The Molecular Mechanisms of Plant-Derived Compounds Targeting Brain Cancer
Abstract
:1. Introduction
2. The Past and Present Use of Natural Products as Cancer Therapies
3. Underlying Molecular Mechanisms for Natural Products Triggering the Death of Cancer Cells
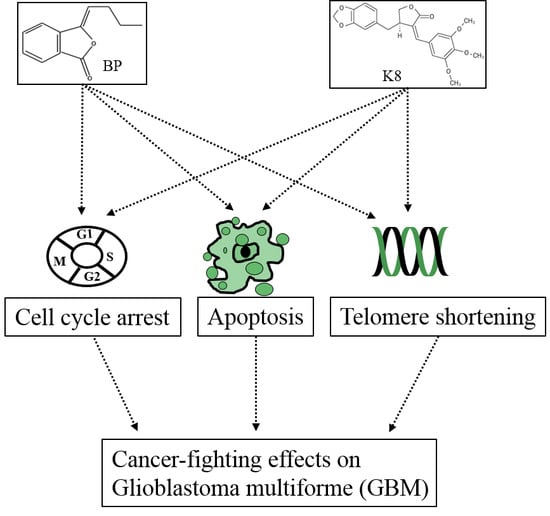
3.1. Cell Cycle Machinery
3.2. Apoptosis
3.3. Telomerase
4. The Use of Plant-Derived Compounds to Target Cancer via Genetic and Epigenetic Alterations
5. Plant-Derived Compounds That Have Recently Exhibited Promise in Treating Brain Cancer
5.1. Effects of Angelica Sinensis (AS) in Brain Cancer Therapy
5.1.1. Penetration through the Blood-Brain Barrier (BBB)
5.1.2. Growth Arrest, Anti-Proliferation, and Apoptosis
5.1.3. Apoptosis and Senescence
5.1.4. Anti-Chemoresistance
5.1.5. Anti-Invasion, Anti-Migration and Anti-Dissemination
5.1.6. N-Butylidenephthalide (BP), a Promising New Anticancer Chemical
5.2. Effects of Bupleurum scorzonerifolium in Brain Cancer Therapy
5.2.1. Telomerase Inhibition and Apoptosis in Lung Cancer
5.2.2. Growth Arrest, Anti-Proliferation, and Apoptosis in Lung Cancer
5.2.3. ICH induced Apoptosis through Nonsteroidal Anti-Inflammatory Drug-Activated Gene (NAG-1) Expression in Lung Cancer
5.2.4. ICF induced Apoptosis in Prostate Cancer Cells by Activating NAG-1
5.2.5. ICF Suppressed GBM Cells Growth by Increasing the Expressions of DNA Damage Inducible Transcript 3 (DDIT3) and NAG-1
5.2.6. Anti-Chemoresistance
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- De Robles, P.; Fiest, K.M.; Frolkis, A.D.; Pringsheim, T.; Atta, C.; St. Germaine-Smith, C.; Day, L.; Lam, D.; Jette, N. The worldwide incidence and prevalence of primary brain tumors: A systematic review and meta-analysis. Neuro Oncol. 2015, 17, 776–783. [Google Scholar] [CrossRef] [PubMed]
- CBTRUS Central Brain Tumor Registry of the United States. CBTRUS Statistical Report: Primary Brain and Central Nervous System Tumors Diagnosed in the United States in 2004–2008; CBTRUS Central Brain Tumor Registry of the United States: Hinsdale, IL, USA, 2014. [Google Scholar]
- Dolecek, T.A.; Propp, J.M.; Stroup, N.E.; Kruchko, C. CBTRUS statistical report: Primary brain and central nervous system tumors diagnosed in the United States in 2005–2009. Neuro.Oncol. 2012, 14 (Suppl. S5), 1–49. [Google Scholar] [CrossRef] [PubMed]
- Chien, L.N.; Gittleman, H.; Ostrom, Q.T.; Hung, K.S.; Sloan, A.E.; Hsieh, Y.C.; Kruchko, C.; Rogers, L.R.; Wang, Y.F.; Chiou, H.Y.; et al. Comparative Brain and Central Nervous System Tumor Incidence and Survival between the United States and Taiwan Based on Population-Based Registry. Front. Public Health. 2016, 4, 151. [Google Scholar] [CrossRef] [PubMed]
- Hegi, M.E.; Diserens, A.C.; Gorlia, T.; Hamou, M.F.; de Tribolet, N.; Weller, M.; Kros, J.M.; Hainfellner, J.A.; Mason, W.; Mariani, L.; et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N. Engl. J. Med. 2005, 352, 997–1003. [Google Scholar] [CrossRef] [PubMed]
- Furnari, F.B.; Fenton, T.; Bachoo, R.M.; Mukasa, A.; Stommel, J.M.; Stegh, A.; Hahn, W.C.; Ligon, K.L.; Louis, D.N.; Brennan, C.; et al. Malignant astrocytic glioma: Genetics, biology, and paths to treatment. Genes Dev. 2007, 21, 2683–2710. [Google Scholar] [CrossRef] [PubMed]
