’Brien, D.A. Classification of mouse sperm motility patterns using an automated multiclass support vector machines model. Biol. Reprod. 2011, 84, 1207–1215. [Google Scholar] [CrossRef] [PubMed]
- Bosakova, Z.; Tockstein, A.; Adamusova, H.; Coufal, P.; Sebkova, N.; Dvorakova-Hortova, K. Kinetic analysis of decreased sperm fertilizing ability by fluorides and fluoroaluminates: A tool for analyzing the effect of environmental substances on biological events. Eur. Biophys. J. 2016, 45, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Dvořáková-Hortová, K.; Šandera, M.; Jursová, M.; Vašinová, J.; Pěknicová, J. The influence of fluorides on mouse sperm capacitation. Anim. Reprod. Sci. 2008, 108, 157–170. [Google Scholar] [CrossRef] [PubMed]
- Davis, B.K.; Byrne, R.; Hungund, B. Studies on the mechanism of capacitation. II. Evidence for lipid transfer between plasma membrane of rat sperm and serum albu min during capacitation in vitro. Biochim. Biophys. Acta 1979, 558, 257–266. [Google Scholar] [CrossRef]
- Dow, M.P.D.; Bavister, B.D. Direct contact is required between serum albu min and hamster spermatozoa for capacitation in vitro. Gamete Res. 1989, 23, 171–180. [Google Scholar] [CrossRef]
- Romeu, A.M.; Martino, E.E.; Stoppani, A.O.M. Structural requirements for the action of steroids as quenchers of albu min fluorescence. Biochim. Biophys. Acta 1975, 409, 376–386. [Google Scholar] [CrossRef]
- Stewart-Savage, J. Effect of bovine serum albu min concentration and source on sperm capacitation in the golden hamster. Biol. Reprod. 1993, 49, 74–81. [Google Scholar] [CrossRef]
- Asquith, K.L.; Baleato, R.M.; McLaughlin, E.A.; Nixon, B.; Aitken, R.J. Tyrosine phosphorylation activates surface chaperones facilitating sperm–zona recognition. J. Cell. Sci. 2004, 117, 3645–3657. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, I.; Ui-Tei, K.; Saigo, K.; Ishii, H.; Sakuma, Y.; Kato, M. 17-Estradiol at physiological concentrations augments Ca2+-activated K+ currents via estrogen receptor in the gonadotropin-releasing hormone neuronal cell line GT1-7. Endocrinology 2008, 149, 774–782. [Google Scholar] [CrossRef] [PubMed]
- Hess, R.A.; Bunick, D.; Bahr, J.M. Sperm, a source of estrogen. Environ. Health Perspect. 1995, 103, 59–62. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, A.A. Estrone and estradiol levels in the ovarian venous blood from rats during the estrous cycle and pregnancy. Biol. Reprod. 1971, 5, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Tarlatzis, B.C.; Pazaitou, K.; Bili, H.; Bontis, J.; Papadimas, J.; Lagos, S.; Spanos, E.; Mantalenakis, S. Growth hormone, oestradiol, progesterone andtestosterone concentrations in follicular fluid after ovarian stimulation with various regimes for assisted reproduction. Hum. Reprod. 1993, 8, 1612–1616. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.; Fujiwara, E.; Kakiuchi, Y.; Okabe, M.; Satouh, Y.; Baba, S.A.; Chiba, K.; Hirohashi, N. Most fertilizing mouse spermatozoa begin their acrosome reaction before contact with the zona pellucida during in vitrofertilization. Proc. Natl. Acad. Sci. USA 2011, 108, 4892–4896. [Google Scholar] [CrossRef] [PubMed]
- Inoue, N.; Satouh, Y.; Ikawa, M.; Okabe, M.; Yanagimachi, R. Acrosome reacted mouse spermatozoa recovered from the perivitelline space can fertilize other eggs. Proc. Natl. Acad. Sci. USA 2001, 108, 20008–20011. [Google Scholar] [CrossRef] [PubMed]
- Mortimer, S.T.; van der Horst, G.; Motimer, D. The future of computer-aided sperm analysis. Asian J. Androl. 2015, 17, 545–553. [Google Scholar] [CrossRef]
- Kozlík, P.; Bosáková, Z.; Tesařová, E.; Coufal, P.; Čabala, R. Development of a solid-phase extraction with capillary liquid chromatography tandem mass spectrometry for analysis of estrogens in environmental water samples. J. Chromatogr. A 2011, 1218, 2127–2132. [Google Scholar] [CrossRef]
- Dvorakova-Hortova, K.; Honetschlägerová, M.; Tesařová, E.; Vašinová, J.; Frolíková, M.; Bosáková, Z. Residual concentration of estriol during mouse sperm capacitation in vitro determined by HPLC method. Folia Zool. 2009, 58, 75–81. [Google Scholar]
