ATP-Binding Cassette Transporter VcaM from Vibrio cholerae is Dependent on the Outer Membrane Factor Family for Its Function
Abstract
:1. Introduction
2. Results and Discussion
2.1. Overexpression and Purification of VcaM in E. coli
2.2. Kinetic Parameters of ATP Hydrolysis by VcaM
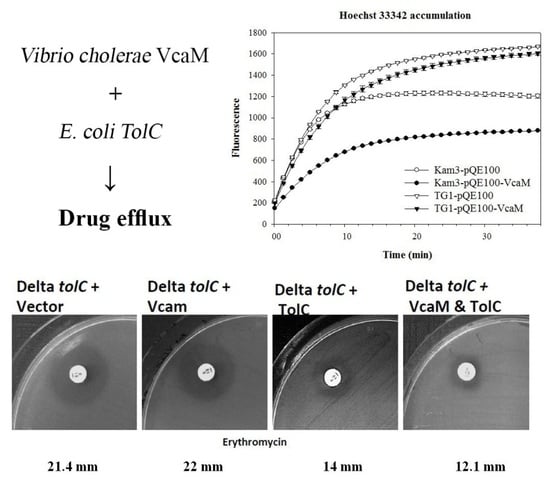
2.3. VcaM Efflux in E. coli Depends on the OMF TolC.
2.4. The Efflux of H33342 Conferred by VcaM/TolC is Independent of Secondary Active Transporters in E. coli
2.5. Dependence on OMF TolC
2.6. Periplasmic Adapter Proteins Might be Needed for V. cholerae VcaM to Provide TolC-Dependent Efflux
3. Materials and Methods
3.1. Bacterial Strains, Media and Chemicals
3.2. Cloning of VcaM
3.3. Expression and Purification
3.4. SDS-PAGE and Western Blot
3.5. ATPase Activity Assay
3.6. Hoechst (H) 33342 Accumulation Assay
3.7. Disk Diffusion Assay
3.8. Prediction of Transmembrane Regions
3.9. Protein Sequence Alignments
3.10. Statistical Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Faruque, S.M.; Albert, M.J.; Mekalanos, J.J. Epidemiology, genetics, and ecology of toxigenic Vibrio cholerae. Microbiol. Mol. Biol. Rev. 1998, 62, 1301–1314. [Google Scholar] [PubMed]
- Morris, J.G., Jr. Cholera and other types of vibriosis: A story of human pandemics and oysters on the half shell. Clin. Infect. Dis. 2003, 37, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.B.; LaRocque, R.C.; Qadri, F.; Ryan, E.T.; Calderwood, S.B. Cholera. Lancet 2012, 379, 2466–2476. [Google Scholar] [CrossRef]
- Kitaoka, M.; Miyata, S.T.; Unterweger, D.; Pukatzki, S. Antibiotic resistance mechanisms of Vibrio cholerae. J. Med. Microbiol. 2011, 60, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Bin Kim, H.; Wang, M.H.; Ahmed, S.; Park, C.H.; LaRocque, R.C.; Faruque, A.S.G.; Salam, M.A.; Khan, W.A.; Qadri, F.; Calderwood, S.B.; et al. Transferable Quinolone Resistance in Vibrio cholerae. Antimicrob. Agents Chemother. 2010, 54, 799–803. [Google Scholar]
- Bina, J.E.; Provenzano, D.; Wang, C.M.; Bina, X.W.R.; Mekalanos, J.J. Characterization of the Vibrio cholerae VexAB and VexCD efflux systems. Arch. Microbiol. 2006, 186, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Yeom, J.H.; Seo, S.; Lee, M.; Kim, S.; Bae, J.; Lee, K.; Hwang, J. Functional analysis of Vibrio vulnificus RND efflux pumps homologous to Vibrio cholerae VexAB and VexCD, and to Escherichia coli AcrAB. J. Microbiol. 2015, 53, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Colmer, J.A.; Fralick, J.A.; Hamood, A.N. Isolation and characterization of a putative multidrug resistance pump from Vibrio cholerae. Mol. Microbiol. 1998, 27, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Federici, L.; Du, D.J.; Walas, F.; Matsumura, H.; Fernandez-Recio, J.; McKeegan, K.S.; Borges-Walmsley, M.I.; Luisi, B.F.; Walmsley, A.R. The crystal structure of the outer membrane protein VceC from the bacterial pathogen Vibrio cholerae at 1.8 angstrom resolution. J. Biol. Chem. 2005, 280, 15307–15314. [Google Scholar] [CrossRef] [PubMed]
