Palladium Nanoparticles: Toxicological Effects and Potential Implications for Occupational Risk Assessment
Abstract
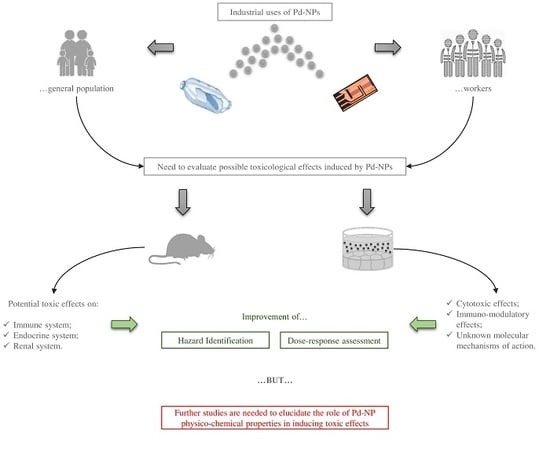
:1. Introduction
2. Possible Pd-NP Applications
3. In Vitro Studies
3.1. Plant Models
3.2. Animal Cells
3.3. Human Cells
3.3.1. Cytotoxic Effects
3.3.2. Immunological and Inflammatory Responses
4. Ex Vivo Studies
5. In Vivo Studies
5.1. Bacteria and Microbial Communities
5.2. Animal Models
6. Discussion
7. Conclusions
Conflicts of Interest
Abbreviations
| 3D model | Three dimensional model |
| A2780 | Human ovarian cancer cells |
| A375 | Human skin malignant melanoma cells |
| A549 | Immortalized alveolar basal human epithelial cells |
| CA | Cysteamine |
| Caco-2 | Human colon adenocarcinoma cells |
| E2 | Estradiol |
| FSH | Follicle-stimulating hormone |
| GM-CSF | Granulocyte-macrophage colony stimulating factor |
| Hacat | Human keratinocyte cells |
| HeLa | Human epithelial cervical cancer cells |
| HepG2 | Human liver cancer cells G2 |
| IL | Interleukin |
| INF-γ | Interferon-γ |
| LC50LH | Lethal concentration 50Luteinizing hormone |
| LPS | Lypopolysaccharide |
| MBC | Mimum bactericidal concentration |
| MDR | Multidrug resistant |
| MIC | Minimum inhibitory concentration |
| MPA | Mercaptopropionic acid |
| n.s. | Not significant |
| MRC-5 | Human fetal lung fibroblast cells |
| NPs | Nanoparticles |
| P | Progesterone |
| Pd | Palladium |
| PBECs | Primary bronchial epithelial cells |
| PGE2 | Prostaglandin E2 |
| PHA | Phytohemagglutinin-L |
| PBMCs | Peripheral blood mononuclear cells |
| Pt | Platinum |
| Rat-1 | Rat embryo fibroblasts |
| RBPROS | Retinol binding proteinReactive oxygen species |
| SD | Standard deviation |
| T | Testosterone |
| THP-1 | Human monocyte leukaemia cells |
References
- Gatoo, M.A.; Naseem, S.; Arfat, M.Y.; Dar, A.M.; Qasim, K.; Zubair, S. Physicochemical properties of nanomaterials: Implication in associated toxic manifestations. BioMed Res. Int. 2014, 2014, 498420. [Google Scholar] [CrossRef] [PubMed]
- Rosi, N.L.; Mirkin, C.A. Nanostructures in biodiagnostics. Chem. Rev. 2005, 105, 1547–1562. [Google Scholar] [CrossRef] [PubMed]
- Roucoux, A.; Schulz, J.; Patin, H. Reduced transition metal colloids: A novel family of reusable catalysts? Chem. Rev. 2002, 102, 3757–3778. [Google Scholar] [CrossRef] [PubMed]
- Gurunathan, S.; Kim, E.; Han, J.W.; Park, J.H.; Kim, J.H. Green chemistry approach for synthesis of effective anticancer palladium nanoparticles. Molecules 2015, 20, 22476–22498. [Google Scholar] [CrossRef] [PubMed]
- Ravindra, K.; Bencs, L.; van Grieken, R. Platinum group elements in the environment and their health risk. Sci. Total Environ. 2004, 318, 1–43. [Google Scholar] [CrossRef]
- Iavicoli, I.; Bocca, B.; Caroli, S.; Caimi, S.; Alimonti, A.; Carelli, G.; Fontana, L. Exposure of Rome city tram drivers to airborne platinum, rhodium, and palladium. J. Occup. Environ. Med. 2008, 50, 1158–1166. [Google Scholar] [CrossRef] [PubMed]
- Iavicoli, I.; Bocca, B.; Carelli, G.; Caroli, S.; Caimi, S.; Alimonti, A.; Fontana, L. Biomonitoring of tram drivers exposed to airborne platinum, rhodium and palladium. Int. Arch. Occup. Environ. Health 2007, 81, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Iavicoli, I.; Bocca, B.; Petrucci, F.; Senofonte, O.; Carelli, G.; Alimonti, A.; Caroli, S. Biomonitoring of traffic police officers exposed to airborne platinum. Occup. Environ. Med. 2004, 61, 636–639. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, R.; El-Sayed, M.A. Catalysis with transition metal nanoparticles in colloidal solution: Nanoparticle shape dependence and stability. J. Phys. Chem. B 2005, 109, 12663–12676. [Google Scholar] [CrossRef] [PubMed]
- Borm, P.J.; Robbins, D.; Haubold, S.; Kuhlbusch, T.; Fissan, H.; Donaldson, K.; Schins, R.; Stone, V.; Kreyling, W.; Lademann, J.; et al. The potential risks of nanomaterials: A review carried out for ECETOC. Part. Fibre Toxicol. 2006, 14, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siddiqi, K.S.; Husen, A. Green Synthesis, Characterization and Uses of palladium/Platinum Nanoparticles. Nanoscale Res. Lett. 2016, 11, 482. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Boone, E.; El-Sayed, M.A. Size effects of PVP-Pd nanoparticles on the catalytic Suzuki reactions in aqueous solution. Langmuir 2002, 18, 4921–4925. [Google Scholar] [CrossRef]