- Weller, M.; Cloughesy, T.; Perry, J.R.; Wick, W. Standards of care for treatment of recurrent glioblastoma—Are we there yet? Neuro. Oncol. 2012, 15, 4–27. [Google Scholar] [CrossRef] [PubMed]
- Nicholas, M.K.; Prados, M.D.; Larson, D.; Black, P.M.; Loeffler, J. Malignant Astrocytomas in Cancer of the Nervous System; Blackwell Publishers: Oxford, UK, 1997; pp. 464–491. [Google Scholar]
- Boiardi, A.; Silvani, A.; Milanesi, I.; Botturi, M.; Broggi, G. Primary glial tumor patients treated by combining cis-platin and etoposide. J. Neurooncol. 1991, 11, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Jeremic, B.; Grujicic, D.; Jevremovic, S.; Stanisavljevic, B.; Milojevic, L.; Djuric, L.; Mijatovic, L. Carboplatin and etoposide chemotherapy regimen for recurrent malignant glioma: A phase II study. J. Clin. Oncol. 1992, 10, 1074–1077. [Google Scholar] [CrossRef] [PubMed]
- Stewart, D.J. A critique of the role of the blood-brain barrier in the chemotherapy of human brain tumors. J. Neurooncol. 1994, 20, 121–139. [Google Scholar] [CrossRef] [PubMed]
- White, L.; Sterling-Levis, K.; Fisher, R.; Tobias, V. Response of brain tumors to chemotherapy, evaluated in a clinically relevant xenograft model. J. Neurooncol. 1995, 25, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Tsai, N.M.; Lin, S.Z.; Lee, C.C.; Chen, S.P.; Su, H.C.; Chang, W.L.; Harn, H.J. The antitumor effects of Angelica sinensis on malignant brain tumors in vitro and in vivo. Clin. Cancer Res. 2005, 11, 3475–3484. [Google Scholar] [CrossRef] [PubMed]
- Cochrane, C.B.; Nair, P.R.; Melnick, S.J.; Resek, A.P.; Ramachandran, C. Anticancer effects of Annonaglabra plant extracts in human leukemia cell lines. Anticancer Res. 2008, 28, 965–971. [Google Scholar] [PubMed]
- Chen, Y.L.; Lin, P.C.; Chen, S.P.; Lin, C.C.; Tsai, N.M.; Cheng, Y.L.; Chang, W.L.; Lin, S.Z.; Harn, H.J. Activation of nonsteroidal anti-inflammatory drug-activated gene-1 via extracellular signal-regulated kinase 1/2 mitogen-activated protein kinase revealed a isochaihulactone-triggered apoptotic pathway in human lung cancer A549 cells. J. Pharmacol. Exp. Ther. 2007, 323, 746–756. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.L.; Su, K.J.; Chen, C.J.; Wei, C.W.; Lin, C.J.; Yiang, G.T.; Lin, S.Z.; Harn, H.J.; Chen, Y.L.S. Synergistic anti-tumor activity of isochaihulactone and paclitaxel on human lung cancer cells. J. Cell. Physiol. 2012, 227, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.Y.; Chen, S.M.; Lu, H.E.; Lai, S.M.; Lai, P.S.; Shen, P.W.; Chen, P.Y.; Shen, C.I.; Harn, H.J.; Lin, S.Z.; et al. N-butylidenephthalide attenuates Alzheimer’s disease-like cytopathy in Down syndrome induced pluripotent stem cell-derived neurons. Sci. Rep. 2015, 5, 8744. [Google Scholar] [CrossRef] [PubMed]
- Rajamani, K.; Liu, J.W.; Wu, C.H.; Chiang, I.T.; You, D.H.; Lin, S.Y.; Hsieh, D.K.; Lin, S.Z.; Harn, H.J.; Chiou, T.W. n-Butylidenephthalide exhibits protection against neurotoxicity through regulation of tryptophan 2, 3 dioxygenase in spinocerebellar ataxia type 3. Neuropharmacology 2017, 117, 434–446. [Google Scholar] [CrossRef] [PubMed]