- Huda, M.N.; Morita, Y.; Kuroda, T.; Mizushima, T.; Tsuchiya, T. Na+-driven multidrug efflux pump VcmA from Vibrio cholerae non-O1, a non-halophilic bacterium. Fems Microbiol. Lett. 2001, 203, 235–239. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.H.; Paulsen, I.T.; Skurray, R.A. The multidrug efflux protein NorM is a prototype of a new family of transporters. Mol. Microbiol. 1999, 31, 394–395. [Google Scholar] [CrossRef] [PubMed]
- Huda, M.N.; Chen, J.; Morita, Y.; Kuroda, T.; Mizushima, T.; Tsuchiya, T. Gene cloning and characterization of VcrM, a Na+-coupled multidrug efflux pump, from Vibrio cholerae non-O1. Microbiol. Immunol. 2003, 47, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Huda, N.; Lee, E.W.; Chen, J.; Morita, Y.; Kuroda, T.; Mizushima, T.; Tsuchiya, T. Molecular cloning and characterization of an ABC multidrug efflux pump, VcaM, in non-O1 Vibrio cholerae. Antimicrob. Agents Chemother. 2003, 47, 2413–2417. [Google Scholar] [CrossRef] [PubMed]
- Paulsen, I.T.; Park, J.H.; Choi, P.S.; Saier, M.H. A family of Gram-negative bacterial outer membrane factors that function in the export of proteins, carbohydrates, drugs and heavy metals from Gram-negative bacteria. Fems Microbiol. Lett. 1997, 156, 1–8. [Google Scholar] [CrossRef]
- Touze, T.; Eswaran, J.; Bokma, E.; Koronakis, E.; Hughes, C.; Koronakis, V. Interactions underlying assembly of the Escherichia coli AcrAB-TolC multidrug efflux system. Mol. Microbiol. 2004, 53, 697–706. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Song, S.; Moeller, A.; Kim, N.; Piao, S.; Sim, S.H.; Kang, M.; Yu, W.; Cho, H.S.; Chang, I.; et al. Functional implications of an intermeshing cogwheel-like interaction between TolC and MacA in the action of macrolide-specific efflux pump MacAB-TolC. J. Biol. Chem. 2011, 286, 13541–13549. [Google Scholar] [CrossRef] [PubMed]
- Tanabe, M.; Szakonyi, G.; Brown, K.A.; Henderson, P.J.F.; Nield, J.; Byrne, B. The multidrug resistance efflux complex, EmrAB from Escherichia coli forms a dimer in vitro. Biochem. Biophys. Res. Commun. 2009, 380, 338–342. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.T.; Bavro, V.N.; Barrera, N.P.; Frankish, H.M.; Velamakanni, S.; van Veen, H.W.; Robinson, C.V.; Borges-Walmsley, M.I.; Walmsley, A.R. MacB ABC transporter is a dimer whose ATPase activity and macrolide-binding capacity are regulated by the membrane fusion protein MacA. J. Biol. Chem. 2009, 284, 1145–1154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hagman, K.E.; Lucas, C.E.; Balthazar, J.T.; Snyder, L.; Nilles, M.; Judd, R.C.; Shafer, W.M. The MtrD protein of Neisseria gonorrhoeae is a member of the resistance/nodulation/division protein family constituting part of an efflux system. Microbiology 1997, 143, 2117–2125. [Google Scholar] [CrossRef] [PubMed]
