Amorphous, Smart, and Bioinspired Polyphosphate Nano/Microparticles: A Biomaterial for Regeneration and Repair of Osteo-Articular Impairments In-Situ
Abstract
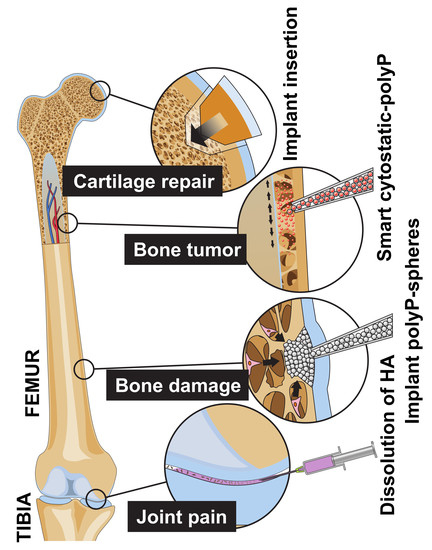
:1. Introduction
2. Results
2.1. Nano/Microparticles with polyP and ZOL
2.2. Composition and Phases of the Particles
2.3. Dose-Finding Assay with Human Mesenchymal Stem Cells
2.4. Incubation of Rat Femur Explants
2.5. Gene Expression Studies: Effect on Osteogenic and Chondrogenic Differentiation in Bone Marrow Cells
2.6. Gene Expression Studies: Effect on Osteoclastic Differentiation
2.7. Mineralization by Bone Marrow Cells in the Presence of polyP and ZOL
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Preparation of Nano/Microparticles
4.3. Electron Microscopy, Energy Dispersive X-ray, and X-ray Diffraction Spectroscopy
4.4. Human Mesenchymal Stem Cells
4.5. Cell Proliferation/Cell Viability Assay
4.6. Preparation and Culture of Femur Explants
4.7. Quantitative Real-Time Polymerase Chain Reaction
4.8. Mineralization by Cells in Femur Explants
4.9. Further Analytical Determination
4.10. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| “Ca-polyP-MP” | Ca-polyP nano/microparticles |
| “Ca-polyP-ZOL-MP” | calcium polyP-zoledronic acid hybrid nano/microparticles |
| “Ca-ZOL-MP” | calcium zoledronic acid nano/microparticles |
| “Na-polyP” | sodium polyP |
| ALP | alkaline phosphatase |
| CatK | cathepsin-K |
| FBS | fetal bovine serum |
| GAPDH | glyceraldehyde 3-phosphate dehydrogenase |
| MSC | mesenchymal stem cells |
| PRP | platelet-rich plasma |
| qRT-PCR | quantitative real-time polymerase chain reaction |
| RANKL | receptor activator of the NF-κB ligand |
| Runx2 | runt related transcription factor |
| Sox9 | sex determining region Y |
| TRAP | tartrate-resistant acid phosphatase |
| TRAP+ | tartrate-resistant acid phosphatase positive |
| XTT | 2,3-bis-(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide |
References
- Tanaka, E.M.; Reddien, P.W. The cellular basis for animal regeneration. Dev. Cell 2011, 21, 172–185. [Google Scholar] [CrossRef] [PubMed]
- Buckwalter, J.A.; Glimcher, M.J.; Cooper, R.R.; Recker, R. Bone biology. Part I: Structure, blood supply, cells, matrix, and mineralization. Instr. Course Lect. 1995, 45, 371–386. [Google Scholar]
- Knothe Tate, M.L.; Falls, T.D.; McBride, S.H.; Atit, R.; Knothe, U.R. Mechanical modulation of osteochondroprogenitor cell fate. Int. J. Biochem. Cell Biol. 2008, 40, 2720–2738. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.; Yu, H.Y.; Heo, J.; Song, M.; Shin, J.H.; Lim, J.; Yoon, S.J.; Kim, Y.; Lee, S.; Kim, S.W.; et al. Mesenchymal stem cells protect against the tissue fibrosis of ketamine-induced cystitis in rat bladder. Sci. Rep. 2016, 6, 30881. [Google Scholar] [CrossRef] [PubMed]
- Norambuena, G.A.; Khoury, M.; Jorgensen, C. Mesenchymal stem cells in osteoarticular pediatric diseases: An update. Pediatr. Res. 2012, 71, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Pavlov, E.; Aschar-Sobbi, R.; Campanella, M.; Turner, R.J.; Gómez-García, M.R.; Abramov, A.Y. Inorganic polyphosphate and energy metabolism in mammalian cells. J. Biol. Chem. 2010, 285, 9420–9428. [Google Scholar] [CrossRef] [PubMed]