- Hsueh, K.W.; Chiou, T.W.; Chiang, S.F.; Yamashita, T.; Abe, K.; Borlongan, C.V.; Sanberg, P.R.; Huang, A.Y.; Lin, S.Z.; Harn, H.J. Autophagic down-regulation in motor neurons remarkably prolongs the survival of ALS mice. Neuropharmacology 2016, 108, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Hande, K.R. Etoposide: Four decades of development of a topoisomerase II inhibitor. Eur. J. Cancer 1998, 34, 1514–1521. [Google Scholar] [CrossRef]
- Francesconi, A.B.; Dupre, S.; Matos, M.; Martin, D.; Hughes, B.G.; Wyld, D.K.; Lickliter, J.D. Carboplatin and etoposide combined with bevacizumab for the treatment of recurrent glioblastomamultiforme. J. Clin. Neurosci. 2010, 17, 970–974. [Google Scholar] [CrossRef] [PubMed]
- Mann, J. Natural products in cancer chemotherapy: Past, present and future. Nat. Rev. Cancer 2002, 2, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Oberlies, N.H.; Kroll, D.J. Camptothecin and Taxol: Historic Achievements in Natural Products Research. J. Nat. Prod. 2004, 67, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Cassileth, B.R.; Chapman, C.C. Alternative and complementary cancer therapies. Cancer 1996, 77, 1026–1034. [Google Scholar] [CrossRef]
- Ernst, E. Complementary cancer treatments: Hope or hazard? Clin. Oncol. 1995, 7, 259–263. [Google Scholar] [CrossRef]
- Denchi, E.L.; Attwooll, C.; Pasini, D.; Helin, K. Deregulated E2F activity induces hyperplasia and senescence-like features in the mouse pituitary gland. Mol. Cell. Biol. 2005, 25, 2660–2672. [Google Scholar] [CrossRef] [PubMed]
- Danaei, G.; Vander Hoorn, S.; Lopez, A.D.; Murray, C.J.; Ezzati, M.; Comparative Risk Assessment Collaborating Group. Causes of cancer in the world: Comparative risk assessment of nine behavioural and environmental risk factors. Lancet 2005, 366, 1784–1793. [Google Scholar] [CrossRef]
- Li, C.M.; Villasante, A.; Strati, K.; Ortega, S.; Cañamero, M.; Blasco, M.A.; Serrano, M. The Ink4/Arf locus is a barrier for iPS cell reprogramming. Nature 2009, 460, 1136–1139. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Nichols, M.A.; Shay, J.W.; Xiong, Y. Transcriptional repression of the d-type cyclin-dependent kinase inhibitor p16 by the retinoblastoma susceptibility gene product pRb. Cancer Res. 1994, 54, 6078–6082. [Google Scholar] [PubMed]
- Hara, E.; Smith, R.; Parry, D.; Tahara, H.; Stone, S.; Peters, G. Regulation of p16CDKN2 expression and its implications for cell immortalization and senescence. Mol. Cell. Biol. 1996, 16, 859–867. [Google Scholar] [CrossRef] [PubMed]
- Meng, A.; Wang, Y.; Van Zant, G.; Zhou, D. Ionizing radiation and busulfan induce premature senescence in murine bone marrow hematopoietic cells. Cancer Res. 2003, 63, 5414–5419. [Google Scholar] [PubMed]
- Wang, Y.; Schulte, B.A.; Larue, A.C.; Ogawa, M.; Zhou, D. Total body irradiation selectively induces murine hematopoietic stem cell senescence. Blood 2006, 107, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, A.; Ohtani, N.; Yamakoshi, K.; Iida, S.; Tahara, H.; Nakayama, K.; Nakayama, K.I.; Ide, T.; Saya, H.; Hara, E. Mitogenic signalling and the p16INK4a-Rb pathway cooperate to enforce irreversible cellular senescence. Nat. Cell Biol. 2006, 8, 1291–1297. [Google Scholar] [CrossRef] [PubMed]