- Janganan, T.K.; Bavro, V.N.; Zhang, L.; Matak-Vinkovic, D.; Barrera, N.P.; Venien-Bryan, C.; Robinson, C.V.; Borges-Walmsley, M.I.; Walmsley, A.R. Evidence for the assembly of a bacterial tripartite multidrug pump with a stoichiometry of 3:6:3. J. Biol. Chem. 2011, 286, 26900–26912. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janganan, T.K.; Zhang, L.; Bavro, V.N.; Matak-Vinkovic, D.; Barrera, N.P.; Burton, M.F.; Steel, P.G.; Robinson, C.V.; Borges-Walmsley, M.I.; Walmsley, A.R. Opening of the outer membrane protein channel in tripartite efflux pumps is induced by interaction with the membrane fusion partner. J. Biol. Chem. 2011, 286, 5484–5493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bina, J.E.; Mekalanos, J.J. Vibrio cholerae TolC is required for bile resistance and colonization. Infect. Immun. 2001, 69, 4681–4685. [Google Scholar] [CrossRef] [PubMed]
- Linares, J.F.; Lopez, J.A.; Camafeita, E.; Albar, J.P.; Rojo, F.; Martinez, J.L. Overexpression of the multidrug efflux pumps MexCD-OprJ and MexEF-OprN is associated with a reduction of type III secretion in Pseudomonas aeruginosa. J. Bacteriol. 2005, 187, 1384–1391. [Google Scholar] [CrossRef] [PubMed]
- Boardman, B.K.; Satchell, K.J.F. Vibrio cholerae strains with mutations in an atypical type I secretion system accumulate RTX toxin intracellularly. J. Bacteriol. 2004, 186, 8137–8143. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.; Holland, I.B.; Schmitt, L. The type 1 secretion pathway—The hemolysin system and beyond. BBA. Mol. Cell Res. 2014, 1843, 1629–1641. [Google Scholar] [CrossRef] [PubMed]
- Aires, J.R.; Nikaido, H. Aminoglycosides are captured from both periplasm and cytoplasm by the AcrD multidrug efflux transporter of Escherichia coli. J. Bacteriol. 2005, 187, 1923–1929. [Google Scholar] [CrossRef] [PubMed]
- Husain, F.; Humbard, M.; Misra, R. Interaction between the TolC and AcrA proteins of a multidrug efflux system of Escherichia coli. J. Bacteriol. 2004, 186, 8533–8536. [Google Scholar] [CrossRef] [PubMed]
- Lau, S.Y.; Zgurskaya, H.I. Cell division defects in Escherichia coli deficient in the multidrug efflux transporter AcrEF-TolC. J. Bacteriol. 2005, 187, 7815–7825. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, A.W.P.; Llabres, S.; Neuberger, A.; Blaza, J.N.; Bai, X.C.; Okada, U.; Murakami, S.; van Veen, H.W.; Zachariae, U.; Scheres, S.H.W.; et al. Structure of the MacAB-TolC ABC-type tripartite multidrug efflux pump. Nat. Microbiol. 2017, 2, 17070. [Google Scholar] [CrossRef] [PubMed]
- Woolley, R.C.; Vediyappan, G.; Anderson, M.; Lackey, M.; Ramasubramanian, B.; Bai, J.P.; Borisova, T.; Colmer, J.A.; Hamood, A.N.; McVay, C.S.; et al. Characterization of the Vibrio cholerae vceCAB multiple-drug resistance efflux operon in Escherichia coli. J. Bacteriol. 2005, 187, 5500–5503. [Google Scholar] [CrossRef] [PubMed]
- Tanabe, H.; Yamasaki, K.; Furue, M.; Yamamoto, K.; Katoh, A.; Yamamoto, M.; Yoshioka, S.; Tagami, H.; Aiba, H.; Utsumi, R. Growth phase-dependent transcription of emrKY, a homolog of multidrug efflux emrAB genes of Escherichia coli, is induced by tetracycline. J. Gen. Appl. Microbiol. 1997, 43, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Tikhonova, E.B.; Devroy, V.K.; Lau, S.Y.; Zgurskaya, H.I. Reconstitution of the Escherichia coli macrolide transporter: The periplasmic membrane fusion protein MacA stimulates the ATPase activity of MacB. Mol. Microbiol. 2007, 63, 895–910. [Google Scholar] [CrossRef] [PubMed]