- Free, M.J.; Jaffe, R.A. Collection of rete testis fluid from rats without previous efferent duct ligation. Biol. Reprod. 1979, 20, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Kelch, R.P.; Jenner, M.R.; Weinstein, R.; Kaplan, S.L.; Grumbach, M.M. Estradiol and testosterone secretion by human, simian, and canine testes, in males with hypogonadism and in male pseudohermaphrodites with the feminizing testes syndrome. J. Clin. Investig. 1972, 51, 824–830. [Google Scholar] [CrossRef] [PubMed]
- Abraham, G.E.; Odell, W.D.; Swerdloff, R.S.; Hopper, K. Simultaneous radioimmunoassay of plasma FSH, LH, progesterone, 17-hydroxyprogesterone, and estradiol-17 during the menstrual cycle. J. Clin. Endocrinol. Metabl. 1972, 34, 312–318. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Zigo, M.; Jonakova, V.; Manaskova-Postlerova, P. Electrophoretic and zymographic characterization of proteins isolated by various extraction methods from ejaculated and capacitated boar sperms. Electrophoresis 2011, 32, 1309–1318. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2017. [Google Scholar]
- Opatova, P.; Ihle, M.; Albrechtova, J.; Tomasek, O.; Kempenaers, B.; Forstmeier, W. Inbreeding depression of sperm traits in the zebra finch Taeniopygia guttata. Ecol. Evol. 2016, 6, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]








| Capacitation Time (min) | Bt | |||||
|---|---|---|---|---|---|---|
| 200 µg/L | 20 µg/L | 2 µg/L | ||||
| BALB/c | C57BL/6N | BALB/c | C57BL/6N | BALB/c | C57BL/6N | |
| 0 | 1.000 ± 0.003 | 1.000 ± 0.003 | 1.000 ± 0.028 | 1.000 ± 0.023 | 1.000 ± 0.050 | 1.000 ± 0.070 |
| 30 | 0.965 ± 0.005 | 0.944 ± 0.004 | 0.965 ± 0.033 | 0.958 ± 0.024 | 0.987 ± 0.055 | 0.988 ± 0.073 |
| 60 | 0.983 ± 0.004 | 0.952 ± 0.003 | 0.919 ± 0.026 | 0.938 ± 0.024 | 0.969 ± 0.061 | 0.982 ± 0.079 |
| 90 | 0.989 ± 0.005 | 0.968 ± 0.005 | 0.874 ± 0.031 | 0.904 ± 0.025 | 0.938 ± 0.055 | 0.923 ± 0.071 |
| 120 | 0.995 ± 0.004 | 0.979 ± 0.004 | 0.886 ± 0.028 | 0.921 ± 0.025 | 0.838 ± 0.046 | 0.858 ± 0.069 |
| 150 | 0.997 ± 0.004 | 0.984 ± 0.005 | 0.914 ± 0.032 | 0.929 ± 0.023 | 0.751 ± 0.062 | 0.826 ± 0.066 |
| 180 | 0.998 ± 0.005 | 0.989 ± 0.005 | 0.958 ± 0.028 | 0.956 ± 0.022 | 0.773 ± 0.058 | 0.947 ± 0.074 |
| Constants | 200 µg/L | 20 µg/L | 2 µg/L | |||
|---|---|---|---|---|---|---|
| BALB/c | C57BL/6N | BALB/c | C57BL/6N | BALB/c | C57BL/6N | |
| D | 1 | 1 | 0.1 | 0.1 | 0.01 | 0.01 |
| n | 12 | 12 | 1.2 | 1.2 | 0.12 | 0.12 |
| K2 | 4.0 | 2.0 | 4.5 | 4.0 | 4.5 | 5.5 |
| K3 | 3.0 | 1.3 | 4.3 | 6.0 | 3.5 | 6.5 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bosakova, T.; Tockstein, A.; Sebkova, N.; Simonik, O.; Adamusova, H.; Albrechtova, J.; Albrecht, T.; Bosakova, Z.; Dvorakova-Hortova, K. New Insight into Sperm Capacitation: A Novel Mechanism of 17β-Estradiol Signalling. Int. J. Mol. Sci. 2018, 19, 4011. https://doi.org/10.3390/ijms19124011
Bosakova T, Tockstein A, Sebkova N, Simonik O, Adamusova H, Albrechtova J, Albrecht T, Bosakova Z, Dvorakova-Hortova K. New Insight into Sperm Capacitation: A Novel Mechanism of 17β-Estradiol Signalling. International Journal of Molecular Sciences. 2018; 19(12):4011. https://doi.org/10.3390/ijms19124011
Chicago/Turabian StyleBosakova, Tereza, Antonin Tockstein, Natasa Sebkova, Ondrej Simonik, Hana Adamusova, Jana Albrechtova, Tomas Albrecht, Zuzana Bosakova, and Katerina Dvorakova-Hortova. 2018. "New Insight into Sperm Capacitation: A Novel Mechanism of 17β-Estradiol Signalling" International Journal of Molecular Sciences 19, no. 12: 4011. https://doi.org/10.3390/ijms19124011
APA StyleBosakova, T., Tockstein, A., Sebkova, N., Simonik, O., Adamusova, H., Albrechtova, J., Albrecht, T., Bosakova, Z., & Dvorakova-Hortova, K. (2018). New Insight into Sperm Capacitation: A Novel Mechanism of 17β-Estradiol Signalling. International Journal of Molecular Sciences, 19(12), 4011. https://doi.org/10.3390/ijms19124011