- Esteller, M. Epigenetics in cancer. N. Engl. J. Med. 2008, 358, 1148–1159. [Google Scholar] [CrossRef] [PubMed]
- Lockwood, W.W.; Zejnullahu, K.; Bradner, J.E.; Varmus, H. Sensitivity of human lung adenocarcinoma cell lines to targeted inhibition of BET epigenetic signaling proteins. Proc. Natl. Acad. Sci. USA 2012, 109, 19408–19413. [Google Scholar] [CrossRef] [PubMed]
- Dawson, M.A.; Prinjha, R.K.; Dittmann, A.; Giotopoulos, G.; Bantscheff, M.; Chan, W.I.; Robson, S.C.; Chung, C.W.; Hopf, C.; Savitski, M.M.; et al. Inhibition of BET recruitment to chromatin as an effective treatment for MLL-fusion leukaemia. Nature 2011, 478, 529–533. [Google Scholar] [CrossRef] [PubMed]
- Puissant, A.; Frumm, S.M.; Alexe, G.; Bassil, C.F.; Qi, J.; Chanthery, Y.H.; Nekritz, E.A.; Zeid, R.; Gustafson, W.C.; Greninger, P. Targeting MYCN in neuroblastoma by BET bromodomain inhibition. Cancer Discov. 2013, 3, 308–323. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, D.W.; Thornberry, N.A. Caspases: Killer proteases. Trends Biochem. Sci. 1997, 22, 299–306. [Google Scholar] [CrossRef]
- Salvesen, G.S.; Dixit, V.M. Caspases: Intracellular signaling by proteolysis. Cell 1997, 91, 443–446. [Google Scholar] [CrossRef]
- Elmore, S. Apoptosis: A Review of Programmed Cell Death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef] [PubMed]
- Robson, M.E.; Storm, C.D.; Weitzel, J.; Wollins, D.S.; Offit, K. American Society of Clinical Oncology policy statement update: Genetic and genomic testing for cancer susceptibility. J. Clin. Oncol. 2010, 28, 893–901. [Google Scholar] [CrossRef] [PubMed]
- Harris, C.C.; Hollstein, M. Clinical implications of the p53 tumor-suppressor gene. N. Engl. J. Med. 1993, 329, 1318–1327. [Google Scholar] [CrossRef] [PubMed]
- Futreal, P.A.; Coin, L.; Marshall, M.; Down, T.; Hubbard, T.; Wooster, R.; Rahman, N.; Stratton, M.R. A census of human cancer genes. Nat. Rev. Cancer 2004, 4, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Ju, Z.; Rudolph, K.L. Telomere shortening and ageing. Z. Gerontol. Geriatr. 2007, 40, 314–324. [Google Scholar] [CrossRef] [PubMed]
- Chadeneau, C.; Siegel, P.; Harley, C.B.; Muller, W.J.; Bacchetti, S. Telomerase activity in normal and malignant murine tissues. Oncogene 1995, 11, 893–898. [Google Scholar] [PubMed]
- Aisner, D.L.; Wright, W.E.; Shay, J.W. Telomerase regulation: Not just flipping the switch. Curr. Opin. Genet. Dev. 2002, 12, 80–85. [Google Scholar] [CrossRef]
- Hsieh, H.F.; Harn, H.J.; Chiu, S.C.; Liu, Y.C.; Lui, W.Y.; Ho, L.I. Telomerase activity correlates with cell cycle regulators in human hepatocellular carcinoma. Liver 2000, 20, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.J.; Tsai, N.M.; Liu, Y.C.; Ho, L.I.; Hsieh, H.F.; Yen, C.Y.; Harn, H.J. Telomerase activity in human hepatocellular carcinoma: Parallel correlation with human telomerase reverse transcriptase (hTERT) mRNA isoform expression but not with cell cycle modulators or c-Myc expression. Eur. J. Surg. Oncol. 2002, 28, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.J.; Kyo, S.; Liu, Y.C.; Cheng, Y.L.; Hsieh, C.B.; Chan, D.C.; Yu, J.C.; Harn, H.J. Modulation of human telomerase reverse transcriptase in hepatocellular carcinoma. World J. Gastroenterol. 2004, 10, 638–642. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.C.; Chen, C.J.; Wu, H.S.; Chan, D.C.; Yu, J.C.; Yang, A.H.; Cheng, Y.L.; Lee, S.C.; Harn, H.J. Telomerase and c-myc expression in hepatocellular carcinomas. Eur. J. Surg. Oncol. 2004, 30, 384–390. [Google Scholar] [CrossRef] [PubMed]