- Dawson, R.J.P.; Locher, K.P. Structure of the multidrug ABC transporter Sav1866 from Staphylococcus aureus in complex with AMP-PNP. Febs Lett. 2007, 581, 935–938. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, H.G.; Tong, Z.; Mathavan, I.; Li, Y.; Iwata, S.; Zirah, S.; Rebuffat, S.; van Veen, H.W.; Beis, K. Structure of an antibacterial peptide ATP-binding cassette transporter in a novel outward occluded state. Proc. Natl. Acad. Sci. USA 2014, 111, 9145–9150. [Google Scholar] [CrossRef] [PubMed]
- Crow, A.; Greene, N.P.; Kaplan, E.; Koronakis, V. Structure and mechanotransmission mechanism of the MacB ABC transporter superfamily. Proc. Natl. Acad. Sci. USA 2017, 114, 12572–12577. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.; Mao, W.M.; Warren, M.S.; Mistry, A.; Hoshino, K.; Okumura, R.; Ishida, H.; Lomovskaya, O. Interplay between efflux pumps may provide either additive or multiplicative effects on drug resistance. J. Bacteriol. 2000, 182, 3142–3150. [Google Scholar] [CrossRef] [PubMed]
- Horiyama, T.; Yamaguchi, A.; Nishino, K. TolC dependency of multidrug efflux systems in Salmonella enterica serovar Typhimurium. J. Antimicrob. Chemother. 2010, 65, 1372–1376. [Google Scholar] [CrossRef] [PubMed]
- Urbatsch, I.L.; Sankaran, B.; Weber, J.; Senior, A.E. P-glycoprotein is stably inhibited by vanadate-induced trapping of nucleotide at a single catalytic site. J. Biol. Chem. 1995, 270, 19383–19390. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Song, S.; Lee, M.; Hwang, S.; Kim, J.S.; Ha, N.C.; Lee, K. Interaction between the α-barrel tip of Vibrio vulnificus TolC homologs and AcrA implies the adapter bridging model. J. Microbiol. 2014, 52, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Horiyama, T.; Nishino, K. AcrB, AcrD, and MdtABC multidrug efflux systems are involved in Enterobactin export in Escherichia coli. PLoS ONE 2014, 9, e108642. [Google Scholar] [CrossRef] [PubMed]
- Nikaido, H.; Basina, M.; Nguyen, V.; Rosenberg, E.Y. Multidrug efflux pump AcrAB of Salmonella typhimurium excretes only those β-lactam antibiotics containing lipophilic side chains. J. Bacteriol. 1998, 180, 4686–4692. [Google Scholar] [PubMed]
- Costa, S.S.; Viveiros, M.; Amaral, L.; Couto, I. Multidrug efflux pumps in Staphylococcus aureus: An update. Open Microbiol. J. 2013, 7, 59–71. [Google Scholar] [CrossRef] [PubMed]
- Opperman, T.J.; Nguyen, S.T. Recent advances toward a molecular mechanism of efflux pump inhibition. Front. Microbiol. 2015, 6, 421. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, E.Y.; Ma, D.; Nikaido, H. AcrD of Escherichia coli is an aminoglycoside efflux pump. J. Bacteriol. 2000, 182, 1754–1756. [Google Scholar] [CrossRef] [PubMed]
- Bohnert, J.A.; Schuster, S.; Fahnrich, E.; Trittler, R.; Kern, W.V. Altered spectrum of multidrug resistance associated with a single point mutation in the Escherichia coli RND-type MDR efflux pump YhiV (MdtF). J. Antimicrob. Chemother. 2007, 59, 1216–1222. [Google Scholar] [CrossRef] [PubMed]