- Morrissey, J.H.; Choi, S.H.; Smith, S.A. Polyphosphate: An ancient molecule that links platelets, coagulation, and inflammation. Blood 2012, 119, 5972–5979. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.H.; Schröder, H.C.; Müller, W.E.G. Enzymatically synthesized inorganic polymers as morphogenetically active bone scaffolds: Application in regenerative medicine. Int. Rev. Cell Mol. Biol. 2014, 313, 27–77. [Google Scholar] [PubMed]
- Kulaev, I.S.; Vagabov, V.; Kulakovskaya, T. The Biochemistry of Inorganic Polyphosphates, 2nd ed.; John Wiley: Chichester, UK, 2004. [Google Scholar]
- Müller, W.E.G.; Tolba, E.; Schröder, H.C.; Wang, X.H. Polyphosphate: A morphogenetically active implant material serving as metabolic fuel for bone regeneration. Macromol. Biosci. 2015, 15, 1182–1197. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.H.; Schröder, H.C.; Müller, W.E.G. Polyphosphate as a metabolic fuel in Metazoa: A foundational breakthrough invention for biomedical applications. Biotechnol. J. 2016, 11, 11–30. [Google Scholar] [CrossRef] [PubMed]
- Solesio, M.E.; Demirkhanyan, L.; Zakharian, E.; Pavlov, E.V. Contribution of inorganic polyphosphate towards regulation of mitochondrial free calcium. Biochim. Biophys. Acta 2016, 1860, 1317–1325. [Google Scholar] [CrossRef] [PubMed]
- Kumble, K.D.; Kornberg, A. Inorganic polyphosphate in mammalian cells and tissues. J. Biol. Chem. 1995, 270, 5818–5822. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, C.; Vetro, R.; Vetro, A.; Mantia, R.; Iovane, A.; Di Gesù, M.; Vasto, S.; Di Noto, L.; Mazzola, G.; Caruso, C. The role of platelet gel in osteoarticular injuries of young and old patients. Immun. Ageing 2014, 11, 21. [Google Scholar] [CrossRef] [PubMed]
- Müller, W.E.G.; Wang, X.H.; Diehl-Seifert, B.; Kropf, K.; Schloßmacher, U.; Lieberwirth, I.; Glasser, G.; Wiens, M.; Schröder, H.C. Inorganic polymeric phosphate/polyphosphate as an inducer of alkaline phosphatase and a modulator of intracellular Ca2+ level in osteoblasts (SaOS-2 cells) in vitro. Acta Biomater. 2011, 7, 2661–2671. [Google Scholar] [CrossRef] [PubMed]
- Müller, W.E.G.; Tolba, E.; Schröder, H.C.; Wang, S.; Glaßer, G.; Muñoz-Espí, R.; Link, T.; Wang, X.H. A new polyphosphate calcium material with morphogenetic activity. Mater. Lett. 2015, 148, 163–166. [Google Scholar] [CrossRef]
- Angelova, P.R.; Baev, A.Y.; Berezhnov, A.V.; Abramov, A.Y. Role of inorganic polyphosphate in mammalian cells: From signal transduction and mitochondrial metabolism to cell death. Biochem. Soc. Trans. 2016, 44, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Müller, W.E.G.; Tolba, E.; Feng, Q.; Schröder, H.C.; Markl, J.S.; Kokkinopoulou, M.; Wang, X.H. Amorphous Ca2+ polyphosphate nanoparticles regulate ATP level in bone-like SaOS-2 cells. J. Cell Sci. 2015, 128, 2202–2207. [Google Scholar] [CrossRef] [PubMed]