- Spear, B.B.; Heath-Chiozzi, M.; Huff, J. Clinical application of pharmacogenetics. Trends Mol. Med. 2001, 7, 201–204. [Google Scholar] [CrossRef]
- Stupp, R.; Mason, W.P.; Van Den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Pommier, Y.; Leo, E.; Zhang, H.; Marchand, C. DNA topoisomerases and their poisoning by anticancer and antibacterial drugs. Chem. Biol. 2010, 17, 421–433. [Google Scholar] [CrossRef] [PubMed]
- Deweese, J.E.; Osheroff, M.A.; Osheroff, N. DNA Topology and Topoisomerases: Teaching a “Knotty” Subject. Biochem. Mol. Biol. Educ. 2008, 37, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Gordaliza, M.; Garcıa, P.A.; Del Corral, J.M.; Castro, M.A.; Gómez-Zurita, M.A. Podophyllotoxin: Distribution, sources, applications and new cytotoxic derivatives. Toxicon 2004, 44, 441–459. [Google Scholar] [CrossRef] [PubMed]
- Pommier, Y. Drugging topoisomerases: Lessons and challenges. ACS Chem. Biol. 2013, 8, 82–95. [Google Scholar] [CrossRef] [PubMed]
- Jordan, M.A. Mechanism of action of antitumor drugs that interact with microtubules and tubulin. Curr. Med. Chem. Anticancer Agents 2002, 2, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Belani, C.P.; Lee, J.S.; Socinski, M.A.; Robert, F.; Waterhouse, D.; Rowland, K.; Ansari, R.; Lilenbaum, R.; Natale, R.B. Randomized phase III trial comparing cisplatin-etoposide to carboplatin-paclitaxel in advanced or metastatic non-small cell lung cancer. Ann. Oncol. 2005, 16, 1069–1075. [Google Scholar] [CrossRef] [PubMed]
- Jeon, H.Y.; Park, C.G.; Ham, S.W.; Choi, S.H.; Lee, S.Y.; Kim, J.Y.; Seo, S.; Jin, X.; Kim, J.K.; Eun, K.; et al. BRM270, a Compound from Natural Plant Extracts, Inhibits Glioblastoma Stem Cell Properties and Glioblastoma Recurrence. J. Med. Food 2017, 20, 838–845. [Google Scholar] [CrossRef] [PubMed]
- Puli, S.; Jain, A.; Lai, J.C.; Bhushan, A. Effect of combination treatment of rapamycin and isoflavones on mtor pathway in human glioblastoma (U87) cells. Neurochem. Res. 2010, 35, 986–993. [Google Scholar] [CrossRef] [PubMed]
- Filippi-Chiela, E.C.; Thomé, M.P.; Bueno, E.; Silva, M.M.; Pelegrini, A.L.; Ledur, P.F.; Garicochea, B.; Zamin, L.L.; Lenz, G. Resveratrol abrogates the Temozolomide-induced G2 arrest leading to mitotic catastrophe and reinforces the Temozolomide-induced senescence in glioma cells. BMC Cancer 2013, 13, 147. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, S.X.; Ma, J.W.; Li, H.Y.; Ye, J.C.; Xie, S.M.; Du, B.; Zhong, X.Y. EGCG inhibits properties of glioma stem-like cells and synergizes with temozolomide through downregulation of P-glycoprotein inhibition. J. Neurooncol. 2015, 121, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Banik, N.L.; Ray, S.K. N-(4-Hydroxyphenyl) retinamide induced both differentiation and apoptosis in human glioblastoma T98G and U87MG cells. Brain Res. 2008, 1227, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Galanti, G.; Fisher, T.; Kventsel, I.; Shoham, J.; Gallily, R.; Mechoulam, R.; Lavie, G.; Amariglio, N.; Rechavi, G.; Toren, A. Delta 9-Tetrahydrocannabinol inhibits cell cycle progression by downregulation of E2F1 in human glioblastoma multiforme cells. Acta Oncol. 2008, 47, 1062–1070. [Google Scholar] [CrossRef] [PubMed]