- Nishino, K.; Yamada, J.; Hirakawa, H.; Hirata, T.; Yamaguchi, A. Roles of TolC-dependent multidrug transporters of Escherichia coli in resistance to β-lactams. Antimicrob. Agents Chemother. 2003, 47, 3030–3033. [Google Scholar] [CrossRef] [PubMed]
- Lomovskaya, O.; Lewis, K. Emr, an Escherichia coli locus for multidrug resistance. Proc. Natl. Acad. Sci. USA 1992, 89, 8938–8942. [Google Scholar] [CrossRef] [PubMed]
- Vediyappan, G.; Borisova, T.; Fralick, J.A. Isolation and characterization of VceC gain-of-function mutants that can function with the AcrAB multiple-drug-resistant efflux pump of Escherichia coli. J. Bacteriol. 2006, 188, 3757–3762. [Google Scholar] [CrossRef] [PubMed]
- Krogh, A.; Larsson, B.; von Heijne, G.; Sonnhammer, E.L.L. Predicting transmembrane protein topology with a hidden Markov model: Application to complete genomes. J. Mol. Biol. 2001, 305, 567–580. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.M.; Xu, Y.B.; Lee, M.; Piao, S.F.; Sim, S.H.; Ha, N.C.; Lee, K. Functional relationships between the AcrA hairpin tip region and the TolC aperture tip region for the formation of the bacterial tripartite efflux pump AcrAB-TolC. J. Bacteriol. 2010, 192, 4498–4503. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Jun, S.Y.; Yoon, B.Y.; Song, S.; Lee, K.; Ha, N.C. Membrane fusion proteins of type I secretion system and tripartite efflux pumps share a binding motif for TolC in Gram-negative bacteria. PLoS ONE 2012, 7, e40460. [Google Scholar] [CrossRef] [PubMed]
- Morgan, J.L.W.; Acheson, J.F.; Zimmer, J. Structure of a type-1 secretion system ABC transporter. Structure 2017, 25, 522–529. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, N.; Nishino, K.; Hirata, T.; Yamaguchi, A. Membrane topology of ABC-type macrolide antibiotic exporter MacB in Escherichia coli. Febs Lett. 2003, 546, 241–246. [Google Scholar] [CrossRef]
- Yum, S.W.; Xu, Y.B.; Piao, S.F.; Sim, S.H.; Kim, H.M.; Jo, W.S.; Kim, K.J.; Kweon, H.S.; Jeong, M.H.; Jeon, H.S.; et al. Crystal structure of the periplasmic component of a tripartite macrolide-specific efflux pump. J. Mol. Biol. 2009, 387, 1286–1297. [Google Scholar] [CrossRef] [PubMed]
- Symmons, M.F.; Marshall, R.L.; Bavro, V.N. Architecture and roles of periplasmic adaptor proteins in tripartite efflux assemblies. Front. Microbiol. 2015, 6, 513. [Google Scholar] [CrossRef] [PubMed]
- Hinchliffe, P.; Greene, N.P.; Paterson, N.G.; Crow, A.; Hughes, C.; Koronakis, V. Structure of the periplasmic adaptor protein from a major facilitator superfamily (MFS) multidrug efflux pump. FEBS Lett. 2014, 588, 3147–3153. [Google Scholar] [CrossRef] [PubMed]
- Levine, M.M.; Kaper, J.B.; Herrington, D.; Losonsky, G.; Morris, J.G.; Clements, M.L.; Black, R.E.; Tall, B.; Hall, R. Volunteer studies of deletion mutants of Vibrio cholerae O1 prepared by recombinant techniques. Infect. Immun. 1988, 56, 161–167. [Google Scholar] [PubMed]
- Morita, Y.; Kodama, K.; Shiota, S.; Mine, T.; Kataoka, A.; Mizushima, T.; Tsuchiya, T. NorM, a putative multidrug efflux protein, of Vibrio parahaemolyticus and its homolog in Escherichia coli. Antimicrob. Agents Chemother. 1998, 42, 1778–1782. [Google Scholar] [PubMed]