- Saldan, I.; Semenyuk, Y.; Marchuk, I.; Reshetnyak, O. Chemical synthesis and application of palladium nanoparticles. J. Mater. Sci. 2015, 50, 2337–2354. [Google Scholar] [CrossRef]
- Patil, A.B.; Bhanage, B.M. Solar energy assisted starch-stabilized palladium nanoparticles and their application in C-C coupling reactions. J. Nanosci. Nanotechnol. 2013, 13, 5061–5068. [Google Scholar] [CrossRef] [PubMed]
- Jamwal, N.; Sodhi, R.K.; Gupta, P.; Paul, S. Nano Pd(0) supported on cellulose: A highly efficient and recyclable heterogeneous catalyst for the Suzuki coupling and aerobic oxidation of benzyl alcohols under liquid phase catalysis. Int. J. Biol. Macromol. 2011, 49, 930–935. [Google Scholar] [CrossRef] [PubMed]
- Lichtenberger, J.; Lee, D.; Iglesia, E. Catalytic oxidation of methanol on Pd metal and oxide clusters at near-ambient temperatures. Phys. Chem. Chem. Phys. 2007, 9, 4902–4906. [Google Scholar] [CrossRef] [PubMed]
- Dumas, A.; Couvreur, P. Palladium: A future key player in the nanomedical field? Chem. Sci. 2015, 6, 2153–2157. [Google Scholar] [CrossRef] [PubMed]
- Hennebel, T.; de Corte, S.; Verstraete, W.; Boon, N. Microbial production and environmental applications of Pd nanoparticles for treatment of halogenated compounds. Curr. Opin. Biotechnol. 2012, 23, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Horinouchi, S.; Yamanoi, Y.; Yonezawa, T.; Mouri, T.; Nishihara, H. Hydrogen storage properties of isocyanide-stabilized palladium nanoparticles. Langmuir 2006, 22, 1880–1884. [Google Scholar] [CrossRef] [PubMed]
- Cookson, J. The Preparation of palladium Nanoparticles. Controlled particle sizes are key to producing more effective and efficient materials. Platin. Met. Rev. 2012, 56, 83–98. [Google Scholar] [CrossRef]
- Cheong, S.; Watt, J.D.; Tilley, R.D. Shape control of platinum and palladium nanoparticles for catalysis. Nanoscale 2010, 2, 2045–2053. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Cheng, L.; Shen, P.; Liu, Y. Methanol and ethanol electrooxidation on Pt and Pd supported on carbon microspheres in alkaline media. Electrochem. Commun. 2007, 9, 997–1001. [Google Scholar] [CrossRef]
- Yamauchi, M.; Ikeda, R.; Kitagawa, H.; Takata, M. Nanosize Effects on Hydrogen Storage in palladium. J. Phys. Chem. C 2008, 112, 3294–3299. [Google Scholar] [CrossRef]
- Mubeen, S.; Zhang, T.; Yoo, G.; Deshusses, M.A.; Myung, N.V. Palladium nanoparticles decorated single-walled carbon nanotube hydrogen sensor. J. Phys. Chem. C 2007, 111, 6321–6327. [Google Scholar] [CrossRef]
- Qiong, Z.; Jin, S.C.; Xiao, F.L.; Hao, T.B.; Jian, H.J. Palladium nanaparticles/chitosan-grafted graphene nanocomposites for construction of a glucose biosensor. Biosens. Bioelectron. 2011, 26, 3456–3463. [Google Scholar] [CrossRef]
- Shah, V.; Belozerova, I. Influence of metal nanoparticles on the soil microbial community and germination of lettuce seeds. Water Air Soil Pollut. 2009, 197, 143–148. [Google Scholar] [CrossRef]
- Speranza, A.; Leopold, K.; Maier, M.; Taddei, A.R.; Scoccianti, V. Pd-nanoparticles cause increased toxicity to kiwifruit pollen compared to soluble Pd(II). Environ. Pollut. 2010, 158, 873–882. [Google Scholar] [CrossRef] [PubMed]
- Battke, F.; Leopold, K.; Maier, M.; Schmidhalter, U.; Schuster, M. palladium exposure of barley: Uptake and effects. Plant Biol. 2008, 10, 272–276. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, K.E.; Palmberg, L.; Witasp, E.; Kupczyk, M.; Feliu, N.; Gerde, P.; Seisenbaeva, G.A.; Fadeel, B.; Dahlén, S.E.; Kessler, V.G. Solution-engineered palladium nanoparticles: Model for health effect studies of automotive particulate pollution. ACS Nano 2011, 5, 5312–5324. [Google Scholar] [CrossRef] [PubMed]
- Iavicoli, I.; Farina, M.; Fontana, L.; Lucchetti, D.; Leso, V.; Fanali, C.; Cufino, V.; Boninsegna, A.; Leopold, K.; Schindl, R.; et al. In vitro evaluation of the potential toxic effects of palladium nanoparticles on fibroblasts and lung epithelial cells. Toxicol. In Vitro 2017, 42, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Petrarca, C.; Clemente, E.; Di Giampaolo, L.; Mariani-Costantini, R.; Leopold, K.; Schindl, R.; Lotti, L.V.; Mangifesta, R.; Sabbioni, E.; Niu, Q.; et al. Palladium nanoparticles induce disturbances in cell cycle entry and progression of peripheral blood mononuclear cells: Paramount role of ions. J. Immunol. Res. 2014, 2014, 295092. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Nitnavare, R.; Dewle, A.; Tomar, G.B.; Chippalkatti, R.; More, P.; Kitture, R.; Kale, S.; Bellare, J.; Chopade, B.A. Novel platinum-palladium bimetallic nanoparticles synthesized by Dioscorea bulbifera: Anticancer and antioxidant activities. Int. J. Nanomed. 2015, 10, 7477–7490. [Google Scholar] [CrossRef]