- Marcu, J.P.; Christian, R.T.; Lau, D.; Zielinski, A.J.; Horowitz, M.P.; Lee, J.; Pakdel, A.; Allison, J.; Limbad, C.; Moore, D.H.; et al. Cannabidiol enhances the inhibitory effects of Δ9-tetrahydrocannabinol on human glioblastoma cell proliferation and survival. Mol. Cancer Ther. 2010, 9, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Yim, T.K.; Wu, W.K.; Pak, W.F.; Mak, D.H.; Liang, S.M.; Ko, K.M. Myocardial protection against ischaemia reperfusion injury by a Polygonum multiflorum extract supplemented “Dang-Gui decoction for enriching blood”, a compound formulation, ex vivo. Phytother. Res. 2002, 14, 195–199. [Google Scholar] [CrossRef]
- Guzmán, M.; Duarte, M.J.; Blázquez, C.; Ravina, J.; Rosa, M.C.; Galve-Roperh, I.; Sánchez, C.; Velasco, G.; González-Feria, L. A pilot clinical study of Δ9-tetrahydrocannabinol in patients with recurrent glioblastoma multiforme. Br. J. Cancer 2006, 95, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.N.; Koo, M.W.; Li, Y.; Matsui, H.; Cho, C.H. Angelica sinensis modulates migration and proliferation of gastric epithelial cells. Life Sci. 2001, 68, 961–968. [Google Scholar] [CrossRef]
- Ye, Y.N.; Liu, E.S.; Li, Y.; So, H.L.; Cho, C.C.; Sheng, H.P.; Lee, S.S.; Cho, C.H. Protective effect of polysaccharides-enriched fraction from Angelica sinensis on hepatic injury. Life Sci. 2001, 69, 637–646. [Google Scholar] [CrossRef]
- Ye, Y.N.; Liu, E.S.; Shin, V.Y.; Koo, M.W.; Li, Y.; Wei, E.Q.; Matsui, H.; Cho, C.H. A mechanistic study of proliferation induced by Angelica sinensis in a normal gastric epithelial cell line. Biochem. Pharmacol. 2001, 61, 1439–1448. [Google Scholar] [CrossRef]
- Ye, Y.N.; So, H.L.; Liu, E.S.; Shin, V.Y.; Cho, C.H. Effect of polysaccharides from Angelica sinensis on gastric ulcer healing. Life Sci. 2003, 72, 925–932. [Google Scholar] [CrossRef]
- Lin, P.C.; Chen, Y.L.; Chiu, S.C.; Yu, Y.L.; Chen, S.P.; Chien, M.H.; Chen, K.Y.; Chang, W.L.; Lin, S.Z.; Chiou, T.W.; et al. Orphan nuclear receptor, Nurr-77 was a possible target gene of butylidenephthalide chemotherapy on glioblastoma multiform brain tumor. J. Neurochem. 2008, 106, 1017–1026. [Google Scholar] [CrossRef] [PubMed]
- Woronicz, J.D.; Calnan, B.; Ngo, V.; Winoto, A. Requirement for the orphan steroid receptor Nurr77 in apoptosis of T-cell hybridomas. Nature 1994, 367, 277–281. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.L.; Jian, M.H.; Lin, C.C.; Kang, J.C.; Chen, S.P.; Lin, P.C.; Hung, P.J.; Chen, J.R.; Chang, W.L.; Lin, S.Z.; et al. The induction of orphan nuclear receptor Nur77 expression by n-butylenephthalide as pharmaceuticals on hepatocellular carcinoma cell therapy. Mol. Pharmacol. 2008, 74, 1046–1058. [Google Scholar] [CrossRef] [PubMed]