- Di Virgilio, F.; Ferrari, D.; Adinolfi, E. P2X7: A growth-promoting receptor-implications for cancer. Purinergic Signal. 2009, 5, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Müller, W.E.G.; Wang, S.; Neufurth, M.; Kokkinopoulou, M.; Feng, Q.; Schröder, H.C.; Wang, X.H. Polyphosphate as a donor of high-energy phosphate for the synthesis of ADP and ATP. J. Cell Sci. 2017, 130, 2747–2756. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.H.; Schröder, H.C.; Diehl-Seifert, B.; Kropf, K.; Schlossmacher, U.; Wiens, M.; Müller, W.E.G. Dual effect of inorganic polymeric phosphate/polyphosphate on osteoblasts and osteoclasts in vitro. J. Tissue Eng. Regen. Med. 2013, 7, 767–776. [Google Scholar] [CrossRef] [PubMed]
- Docampo, R. Acidocalcisomes and polyphosphate granules. In Microbiology Monographs; Springer-Verlag: Berlin, Germany, 2006; pp. 53–70. [Google Scholar]
- Polascik, T.J.; Mouraviev, V. Zoledronic acid in the management of metastatic bone disease. Ther. Clin. Risk Manag. 2008, 4, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Suva, L.J.; Washam, C.; Nicholas, R.W.; Griffin, R.J. Bone metastasis: Mechanisms and therapeutic opportunities. Nat. Rev. Endocrinol. 2011, 7, 208–218. [Google Scholar] [CrossRef] [PubMed]
- Zekri, J.; Mansour, M.; Karim, S.M. The anti-tumour effects of zoledronic acid. J. Bone Oncol. 2014, 3, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Herlofsen, S.R.; Küchler, A.M.; Melvik, J.E.; Brinchmann, J.E. Chondrogenic differentiation of human bone marrow-derived mesenchymal stem cells in self-gelling alginate discs reveals novel chondrogenic signature gene clusters. Tissue Eng. Part A 2011, 17, 1003–1013. [Google Scholar] [CrossRef] [PubMed]
- Zou, L.; Kidwai, F.K.; Kopher, R.A.; Motl, J.; Kellum, C.A.; Westendorf, J.J.; Kaufman, D.S. Use of RUNX2 expression to identify osteogenic progenitor cells derived from human embryonic stem cells. Stem Cell Rep. 2015, 4, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Murakami, S.; Kan, M.; McKeehan, W.L.; de Crombrugghe, B. Up-regulation of the chondrogenic Sox9 gene by fibroblast growth factors is mediated by the mitogen-activated protein kinase pathway. Proc. Natl. Acad. Sci. USA 2000, 97, 1113–1118. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Kienzle, A.; Liu, X.J.; Müller, W.E.G.; Feng, Q.L. In vitro 30 nm silver nanoparticles promote chondrogenesis of human mesenchymal stem cells. RSC Adv. 2015, 5, 49809–49818. [Google Scholar] [CrossRef]
- Li, C.Y.; Yam, L.T.; Lam, K.W. Studies of acid phosphatase isoenzymes in human leukocytes. Demonstration of isoenzyme cell specificity. J. Histochem. Cytochem. 1970, 18, 901–910. [Google Scholar] [CrossRef] [PubMed]
- Walsh, N.C.; Cahill, M.; Carninci, P.; Kawai, J.; Okazaki, Y.; Hayashizaki, Y.; Hume, D.A.; Cassady, A.I. Multiple tissue-specific promoters control expression of the murine tartrate-resistant acid phosphatase gene. Gene 2003, 307, 111–123. [Google Scholar] [CrossRef]
- Blumer, M.J.; Hausott, B.; Schwarzer, C.; Hayman, A.R.; Stempel, J.; Fritsch, H. Role of tartrate-resistant acid phosphatase (TRAP) in long bone development. Mech. Dev. 2012, 129, 162–176. [Google Scholar] [CrossRef] [PubMed]
- Gradin, P.; Hollberg, K.; Cassady, A.I.; Lång, P.; Andersson, G. Transgenic overexpression of tartrate-resistant acid phosphatase is associated with induction of osteoblast gene expression and increased cortical bone mineral content and density. Cells Tissues Org. 2012, 196, 68–81. [Google Scholar] [CrossRef] [PubMed]