- Rajakumar, G.; Rahuman, A.A.; Chung, I.M.; Kirthi, A.V.; Marimuthu, S.; Anbarasan, K. Antiplasmodial activity of eco-friendly synthesized palladium nanoparticles using Eclipta prostrata extract against Plasmodium berghei in Swiss albino mice. Parasitol. Res. 2015, 114, 1397–1406. [Google Scholar] [CrossRef] [PubMed]
- Dahal, E.; Curtiss, J.; Subedi, D.; Chen, G.; Houston, J.P.; Smirnov, S. Evaluation of the catalytic activity and cytotoxicity of palladium nanocubes: The role of oxygen. ACS Appl. Mater. Interfaces 2015, 7, 9364–9371. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.T.; Smith, C.E.; Kwok, K.S.; Chen, J.; Kong, H.; Yang, H. Functionalized ultrathin palladium nanosheets as patches for HepG2 cancer cells. Chem. Commun. 2015, 51, 14171–14174. [Google Scholar] [CrossRef] [PubMed]
- Alarifi, S.; Ali, D.; Alkahtani, S.; Almeer, R.S. ROS-Mediated Apoptosis and Genotoxicity Induced by palladium Nanoparticles in Human Skin Malignant Melanoma Cells. Oxid. Med. Cell. Longev. 2017, 2017, 8439098. [Google Scholar] [CrossRef] [PubMed]
- Boscolo, P.; Bellante, V.; Leopold, K.; Maier, M.; di Giampaolo, L.; Antonucci, A.; Iavicoli, I.; Tobia, L.; Paoletti, A.; Montalti, M.; et al. Effects of palladium nanoparticles on the cytokine release from peripheral blood mononuclear cells of non-atopic women. J. Biol. Regul. Homeost. Agents 2010, 24, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Reale, M.; Vianale, G.; Lotti, L.V.; Mariani-Costantini, R.; Perconti, S.; Cristaudo, A.; Leopold, K.; Antonucci, A.; di Giampaolo, L.; Iavicoli, I.; et al. Effects of palladium nanoparticles on the cytokine release from peripheral blood mononuclear cells of palladium-sensitized women. J. Occup. Environ. Med. 2011, 53, 1054–1060. [Google Scholar] [CrossRef] [PubMed]
- Bellani, L.; Giorgetti, L.; Riela, S.; Lazzara, G.; Scialabba, A.; Massaro, M. Ecotoxicity of halloysite nanotube-supported palladium nanoparticles in Raphanus sativus L. Environ. Toxicol. Chem. 2016, 35, 2503–2510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hildebrand, H.; Kühnel, D.; Potthoff, A.; Mackenzie, K.; Springer, A.; Schirmer, K. Evaluating the cytotoxicity of palladium/magnetite nano-catalysts intended for wastewater treatment. Environ. Pollut. 2010, 158, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Neubauer, N.; Palomaeki, J.; Karisola, P.; Alenius, H.; Kasper, G. Size-dependent ROS production by palladium and nickel nanoparticles in cellular and acellular environments—An indication for the catalytic nature of their interactions. Nanotoxicology 2015, 9, 1059–1066. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.; Hedelin, A.; Malmlöf, M.; Kessler, V.; Seisenbaeva, G.; Gerde, P.; Palmberg, L. Development of combining of human bronchial mucosa models with XPOSEALI® for exposure of air pollution nanoparticles. PLoS ONE 2017, 12, e0170428. [Google Scholar] [CrossRef] [PubMed]
- Chhay, P.; Murphy-Marion, M.; Samson, Y.; Girard, D. Activation of human eosinophils with palladium nanoparticles (Pd NPs): Importance of the actin cytoskeleton in Pd NPs-induced cellular adhesion. Environ. Toxicol. Pharmacol. 2017, 57, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Boczkowski, J.; Hoet, P. What’s new in nanotoxicology? Implications for public health from a brief review of the 2008 literature. Nanotoxicology 2010, 4, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Faurschou, A.; Menné, T.; Johansen, J.D.; Thyssen, J.P. Metal allergen of the 21st century—A review on exposure, epidemiology and clinical manifestations of palladium allergy. Contact Dermat. 2011, 64, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Muris, J.; Goossens, A.; Gonçalo, M.; Bircher, A.J.; Giménez-Arnau, A.; Foti, C.; Bruze, M.; Andersen, K.E.; Rustemeyer, T.; Feilzer, A.J.; et al. Sensitization to palladium in Europe. Contact Dermat. 2015, 72, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Muris, J.; Goossens, A.; Gonçalo, M.; Bircher, A.J.; Giménez-Arnau, A.; Foti, C.; Rustemeyer, T.; Feilzer, A.J.; Kleverlaan, C.J. Sensitization to palladium and nickel in Europe and the relationship with oral disease and dental alloys. Contact Dermat. 2015, 72, 286–296. [Google Scholar] [CrossRef] [PubMed]