- Dalmas, O.; Do Cao, M.A.; Lugo, M.R.; Sharom, F.J.; Di Pietro, A.; Jault, J.M. Time-resolved fluorescence resonance energy transfer shows that the bacterial multidrug ABC half-transporter BmrA functions as a homodimer. Biochemistry 2005, 44, 4312–4321. [Google Scholar] [CrossRef] [PubMed]
- Geladopoulos, T.P.; Sotiroudis, T.G.; Evangelopoulos, A.E. A malachite green colorimetric assay for protein phosphatase activity. Anal. Biochem. 1991, 192, 112–116. [Google Scholar] [CrossRef]
- Richmond, G.E.; Chua, K.L.; Piddock, L.J.V. Efflux in Acinetobacter baumannii can be determined by measuring accumulation of H33342 (bis-benzamide). J. Antimicrob. Chemother. 2013, 68, 1594–1600. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janganan, T.K.; Bavro, V.N.; Zhang, L.; Borges-Walmsley, M.I.; Walmsley, A.R. Tripartite efflux pumps: Energy is required for dissociation, but not assembly or opening of the outer membrane channel of the pump. Mol. Microbiol. 2013, 88, 590–602. [Google Scholar] [CrossRef] [PubMed]
- Sievers, F.; Wilm, A.; Dineen, D.; Gibson, T.J.; Karplus, K.; Li, W.Z.; Lopez, R.; McWilliam, H.; Remmert, M.; Soding, J.; et al. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol. Syst. Biol. 2011, 7, 539. [Google Scholar] [CrossRef] [PubMed]








| PAPs | Inner Membrane Transporter | PAP Homologues in V. cholera (SEQUENCE Identity %) | Reference |
|---|---|---|---|
| RND family | |||
| AcrA | AcrB; AcrD | MexC, 36% | [26,27] |
| AcrE | AcrF | MexC, 36% | [28] |
| ABC family | |||
| MacA | MacB | Hemolysin D, 38% | [29] |
| MFS family | |||
| EmrA | EmrB | VceA, 39% | [17,30] |
| EmrK | EmrY | VceA, 40% | [30,31] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, W.-J.; Lin, H.-J.; Janganan, T.K.; Li, C.-Y.; Chin, W.-C.; Bavro, V.N.; Lin, H.-T.V. ATP-Binding Cassette Transporter VcaM from Vibrio cholerae is Dependent on the Outer Membrane Factor Family for Its Function. Int. J. Mol. Sci. 2018, 19, 1000. https://doi.org/10.3390/ijms19041000
Lu W-J, Lin H-J, Janganan TK, Li C-Y, Chin W-C, Bavro VN, Lin H-TV. ATP-Binding Cassette Transporter VcaM from Vibrio cholerae is Dependent on the Outer Membrane Factor Family for Its Function. International Journal of Molecular Sciences. 2018; 19(4):1000. https://doi.org/10.3390/ijms19041000
Chicago/Turabian StyleLu, Wen-Jung, Hsuan-Ju Lin, Thamarai K. Janganan, Cheng-Yi Li, Wei-Chiang Chin, Vassiliy N. Bavro, and Hong-Ting Victor Lin. 2018. "ATP-Binding Cassette Transporter VcaM from Vibrio cholerae is Dependent on the Outer Membrane Factor Family for Its Function" International Journal of Molecular Sciences 19, no. 4: 1000. https://doi.org/10.3390/ijms19041000
APA StyleLu, W. -J., Lin, H. -J., Janganan, T. K., Li, C. -Y., Chin, W. -C., Bavro, V. N., & Lin, H. -T. V. (2018). ATP-Binding Cassette Transporter VcaM from Vibrio cholerae is Dependent on the Outer Membrane Factor Family for Its Function. International Journal of Molecular Sciences, 19(4), 1000. https://doi.org/10.3390/ijms19041000