- Mason, W.P.; Cairncross, J.G. Drug insight: Temozolomide as a treatment for malignant glioma—Impact of a recent trial. Nat. Clin. Pract. Neurol. 2005, 1, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Harn, H.J.; Chen, S.R.; Huang, M.H.; Lin, P.C.; Syu, F.J.; Hsieh, D.K.; Yen, S.Y.; Lin, S.Z.; Chiou, T.W. (Z)-Butylidenephthalide restores temozolomide sensitivity to temozolomide-resistant malignant glioma cells by downregulating expression of the DNA repair enzyme MGMT. J. Pharm. Pharmacol. 2013, 1, 36–49. [Google Scholar]
- Holland, E.C. Glioblastomamultiforme: The terminator. Proc. Natl. Acad. Sci. USA 2000, 97, 6242–6244. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, R.; Weinberg, R.A. The basics of epithelial-mesenchymal transition. J. Clin. Investig. 2009, 119, 1420–1428. [Google Scholar] [CrossRef] [PubMed]
- Keating, A.K.; Kim, G.K.; Jones, A.E.; Donson, A.M.; Ware, K.; Mulcahy, J.M.; Salzberg, D.B.; Foreman, N.K.; Liang, X.; Thorburn, A.; et al. Inhibition of Mer and Axl receptor tyrosine kinases in astrocytoma cells leads to increased apoptosis and improved chemosensitivity. Mol. Cancer Ther. 2010, 9, 1298–1307. [Google Scholar] [CrossRef] [PubMed]
- Harn, H.J.; Chuang, H.M.; Hsieh, J.; Huang, M.H.; Chuang, S.E.; Chen, S.R.; Yen, S.Y.; Lin, S.Z.; Chiou, T.W.; Li, Y.S. Biodegradable interstitial release polymer loading a novel small molecule targeting Axl receptor tyrosine kinase and reducing brain tumor migration and invasion. Oncogene 2015, 35, 2156–2165. [Google Scholar]
- Ott, M.; Litzenburger, U.M.; Sahm, F.; Rauschenbach, K.J.; Tudoran, R.; Hartmann, C.; Marquez, V.E.; von Deimling, A.; Wick, W.; Platten, M. Promotion of glioblastoma cell motility by enhancer of zeste homolog 2 (EZH2) is mediated by axl receptor kinase. PLoS ONE 2012, 7, e47663. [Google Scholar] [CrossRef] [PubMed]
- Orzan, F.; Pellegatta, S.; Poliani, P.L.; Pisati, F.; Caldera, V.; Menghi, F.; Kapetis, D.; Marras, C.; Schiffer, D.; Finocchiaro, G. Enhancer of Zeste 2 (EZH2) is up-regulated in malignant gliomas and in glioma stem-like cells. Neuropathol. Appl. Neurobiol. 2011, 37, 381–394. [Google Scholar] [CrossRef] [PubMed]
- Yen, S.Y.; Chuang, H.M.; Huang, M.H.; Lin, S.Z.; Chiou, T.W.; Harn, H.J. n-Butylidenephthalide Regulated Tumor Stem Cell Genes EZH2/AXL and Reduced Its Migration and Invasion in Glioblastoma. Int. J. Mol. Sci. 2017, 18, 372. [Google Scholar] [CrossRef] [PubMed]
- Ashour, M.L.; Wink, M. GenusBupleurum: A review of its phytochemistry, pharmacology and modes of action. J. Pharm. Pharmacol. 2011, 63, 305–321. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.L.; Chang, W.L.; Lee, S.C.; Liu, Y.G.; Lin, H.C.; Chen, C.J.; Yen, C.Y.; Yu, D.S.; Lin, S.Z.; Harn, H.J. Acetone extract of Bupleurum scorzonerifolium inhibits proliferation of A549 human lung cancer cells via inducing apoptosis and suppressing telomerase activity. Life Sci. 2003, 73, 2383–2394. [Google Scholar] [CrossRef]
- Cheng, Y.L.; Lee, S.C.; Lin, S.Z.; Chang, W.L.; Chen, Y.L.; Tsai, N.M.; Liu, Y.C.; Tzao, C.; Yu, D.S.; Harn, H.J. Anti-proliferative activity of Bupleurum scrozonerifolium in A549 human lung cancer cells in vitro and in vivo. Cancer Lett. 2005, 222, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Krishnaraju, K.; Nguyen, H.Q.; Liebermann, D.A.; Hoffman, B. The zinc finger transcription factor EGR-1 potentiates macrophage differentiation of hematopoietic cells. Mol. Cell. Biol. 1995, 15, 5499–5507. [Google Scholar] [CrossRef] [PubMed]