- Larese Filon, F.; Maina, G.; Adami, G.; Venier, M.; Coceani, N.; Bussani, R.; Massiccio, M.; Barbieri, P.; Spinelli, P. In vitro percutaneous absorption of cobalt. Int. Arch. Occup. Environ. Health 2004, 77, 85–99. [Google Scholar] [CrossRef] [PubMed]
- Ponti, J.; Sabbioni, E.; Munaro, B.; Broggi, F.; Marmorato, P.; Franchini, F.; Colognato, R.; Rossi, F. Genotoxicity and morphological transformation induced by cobalt nanoparticles and cobalt chloride: An in vitro study in Balb/3T3 mouse fibroblasts. Mutagenesis 2009, 24, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Larese Filon, F.; Crosera, M.; Mauro, M.; Baracchini, E.; Bovenzi, M.; Montini, T.; Fornasiero, P.; Adami, G. palladium nanoparticles exposure: Evaluation of permeation through damaged and intact human skin. Environ. Pollut. 2016, 214, 497–503. [Google Scholar] [CrossRef] [PubMed]
- Adams, C.P.; Walker, K.A.; Obare, S.O.; Docherty, K.M. Size-dependent antimicrobial effects of novel palladium nanoparticles. PLoS ONE 2014, 9, e85981. [Google Scholar] [CrossRef] [PubMed]
- Iavicoli, I.; Fontana, L.; Corbi, M.; Leso, V.; Marinaccio, A.; Leopold, K.; Schindl, R.; Sgambato, A. Exposure to palladium nanoparticles affects serum levels of cytokines in female wistar rats. PLoS ONE 2015, 10, e0143801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iavicoli, I.; Fontana, L.; Leso, V.; Corbi, M.; Marinaccio, A.; Leopold, K.; Schindl, R.; Lucchetti, D.; Calapà, F.; Sgambato, A. Subchronic exposure to palladium nanoparticles affects serum levels of cytokines in female Wistar rats. Hum. Exp. Toxicol. 2017, 3, 309–320. [Google Scholar] [CrossRef] [PubMed]
- Fontana, L.; Leso, V.; Marinaccio, A.; Cenacchi, G.; Papa, V.; Leopold, K.; Schindl, R.; Bocca, B.; Alimonti, A.; Iavicoli, I. The effects of palladium nanoparticles on the renal function of female Wistar rats. Nanotoxicology 2015, 9, 843–851. [Google Scholar] [CrossRef] [PubMed]
- Leso, V.; Fontana, L.; Marinaccio, A.; Leopold, K.; Fanali, C.; Lucchetti, D.; Sgambato, A.; Iavicoli, I. The effects of palladium nanoparticles on the endocrine reproductive system of female Wistar rats. Hum. Exp. Toxicol. 2018, in press. [Google Scholar] [CrossRef] [PubMed]
- Murugesan, K.; Bokare, V.; Jeon, J.R.; Kim, E.J.; Kim, J.H.; Chang, Y.S. Effect of Fe-Pd bimetallic nanoparticles on Sphingomonas sp. PH-07 and a nano-bio hybrid process for triclosan degradation. Bioresour. Technol. 2011, 102, 6019–6025. [Google Scholar] [CrossRef] [PubMed]
- Boomi, P.; Gurumallesh Prabu, H.; Mathiyarasu, J. Original article synthesis, characterization and antibacterial activity of polyaniline/Pt-Pd nanocomposite. Eur. J. Med. Chem. 2014, 72, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Surendra, T.V.; Roopan, S.M.; Arasu, M.V.; Al-Dhabi, N.A.; Rayalu, G.M. RSM optimized Moringa oleifera peel extract for green synthesis of M. oleifera capped palladium nanoparticles with antibacterial and hemolytic property. J. Photochem. Photobiol. B 2016, 162, 550–557. [Google Scholar] [CrossRef] [PubMed]
- Nuzzo, A.; Hosseinkhani, B.; Boon, N.; Zanaroli, G.; Fava, F. Impact of bio-palladium nanoparticles (bio-Pd NPs) on the activity and structure of a marine microbial community. Environ. Pollut. 2017, 220, 1068–1078. [Google Scholar] [CrossRef] [PubMed]
- Hazarika, M.; Borah, D.; Bora, P.; Silva, A.R.; Das, P. Biogenic synthesis of palladium nanoparticles and their applications as catalyst and antimicrobial agent. PLoS ONE 2017, 12, e0184936. [Google Scholar] [CrossRef] [PubMed]
- Gizdavic-Nikolaidis, M.R.; Bennett, J.R.; Swift, S.; Easteal, A.J.; Ambrose, M. Broad spectrum antimicrobial activity of functionalized polyanilines. Acta Biomater. 2011, 7, 4204–4209. [Google Scholar] [CrossRef] [PubMed]
- Iavicoli, I.; Fontana, L.; Nordberg, G. The effects of nanoparticles on the renal system. Crit. Rev. Toxicol. 2016, 46, 490–560. [Google Scholar] [CrossRef] [PubMed]
- Iavicoli, I.; Leso, V.; Manno, M.; Schulte, P.A. Biomarkers of nanomaterial exposure and effect: Current status. J. Nanopart. Res. 2014, 16, 2302. [Google Scholar] [CrossRef]
- Iavicoli, I.; Leso, V.; Schulte, P.A. Biomarkers of susceptibility: State of the art and implications for occupational exposure to engineered nanomaterials. Toxicol. Appl. Pharmacol. 2016, 299, 112–124. [Google Scholar] [CrossRef] [PubMed]