- Drake, F.H.; Dodds, R.A.; James, I.E.; Connor, J.R.; Debouck, C.; Richardson, S.; Lee-Rykaczewski, E.; Coleman, L.; Rieman, D.; Barthlow, R.; et al. Cathepsin K, but not cathepsins B, L, or S, is abundantly expressed in human osteoclasts. J. Biol. Chem. 1996, 271, 12511–12516. [Google Scholar] [CrossRef] [PubMed]
- Mundy, G.R. Metastasis to bone: Causes, consequences and therapeutic opportunities. Nat. Rev. Cancer 2002, 2, 584–593. [Google Scholar] [CrossRef] [PubMed]
- Amend, S.R.; Valkenburg, K.C.; Pienta, K.J. Murine hind limb long bone dissection and bone marrow isolation. J. Vis. Exp. 2016, 110, e53936. [Google Scholar] [CrossRef] [PubMed]
- Weilbaecher, K.N.; Guise, T.A.; McCauley, L.K. Cancer to bone: A fatal attraction. Nat. Rev. Cancer 2011, 11, 411–425. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Kramer, S.A.; Huxford-Phillips, R.C.; Wang, S.; Della Rocca, J.; Lin, W. Coercing bisphosphonates to kill cancer cells with nanoscale coordination polymers. Chem. Commun. 2012, 48, 2668–2670. [Google Scholar] [CrossRef]
- Khajuria, D.K.; Razdan, R.; Mahapatra, D.R. Development, in vitro and in vivo characterization of zoledronic acid functionalized hydroxyapatite nanoparticle based formulation for treatment of osteoporosis in animal model. Eur. J. Pharm. Sci. 2015, 66, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Roca-Cusachs, P.; del Rio, A.; Puklin-Faucher, E.; Gauthier, N.C.; Biais, N.; Sheetz, M.P. Integrin-dependent force transmission to the extracellular matrix by α-actinin triggers adhesion maturation. Proc. Natl. Acad. Sci. USA. 2013, 110, E1361–E1370. [Google Scholar] [CrossRef] [PubMed]
- Lafage-Proust, M.H.; Roche, B.; Langer, M.; Cleret, D.; Vanden Bossche, A.; Olivier, T.; Vico, L. Assessment of bone vascularization and its role in bone remodeling. BoneKEy Rep. 2015, 4. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.H.; Ackermann, M.; Tolba, E.; Neufurth, M.; Wurm, F.; Feng, Q.; Wang, S.; Schröder, H.C.; Müller, W.E.G. Artificial cartilage bio-matrix formed of hyaluronic acid and Mg2+-polyphosphate. Eur. Cell Mater. 2016, 32, 271–283. [Google Scholar] [CrossRef] [PubMed]
- Müller, W.E.G.; Ackermann, M.; Tolba, E.; Neufurth, M.; Wang, S.; Schröder, H.C.; Wang, X.H. A bio-imitating approach to fabricate an artificial matrix for cartilage tissue engineering using magnesium-polyphosphate and hyaluronic acid. RSC Adv. 2016, 6, 88559–88570. [Google Scholar] [CrossRef]
- Müller, W.E.G.; Schröder, H.C.; Tolba, E.; Diehl-Seifert, B.; Wang, X.H. Mineralization of bone-related SaOS-2 cells under physiological hypoxic conditions. FEBS J. 2016, 283, 74–87. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.H.; Ackermann, M.; Wang, S.; Tolba, E.; Neufurth, M.; Feng, Q.; Schröder, H.C.; Müller, W.E.G. Amorphous polyphosphate/amorphous calcium carbonate implant material with enhanced bone healing efficacy in a critical-size-defect in rats. Biomed. Mater. 2016, 11, 035005. [Google Scholar] [CrossRef] [PubMed]
- Neufurth, M.; Wang, X.H.; Schröder, H.C.; Feng, Q.L.; Diehl-Seifert, B.; Ziebart, T.; Steffen, R.; Wang, S.F.; Müller, W.E.G. Engineering a morphogenetically active hydrogel for bioprinting of bioartificial tissue derived from human osteoblast-like SaOS-2 cells. Biomaterials 2014, 35, 8810–8819. [Google Scholar] [CrossRef] [PubMed]