- Baek, S.J.; Horowitz, J.M.; Eling, T.E. Molecular cloning and characterization of human nonsteroidal anti-inflammatory drug-activated gene promoter. J. Biol. Chem. 2001, 276, 33384–33392. [Google Scholar] [CrossRef] [PubMed]
- Chiu, S.C.; Wang, M.J.; Yang, H.H.; Chen, S.P.; Huang, S.Y.; Chen, Y.L.; Lin, S.Z.; Harn, H.J.; Pang, C.Y. Activation of NAG-1 via JNK signaling revealed an isochaihulactone-triggered cell death in human LNCaP prostate cancer cells. BMC Cancer 2011, 11, 146. [Google Scholar] [CrossRef] [PubMed]
- Tsai, S.F.; Tao, M.; Ho, L.I.; Chiou, T.W.; Lin, S.Z.; Su, H.L.; Harn, H.J. Isochaihulactone-induced DDIT3 causes ER stress-PERK independent apoptosis in glioblastoma multiforme cells. Oncotarget 2017, 8, 4051–4061. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.L.; Lin, S.Z.; Chang, J.Y.; Cheng, Y.L.; Tsai, N.M.; Chen, S.P.; Chang, W.L.; Harn, H.J. In vitro and in vivo studies of a novel potential anticancer agent of isochaihulactone on human lung cancer A549 cells. Biochem. Pharmacol. 2006, 72, 308–319. [Google Scholar] [CrossRef] [PubMed]
- Martello, L.A.; Verdier-Pinard, P.; Shen, H.J.; He, L.; Torres, K.; Orr, G.A.; Horwitz, S.B. Elevated level of microtubule destabilizing factors in a taxol-resistant/dependent A549 cell line with a-tubulin mutation. Cancer Res. 2003, 63, 448–454. [Google Scholar]
 Pathway;
Pathway;  Inhibition;
Inhibition;  Final effect. Please see the content in Section 5.1.
Final effect. Please see the content in Section 5.1.
 Pathway;
Pathway;  Inhibition;
Inhibition;  Final effect. Please see the content in Section 5.1.
Final effect. Please see the content in Section 5.1.
 Pathway;
Pathway;  Inhibition;
Inhibition;  Final effect. Please see the content in Section 5.2.
Final effect. Please see the content in Section 5.2.
 Pathway;
Pathway;  Inhibition;
Inhibition;  Final effect. Please see the content in Section 5.2.
Final effect. Please see the content in Section 5.2.
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, H.-C.; Chi, C.-S.; Chang, Y.-K.; Tung, M.-C.; Lin, S.-Z.; Harn, H.-J. The Molecular Mechanisms of Plant-Derived Compounds Targeting Brain Cancer. Int. J. Mol. Sci. 2018, 19, 395. https://doi.org/10.3390/ijms19020395
Fan H-C, Chi C-S, Chang Y-K, Tung M-C, Lin S-Z, Harn H-J. The Molecular Mechanisms of Plant-Derived Compounds Targeting Brain Cancer. International Journal of Molecular Sciences. 2018; 19(2):395. https://doi.org/10.3390/ijms19020395
Chicago/Turabian StyleFan, Hueng-Chuen, Ching-Shiang Chi, Yu-Kang Chang, Min-Che Tung, Shinn-Zong Lin, and Horng-Jyh Harn. 2018. "The Molecular Mechanisms of Plant-Derived Compounds Targeting Brain Cancer" International Journal of Molecular Sciences 19, no. 2: 395. https://doi.org/10.3390/ijms19020395
APA StyleFan, H. -C., Chi, C. -S., Chang, Y. -K., Tung, M. -C., Lin, S. -Z., & Harn, H. -J. (2018). The Molecular Mechanisms of Plant-Derived Compounds Targeting Brain Cancer. International Journal of Molecular Sciences, 19(2), 395. https://doi.org/10.3390/ijms19020395