| Palladium-NP Characterization | Investigated Models | Experimental Design | Endpoints | Results | References |
|---|---|---|---|---|---|
| Plants | |||||
| Size: 1–12 nm | Barley (Hordeum vulgare) plants | Seeds were cultivated in a floating hydroponic system containing a nutrient solution (0–50 μmol Pd/L) for 1 week | Leaf length | Leaf length significantly decreased according to the increased exposure concentrations (105–115 mm after 50 μmol Pd vs. 120–125 mm of unexposed plants). | Battke et al. [28] |
| Pd-NPs entrapped in an aluminum hydroxide matrix | Lettuce seeds | Seeds were planted immediately (day 0) or 15 day after adding NPs to the soil (0.013 and 0.066% w/w) | Seed germination and growth | Shoot/root ratio: no significant influence. on the difference in the ratio when the seeds were planted on day 0. palladium-NPs at low concentration significantly increased the ratio (2.5 cm) compared to controls (1.41 cm) in soils incubated with NPs for 15 d. | Shah and Bazelerova, [26] |
| Size: 5–10 nm | Kiwifruit pollen from plants of the male genotype of Actinidia deliciosa var. deliciosa | Pollen was exposed to 0–7 mg/L NPs for up to 90 min | Pollen performance and lethality | Tube emergence and growth: significant inhibition began at 0.1 mg/L, complete growth cessation at 0.4 mg/L. Lethality: as concentration increased, viability exponentially decreased (LC50 at 90 min: 1.0 ± 0.3 mg/L). | Speranza et al. [27] |
| Halloysite supported Pd-NPs. Tube diameter: 50 nm; Inner lumen diameter: 15 nm; Surface area: 65 m2/g | High and low vigor R. sativus | Radish seeds were exposed to 10 mL of nanomaterials at concentrations ranging 0–1500 mg/L for up to 72 h | Germination and seedling development | Exposure to Halloysite -PdNPs had no significant influence on germination, seedling development, xylem differentiation, or mitotic index in both lots. | Bellani et al. [39] |
| Animal cells | |||||
| Chemical composition: Pd/magnetite; Size: 20–30 nm; Purity: ≥98% Surface area: 46.4 ± 0.2 m2/g | RTgill-W1 | Cells were exposed to 5–25 mg/L NPs for 1 h and 3 days | Cell viability | Metabolic activity and membrane integrity showed a significant decrease after 1 h exposure due to cellular stress. Cell viability was full restored after 3 days. | Hildebrand et al. [40] |
| Size: 10 ± 6 nm | Rat-1 | Cells were exposed to 1 and 2 μg/mL NPs for 2–120 h | Cell viability; Oxidative stress reaction; cell cycle distribution; DNA damage | Cell viability was significantly reduced by both concentrations at 120 h. Cell cycle distribution: time-dependent increase in G0/G1 phase (from 45% in controls to 70% following 2 μg/mL for 120 h). Decrease of cell percentage in the S phase (from 30% in controls to 15% following 2 μg/mL for 120 h). DNA damage: a significant effect was evident only following treatment with 2 μg/mL for 120 h. ROS production: significant increase compared to controls only after 2 μg/mL NPs for 2 and 4 h. | Iavicoli et al. [30] |
| Human cells | |||||
| Size: 5–10 nm | PBMCs from 8 non-atopic healthy donors | Cells were overnight incubated with NPs (10−5, 10−6 M), with or without 10 μg/mL LPS stimulation | Cell viability; Cytokine release | Cell viability: not affected by all conditions of exposure. Cytokine release (no LPS): 10−5 M Pd-NPs significantly inhibited TNF-α and IL-17. Cytokine release (with LPS): 10−5 M Pd-NPs significantly inhibited TNF-α and IL-17 and increased INF-γ. Pd-NPs (10−6 M) significantly inhibited IL-17. | Boscolo et al. [37] |
| Size: 5–10 nm | PBMCs from 12 non-atopic and 8 atopic donors | Cells were overnight incubated with NPs (10−5 M), with or without 10 μg/mL LPS stimulation | Cell viability; Cytokine release | Cell viability: not affected by all conditions of exposure. Cytokine release (no LPS): no significant effects on cytokine release. Cytokine release (with LPS): NPs significantly reduced TNF-α and increased INF-γ levels in cells from non-atopic subjects. NPs reduced TNF-α in cells from atopic subjects. | Reale et al. [38] |
| Chemical composition: Pd/magnetite; Size: 20–30 nm; Purity: ≥98% Surface area: 46.4 ± 0.2 m2/g | Caco-2, Hacat | Cells were exposed to 5–25 mg/L NPs for 1 h and 3 days | Cell viability; Oxidative stress reaction | Hacat cells: viability was reduced to app. 91 ± 3% for all tested concentrations. After 3 days cell viability recovered up to 94% except with highest dose. Caco-2 cells showed similar tendency. No significant ROS production was demonstrated in comparison to controls. | Hildebrand et al. [40] |