- Weiner, S.; Mahamid, J.; Politi, Y.; Ma, Y.; Addadi, L. Overview of the amorphous precursor phase strategy in biomineralization. Front. Mater. Sci. 2009, 3, 104–108. [Google Scholar] [CrossRef]
- Zhou, G.; Zheng, Q.; Engin, F.; Munivez, E.; Chen, Y.; Sebald, E.; Krakow, D.; Lee, B. Dominance of SOX9 function over RUNX2 during skeletogenesis. Proc. Natl. Acad. Sci. USA 2006, 103, 19004–19009. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, Y.; Choukroun, J.; Ghanaati, S.; Miron, R.J. Effects of an injectable platelet-rich fibrin on osteoblast behavior and bone tissue formation in comparison to platelet-rich plasma. Platelets 2017, 29, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kabiri, A.; Esfandiari, E.; Esmaeili, A.; Hashemibeni, B.; Pourazar, A.; Mardani, M. Platelet-rich plasma application in chondrogenesis. Adv. Biomed. Res. 2014, 3, 138. [Google Scholar] [CrossRef] [PubMed]
- Zhong, W.; Sumita, Y.; Ohba, S.; Kawasaki, T.; Nagai, K.; Ma, G.; Asahina, I. In vivo comparison of the bone regeneration capability of human bone marrow concentrates vs. platelet-rich plasma. PLoS ONE 2012, 7, e40833. [Google Scholar] [CrossRef] [PubMed]
- Little, D.G.; Ramachandran, M.; Schindeler, A. The anabolic and catabolic responses in bone repair. J. Bone Jt. Surg. Br. 2007, 89, 425–433. [Google Scholar] [CrossRef] [PubMed]
- Abrams, G.D.; Frank, R.M.; Fortier, L.A.; Cole, B.J. Platelet-rich plasma for articular cartilage repair. Sports Med. Arthrosc. 2013, 21, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Mithoefer, K.; McAdams, T.R.; Scopp, J.M.; Mandelbaum, B.R. Emerging options for treatment of articular cartilage injury in the athlete. Clin. Sports Med. 2009, 28, 25–40. [Google Scholar] [CrossRef] [PubMed]
- Cenni, E.; Avnet, S.; Fotia, C.; Salerno, M.; Baldini, N. Platelet-rich plasma impairs osteoclast generation from human precursors of peripheral blood. J. Orthop. Res. 2010, 28, 792–797. [Google Scholar] [CrossRef] [PubMed]
- De Souza Faloni, A.P.; Schoenmaker, T.; Azari, A.; Katchburian, E.; Cerri, P.S.; de Vries, T.J.; Everts, V. Jaw and long bone marrows have a different osteoclastogenic potential. Calcif. Tissue Int. 2011, 88, 63–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosen, L.S.; Gordon, D.; Tchekmedyian, S.; Yanagihara, R.; Hirsh, V.; Krzakowski, M.; Pawlicki, M.; de Souza, P.; Zheng, M.; Urbanowitz, G.; et al. Zoledronic acid versus placebo in the treatment of skeletal metastases in patients with lung cancer and other solid tumors: A phase III, double-blind, randomized trial—The zoledronic acid lung cancer and other solid tumors study group. J. Clin. Oncol. 2003, 21, 3150–3157. [Google Scholar] [CrossRef] [PubMed]
- Theriault, R.L.; Theriault, R.L. Biology of bone metastases. Cancer Control 2012, 19, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.H.; Schröder, H.C.; Müller, W.E.G. Enzyme-based biosilica and biocalcite: Biomaterials for the future in regenerative medicine. Trends Biotechnol. 2014, 32, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Hirsh, V. Targeted treatments of bone metastases in patients with lung cancer. Front. Oncol. 2014, 4, 146. [Google Scholar] [CrossRef] [PubMed]