| Size: 10.4 ± 2.7 nm; Shape: spherical | PBECs, A549 | Cells were exposed to 0–25 μg/mL NPs for 24 h | Cell viability; Oxidative stress reaction; inflammatory response | Cell viability: no alterations up to 10 μg/mL, decreased by higher concentrations. PBEC apoptosis: dose-dependently induced. ROS: no significant induction. IL-8: significantly increased at 0.01 and 10 μg/mL, and decreased at 0.1 μg/mL in A549 cells; significantly decreased at 1 μg/mL for PBECs compared to controls. PGE2: significantly decreased at 0.1 and 10 μg/mL in A549 cells; significantly decreased at all the concentrations tested for PBECs compared to controls. | Wilkinson et al. [29] |
| Zerovalent NPs; Size: 2–8 nm | PBMCs from healthy donors | Cells were exposed to 0–80 μg/mL NPs for 4–72 h following PHA stimulation (48 h) | Cell viability; Oxidative stress reaction; Cell cycle distribution | No alterations in cell viability (4 h); Significant reduction at 80 (24 h), 20 (48 h) and 10 μg/mL (72 h). A moderate (n.s.) intracellular ROS increase was induced by NP exposure. Cell cycle distribution: PHA-stimulated PBMCs (G0/G1: 43.1 ± 1.9%; S: 48.8 ± 2.3%; G2/M: 8.1 ± 1.2%); 10 μg/mL NPs (G0/G1: 59.8 ± 1.8%; S: 35.4 ± 1.5%; G2/M: 4.8 ± 1.2%). | Petrarca et al. [31] |
| Size: 3.8 ± 0.4–26.6 ± 2.2 nm; Agglomerate size: 50–150 nm | THP-1 | Cells were exposed to 0–450 μg/mL NPs | Oxidative stress reaction | A linear dependence of ROS production was evident up to 100 μg/mL. Above this level ROS levels were leveled off. | Neubauer et al. [41] |
| Pd-NPs obtained through leaf extracts of Evolvulus alsinoides. Shape: spherical; Size: 27 ± 2 nm | A2780 | Cells were exposed to 0–10 μg/mL NPs for 24 h | Cell viability; Oxidative stress reaction | Cell viability: doses greater than 6 μg/mL NPs induced a significant reduction compared to controls. ROS production: dose-dependent, significant increase in treated cells compared to controls. Apoptosis: induced by NP treatment compared to controls. | Gurunathan et al. [4] |
| Pd nanosheets; positively charged CA-Pd nanosheets; Negatively charged 3-MPA-Pd-nanosheets | HepG2 | Cells were incubated with nanosheets at 0–100 ppm for 24 h | Cell viability | Pd nano-sheets: 93.2 ± 6.8% at 50 ppm; 74.7 ± 8.5% at 100 ppm. CA-Pd nano-sheets: 42.4 ± 11.9% at 50 ppm; 17.93 ± 3.74% at 100 ppm. MPA-Pd nano-sheets: 83.7 ± 8.54% at 50 ppm; 68.4 ± 3.9% at 100 ppm. | Pan et al. [35] |
| Pd-NPs synthesized through leaf extract of Eclipta prostrate. Size: 18–64 nm (average size 27 ± 1.3 nm); Aggregate sizes: 63 ± 1.4 nm | HepG2 | Cells were incubated with nanosheets at 0–500 μg/mL | Cell viability | Cytotoxicity was 8.5, 24, 48, 65, and 76.5% of cells treated with 1, 10, 100, 250, 500 μg/mL. | Rajakumar et al. [33] |
| Pd-nanocubes Size: ~10 nm | HeLa | Cells were exposed to 425 μg/mL Pd-nanocubes for 24 h | Cell viability | Percent of non-viable cells (necrotic or late apoptotic): 6.6% with serum, 14.7% without serum. Percent of apoptotic cells: ~7% with serum, 23% without serum (DNA fragmentation test). | Dahal et al. [34] |
| Pd-NPs and bimetallic Pt-Pd-NPs obtained through the extract of Dioscorea bulbifera. Pd-NP shape: spherical and blunt ended cubes; Pt-Pd-NP shape: irregular; Size: 10–25 nm | HeLa | Cells were exposed to 10 μg/mL NPs for 48 h | Cell viability | Reduced by 33.15% by Pd-NPs and by 74.25% by Pt-Pd-NPs. Immunofluorescence analysis indicated apoptosis of the treated cells (higher in geoups treated with bimetallic NPs than with Pd-NPs). Anti-scavenging activity: greater for bimetallic NPs than individual Pd-NPs. | Ghosh et al. [32] |
| Size: 10 ± 6 nm | A549 | Cells were exposed to 1 and 2 μg/mL NPs for 4–120 h | Cell viability; Oxidative stress reaction; cell cycle distribution; DNA damage | Cell viability was significantly reduced by 2 μg/mL NPs at 96 and 120 h. Cell cycle distribution: time-dependent increase in G0/G1 phase (40% in controls to 80% following 2 μg/mL for 120 h). Decrease of cell percentage in the S phase (from 30% in controls to 15% following 2 μg/mL for 120 h). DNA damage: a significant effect was evident after 2 μg/mL at all time points. ROS production: significant increase compared to controls only for 2 μg/mL NPs for 4 h. | Iavicoli et al. [30] |