- Fischer, V.; Lieberwirth, I.; Jakob, G.; Landfester, K.; Muñoz-Espí, R. Metal oxide/polymer hybrid nanoparticles with versatile functionality prepared by controlled surface crystallization. Adv. Funct. Mater. 2013, 23, 451–466. [Google Scholar] [CrossRef]
- Westhrin, M.; Xie, M.; Olderøy, M.Ø.; Sikorski, P.; Strand, B.L.; Standal, T. Osteogenic differentiation of human mesenchymal stem cells in mineralized alginate matrices. PLoS ONE 2015, 10, e0120374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mori, K.; Berreur, M.; Blanchard, F.; Chevalier, C.; Guisle-Marsollier, I.; Masson, M.; Rédini, F.; Heymann, D. Receptor activator of nuclear factor-kappaB ligand (RANKL) directly modulates the gene expression profile of RANK-positive Saos-2 human osteosarcoma cells. Oncol. Rep. 2007, 18, 1365–1371. [Google Scholar] [PubMed]
- Templeton, Z.S.; Bachmann, M.H.; Alluri, R.V.; Maloney, W.J.; Contag, C.H.; King, B.L. Methods for culturing human femur tissue explants to study breast cancer cell colonization of the metastatic niche. J. Vis. Exp. 2015, 97. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Quan, N. Immune cell isolation from mouse femur bone marrow. Bio-Protocol 2015, 5. [Google Scholar] [CrossRef]
- Wiens, M.; Wang, X.H.; Schröder, H.C.; Kolb, U.; Schloßmacher, U.; Ushijima, H.; Müller, W.E.G. The role of biosilica in the osteoprotegerin/RANKL ratio in human osteoblast-like cells. Biomaterials 2010, 31, 7716–7725. [Google Scholar] [CrossRef] [PubMed]
- Bowles, J.; Feng, C.W.; Knight, D.; Smith, C.A.; Roeszler, K.N.; Bagheri-Fam, S.; Harley, V.R.; Sinclair, A.H.; Koopman, P. Male-specific expression of Aldh1a1 in mouse and chicken fetal testes: Implications for retinoid balance in gonad development. Dev. Dyn. 2009, 238, 2073–2080. [Google Scholar] [CrossRef] [PubMed]
- Endo, T.; Kobayashi, T. Runx2 deficiency in mice causes decreased thyroglobulin expression and hypothyroidism. Mol. Endocrinol. 2010, 24, 1267–1273. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Gao, J.; Lv, X.; Yang, W.; Wen, S.; Tong, H.; Tang, C. Quantitative evaluation and selection of reference genes for quantitative RT-PCR in mouse acute pancreatitis. Biomed. Res. Int. 2016, 2016, 8367063. [Google Scholar] [CrossRef] [PubMed]
- Cuetara, B.L.; Crotti, T.N.; O’Donoghue, A.J.; McHugh, K.P. Cloning and characterization of osteoclast precursors from the RAW264.7 cell line. In Vitro. Cell. Dev. Biol. Anim. 2006, 42, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Gregory, C.A.; Gunn, W.G.; Peister, A.; Prockop, D.J. An Alizarin red-based assay of mineralization by adherent cells in culture: Comparison with cetylpyridinium chloride extraction. Anal. Biochem. 2004, 329, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Schröder, H.C.; Borejko, A.; Krasko, A.; Reiber, A.; Schwertner, H.; Müller, W.E.G. Mineralization of SaOS-2 cells on enzymatically (Silicatein) modified bioactive osteoblast-stimulating surfaces. J. Biomed. Mat. Res. Part B 2005, 75B, 387–392. [Google Scholar] [CrossRef] [PubMed]
- Petrie, A.; Watson, P. Statistics for Veterinary and Animal Science; Wiley-Blackwell: Oxford, UK, 2013; pp. 85–99. [Google Scholar]
- Clézardin, P. Mechanisms of action of bisphosphonates in oncology: A scientific concept evolving from antiresorptive to anticancer activities. BoneKEy Rep. 2013, 2. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.H.; Wang, S.; He, F.; Tolba, E.; Schröder, H.C.; Diehl-Seifert, B.; Müller, W.E.G. Polyphosphate as a bioactive and biodegradable implant material: Induction of bone regeneration in rats. Adv. Eng. Mater. 2016, 18, 1406–1417. [Google Scholar] [CrossRef]
- Müller, W.E.G.; Relkovic, D.; Ackermann, M.; Wang, S.; Neufurth, M.; Paravic-Radicevic, A.; Ushijima, H.; Schröder, H.C.; Wang, X.H. Enhancement of wound healing in normal and diabetic mice by topical application of amorphous polyphosphate—Superior effect of the host-guest composite material composed of collagen (host) and polyphosphate (guest). Polymers 2017, 9, 300. [Google Scholar] [CrossRef]
- Müller, W.E.G.; Wang, S.; Wiens, M.; Neufurth, M.; Ackermann, M.; Relkovic, D.; Kokkinopoulou, M.; Feng, Q.; Schröder, H.C.; Wang, X.H. Uptake of polyphosphate microparticles in vitro (SaOS-2 and HUVEC cells) followed by an increase of the intracellular ATP pool size. PLoS ONE 2017, 12, e0188977. [Google Scholar] [CrossRef] [PubMed]
- Müller, W.E.G.; Tolba, E.; Ackermann, M.; Neufurth, M.; Wang, S.; Feng, Q.; Schröder, H.C.; Wang, X.H. Fabrication of amorphous strontium polyphosphate microparticles that induce mineralization of bone cells in vitro and in vivo. Acta Biomater. 2017, 50, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Saeed, H.; Ahsan, M.; Saleem, Z.; Iqtedar, M.; Islam, M.; Danish, Z.; Khan, A.M. Mesenchymal stem cells (MSCs) as skeletal therapeutics—An update. J. Biomed. Sci. 2016, 23, 41. [Google Scholar] [CrossRef] [PubMed]









© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Müller, W.E.G.; Neufurth, M.; Wang, S.; Ackermann, M.; Muñoz-Espí, R.; Feng, Q.; Lu, Q.; Schröder, H.C.; Wang, X. Amorphous, Smart, and Bioinspired Polyphosphate Nano/Microparticles: A Biomaterial for Regeneration and Repair of Osteo-Articular Impairments In-Situ. Int. J. Mol. Sci. 2018, 19, 427. https://doi.org/10.3390/ijms19020427
Müller WEG, Neufurth M, Wang S, Ackermann M, Muñoz-Espí R, Feng Q, Lu Q, Schröder HC, Wang X. Amorphous, Smart, and Bioinspired Polyphosphate Nano/Microparticles: A Biomaterial for Regeneration and Repair of Osteo-Articular Impairments In-Situ. International Journal of Molecular Sciences. 2018; 19(2):427. https://doi.org/10.3390/ijms19020427
Chicago/Turabian StyleMüller, Werner E. G., Meik Neufurth, Shunfeng Wang, Maximilian Ackermann, Rafael Muñoz-Espí, Qingling Feng, Qiang Lu, Heinz C. Schröder, and Xiaohong Wang. 2018. "Amorphous, Smart, and Bioinspired Polyphosphate Nano/Microparticles: A Biomaterial for Regeneration and Repair of Osteo-Articular Impairments In-Situ" International Journal of Molecular Sciences 19, no. 2: 427. https://doi.org/10.3390/ijms19020427
APA StyleMüller, W. E. G., Neufurth, M., Wang, S., Ackermann, M., Muñoz-Espí, R., Feng, Q., Lu, Q., Schröder, H. C., & Wang, X. (2018). Amorphous, Smart, and Bioinspired Polyphosphate Nano/Microparticles: A Biomaterial for Regeneration and Repair of Osteo-Articular Impairments In-Situ. International Journal of Molecular Sciences, 19(2), 427. https://doi.org/10.3390/ijms19020427