| Shape: spherical; Size: 6–10 nm Hydrodynamic diameter: 138 ± 3 and 151 ± 6 nm in ethanol and KSFM medium, respectively | Bronchial mucosa 3D model: PBECs and MRC-5 | Bronchial cells [stimulated or not with IL-13 (1 ng/mL)] were exposed to aerosolized NPs at 250, 400 and 650 ng/cm2 for 24 h | Cell viability; Release of inflammatory mediators | No alterations in cell viability. IL-8 release: significantly increased in the basal medium following the medium dose exposure in the normal model and the highest concentration in IL-13 stimulated model, compared to controls. | Ji et al. [42] |
| Mean size (±SD): 14.70 ± 2.30 nm | A375 | Cells were exposed to 0–40 μg/mL NPs for 24 and 48 h | Cell viability; Oxidative stress reaction; Cell cycle | Cell viability: dose- and time-dependent significant reduction (62.3% and 75.94% decrease at 24 and 48 h, compared to controls). ROS production: significantly increased by all concentrations of Pd-NPs. A significant reduction of GSH, 15.76% and 49.73%, compared to controls was induced by 40 μg/mL at 24 and 48 h, respectively. Cell cycle (24 h): cells in sub-G1 stage increased from 0.78% (untreated group) to 5.59% and 15.28%, at 20 μg/mL and 40 μg/mL concentrations, respectively. | Alarifi et al. [36] |
| Size: 1–10 nm; Hydrodynamic diameter in water: 446.9 ± 14.1 nm | Human eosinophilic cell line AML14.3D10 and freshly isolated eosinophils | Cells were incubated with 0–150 μg/mL NPs for up to 24 h | Cell viability; Oxidative stress reaction | Cell viability: no significant alterations in necrotic and apoptotic cells. ROS production: no significant alterations. Cytoskeleton rearrangement: actin was diffusely stained in the cytoplasm after NP treatment. Adhesion to endothelial cells: increased by a factor of 1.6 ± 0.2 (3D10 cell line treated with NPs); 1.7 ± 0.4 (freshly eosinophils treated with NPs) which are close to 1.8 ± 0.2 of positive controls. | Chhay et al. [43] |
| Palladium-NP Characterization | Investigated Models | Experimental Designs | Endpoints | Results | References |
|---|---|---|---|---|---|
| Bacterial models | |||||
| Pd-NPs entrapped in an aluminum hydroxide matrix | Microcosm soil | NPs were added to the soil at a final concentration of 0.013% or 0.066% (w/w) | Bacterial growth | No significant effects on the number of colony forming units or on the total soil community metabolic fingerprint. | Shah and Belozerova, [27] |
| Bimetallic Pd-iron NPs | Sphingomonas sp. PH-07 | Bacterial strain was exposed to 0–0.5 g/L | Bacterial growth | No differences compared to controls were evident up to 0.1 g/L, whereas bacterial growth was significantly inhibited with greater NP concentrations. | Murugesan et al. [56] |
| Size: 2.0 ± 0.1; 2.5 ± 0.2; 3.1 ± 0.2 nm. | Gram negative E. Coli, Gram positive S. aureus bacterial strains | Bacteria were exposed to 2.5 × 10−4, 10−5, 10−6, 10−7, 10−8, and 10−9 M for each size of NPs for 24 h | Bacterial growth | A significant decrease in the amount of colonies/mL over the 24 h exposure time, at the 10−4–10−6 M concentrations was evident for both microorganisms. | Adams et al. [51] |
| Pt-Pd polyaniline nanocomposites | Gram positive (Streptococcus sp. and Staphylococcus sp.) and Gram-negative bacteria (E. coli and Klebsiella sp.) | Bacteria were incubated with 0–150 μg/mL pristine anyline, Pt-Pd bimetal NPs and Pt-Pd polyaniline nanocomposites for 24 h | Bacterial growth | The maximum zone of inhibition (mm in diameter) was observed for polyaniline/Pt-Pd nanocomposite against Staphylococcus sp. (28 ± 0.70 mm) followed by Klebsiella sp. (25 ± 0.85 mm), E. coli (22 ± 0.36 mm) and Streptococcus sp. (21 ± 0.30 mm). MIC: 25 and 75 μg/mL for anyline and Pt-Pd nanocomposites. Pt-Pd colloids did not show the zone of inhibition against investigated pathogens. | Boomi et al. [57] |
| Pd-NPs obtained throuh methanolic extraction from M. oleifera peel. Shape: spherical; Size: 27 ± 2 nm | Gram negative E. coli, Gram positive S. aureus bacterial strains | - | Bacterial growth | Good antibacterial activity was reported for both Gram positive and Gram negative bacterial strains. | Surendra et al. [58] |
| Pd-NPs obtained through bio-precipitation in bacterial cultures | Marine Gram negative bacterium V. fischeri; PCB-dechlorinating marine microbial community cultures from a sediment collected in the Venice lagoon, Italy | V. fischeri were exposed to 0–9 μg/mL for 5–30 min. Marine cultures were exposed to 5 and 50 mg/kgdw for upt to 18 weeks | Bacterial growth of V. fischeri; Respiratory metabolisms and structure of the marine microbial community | Acute toxicity: no significant effects. Respiratory metabolisms: Pd-NPs did not impact the dechlorination activities; while dose-dependently inhibited the sulfate reduction and methanogenesis. Pd-NPs increased the richness of the microbial community. | Nuzzo et al. [59] |
| Pd-NPs obtained through leaf extract of Garciniapedunculata; Shape: spherical and non-spherical; Size: ~2 ± 4 nm; Hydrodynamic diameter: 322 nm | MDR clinical isolate Cronobacter sakazakii strain AMD04 | Bacterial cultures (1 mL) were incubated with 0–0.65 mM NPs up to 24 h | Bacterial and biofilm growth | Bactericidal activity: a 31.67 ± 1.53 mm diameter zone of inhibition was evident around the Pd-NP well. MIC: 0.06 mM; MBC: 0.12 mM. Biofilm activity: maximum inhibition at 0.26 ± 0.02 mM. Comparable biomass formation decrease at 0.39 and 0.52 mM. | Hazarika et al. [60] |
| Animal models | |||||
| Size: 10 ± 6 nm | Female Wistar rats (n. 4 per group) | Animals were exposed to a single intravenous injection of Pd-NPs at 0–12 µg/kg dose. Serum cytokine concentrations were assessed at the 14th day post-exposure | Effects on the immune system | The highest dose of Pd-NPs (12 μg/kg) induced a significant increase of IL-1α, IL-4, IL-6, IL-10, IL-12, GM-CSF and INF-γ compared to controls. The 0.12 μg/kg dose induced a significant increase of IL-2, IL-4, IL-10 serum concentrations. | Iavicoli et al. [52] |
| Female Wistar rats (n. 5 per group) | Animals were exposed to repeated (on day 1, 30 and 60) intravenous injections of Pd-NPs at 0–12 µg/kg dose. Serum cytokine concentrations were assessed at the 90th day post-exposure | Effects on the immune system | The highest dose of Pd-NPs (12 μg/kg) induced a significant reduction of IL-1α, IL-4, IL-6, IL-10, IL-12, and GM-CSF compared to controls. The 1.2 μg/kg dose induced also a significant reduction of IL-1α. Interleukin-10 was significantly reduced at all the investigated concentrations. | Iavicoli et al. [53] | |
| Female Wistar rats (n. 5 per group) | Animals were exposed to a single intravenous injection of Pd-NPs at 0–12 µg/kg dose. Urinary protein concentrations were analyzed at the 14th day post exposure | Effects on the renal system | RBP: significantly increased in the 12 µg/kg group (0.23 ± 0.07 mg/mL) compared to controls (0.11 ± 0.03 mg/mL). Beta2-microglobulin: significantly increased in the 12 µg/kg group (0.97 ± 0.23 mg/mL) compared to controls (0.40 ± 0.07 mg/mL). | Fontana et al. [54] | |
| Female Wistar rats (n. 4 per group) | Animals were exposed to a single intravenous injection of Pd-NPs at 0–12 µg/kg dose. E2, FSH, LH, P and T levels were determined in the serum samples collected 14 days after exposure | Effects on the endocrine system | Dose 12 μg/kg: a significant reduction of E2 (0.012 ± 0.002 ng/mL) and T (0.47 ± 0.04 ng/mL) concentrations and an increase of LH levels (23.09 ± 1.07 ng/mL) compared to controls (E2: 0.032 ± 0.006; T: 0.80 ± 0.76; LH: 16.48 ± 1.31 ng/mL). Dose 1.2 μg/kg: significant reduction for E2 (0.012 ± 0.005 ng/mL) and increase for LH (20.11 ± 1.14 ng/mL) compared to controls. | Leso et al. [55] | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leso, V.; Iavicoli, I. Palladium Nanoparticles: Toxicological Effects and Potential Implications for Occupational Risk Assessment. Int. J. Mol. Sci. 2018, 19, 503. https://doi.org/10.3390/ijms19020503
Leso V, Iavicoli I. Palladium Nanoparticles: Toxicological Effects and Potential Implications for Occupational Risk Assessment. International Journal of Molecular Sciences. 2018; 19(2):503. https://doi.org/10.3390/ijms19020503
Chicago/Turabian StyleLeso, Veruscka, and Ivo Iavicoli. 2018. "Palladium Nanoparticles: Toxicological Effects and Potential Implications for Occupational Risk Assessment" International Journal of Molecular Sciences 19, no. 2: 503. https://doi.org/10.3390/ijms19020503
APA StyleLeso, V., & Iavicoli, I. (2018). Palladium Nanoparticles: Toxicological Effects and Potential Implications for Occupational Risk Assessment. International Journal of Molecular Sciences, 19(2), 503. https://doi.org/10.3390/ijms19020503