Metabolomics and Transcriptomics Identify Multiple Downstream Targets of Paraburkholderia phymatum σ54 During Symbiosis with Phaseolus vulgaris
Abstract
:1. Introduction
2. Results and Discussion
2.1. Metabolomic Analysis of P. vulgaris Root Nodules Infected with P. phymatum STM815T Wild-Type and with a rpoN Mutant
2.2. RpoN Regulon in Symbiosis, as Determined by Transcriptomics
2.3. In Silico Identification of RpoN Binding Sites
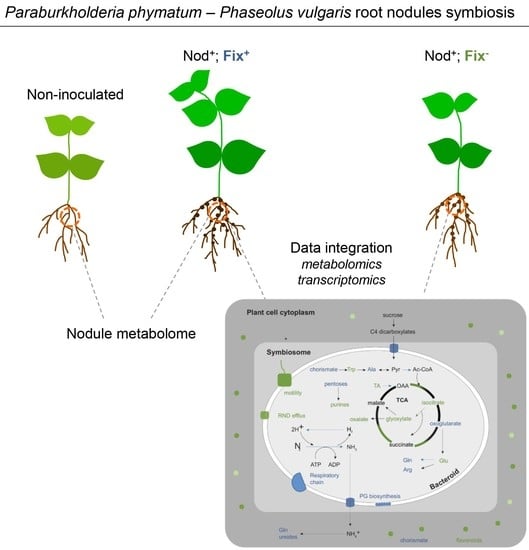
2.4. Integration of Metabolomics and Transcriptome Data
2.5. Construction and Phenotypical Analysis of an ntrB Mutant
2.5.1. Role of ntrB during Symbiosis
2.5.2. Role of ntrB for Nitrogen Uptake in Free-Living Conditions
3. Materials and Methods
3.1. Bacterial Strains, Media, and Cultivation
3.2. Plant Growth Conditions
3.3. Plant Harvesting and Metabolite Extraction
3.4. Metabolite Data Analysis
3.5. RNA-Sequencing and Data Processing
3.6. q-PCR Analysis
3.7. Genome-Wide In Silico Prediction of RpoN-Binding Sequences
3.8. Construction of a Paraburkholderia Phymatum STM815T ntrB Deletion Mutant
3.9. Determination of Symbiotic Properties
3.10. Biolog Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Moulin, L.; Munive, A.; Dreyfus, B.; Boivin-Masson, C. Nodulation of legumes by members of the β-subclass of Proteobacteria. Nature 2001, 411, 948–950. [Google Scholar] [CrossRef] [PubMed]
- Elliott, G.N.; Chen, W.-M.; Chou, J.-H.; Wang, H.-C.; Sheu, S.-Y.; Perin, L.; Reis, V.M.; Moulin, L.; Simon, M.F.; Bontemps, C.; et al. Burkholderia phymatum is a highly effective nitrogen-fixing symbiont of Mimosa spp. and fixes nitrogen ex planta. New Phytol. 2007, 173, 168–180. [Google Scholar] [CrossRef] [PubMed]
- Mishra, R.P.N.; Tisseyre, P.; Melkonian, R.; Chaintreuil, C.; Miché, L.; Klonowska, A.; Gonzalez, S.; Bena, G.; Laguerre, G.; Moulin, L. Genetic diversity of Mimosa pudica rhizobial symbionts in soils of French Guiana: Investigating the origin and diversity of Burkholderia phymatum and other β-rhizobia. FEMS Microbiol. Ecol. 2012, 79, 487–503. [Google Scholar] [CrossRef] [PubMed]
- Dos Reis, F.B., Jr.; Simon, M.F.; Gross, E.; Boddey, R.M.; Elliott, G.N.; Neto, N.E.; de Fatima Loureiro, M.; de Queiroz, L.P.; Scotti, M.R.; Chen, W.-M.; et al. Nodulation and nitrogen fixation by Mimosa spp. in the Cerrado and Caatinga biomes of Brazil. New Phytol. 2010, 186, 934–946. [Google Scholar] [CrossRef] [PubMed]
- Moulin, L.; Klonowska, A.; Caroline, B.; Booth, K.; Vriezen, J.A.C.; Melkonian, R.; James, E.K.; Young, J.P.W.; Bena, G.; Hauser, L.; et al. Complete Genome sequence of Burkholderia phymatum STM815T, a broad host range and efficient nitrogen-fixing symbiont of Mimosa species. Stand. Genom. Sci. 2014, 9, 763–774. [Google Scholar] [CrossRef] [PubMed]
- Talbi, C.; Delgado, M.J.; Girard, L.; Ramirez-Trujillo, A.; Caballero-Mellado, J.; Bedmar, E.J. Burkholderia phymatum strains capable of nodulating Phaseolus vulgaris are present in Moroccan soils. Appl. Environ. Microbiol. 2010, 76, 4587–4591. [Google Scholar] [CrossRef] [PubMed]
- Gyaneshwar, P.; Hirsch, A.M.; Moulin, L.; Chen, W.-M.; Elliott, G.N.; Bontemps, C.; Estrada-de los Santos, P.; Gross, E.; dos Reis, F.B.; Sprent, J.I.; et al. Legume-nodulating betaproteobacteria: Diversity, host range, and future prospects. Mol. Plant Microbe Interact. 2011, 24, 1276–1288. [Google Scholar] [CrossRef] [PubMed]
- Lodwig, E.M.; Hosie, A.H.F.; Bourdès, A.; Findlay, K.; Allaway, D.; Karunakaran, R.; Downie, J.A.; Poole, P.S. Amino-acid cycling drives nitrogen fixation in the legume—Rhizobium symbiosis. Nature 2003, 422, 722–726. [Google Scholar] [CrossRef] [PubMed]
- Prell, J.; Poole, P. Metabolic changes of rhizobia in legume nodules. Trends Microbiol. 2006, 14, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Baral, B.; Teixeira da Silva, J.A.; Izaguirre-Mayoral, M.L. Early signaling, synthesis, transport and metabolism of ureides. J. Plant Physiol. 2016, 193, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Pessi, G.; Ahrens, C.H.; Rehrauer, H.; Lindemann, A.; Hauser, F.; Fischer, H.-M.; Hennecke, H. Genome-wide transcript analysis of Bradyrhizobium japonicum bacteroids in soybean root nodules. Mol. Plant Microbe Interact. 2007, 20, 1353–1363. [Google Scholar] [CrossRef] [PubMed]
- Karunakaran, R.; Ramachandran, V.K.; Seaman, J.C.; East, A.K.; Mouhsine, B.; Mauchline, T.H.; Prell, J.; Skeffington, A.; Poole, P.S. Transcriptomic analysis of Rhizobium leguminosarum biovar viciae in symbiosis with host plants Pisum sativum and Vicia cracca. J. Bacteriol. 2009, 191, 4002–4014. [Google Scholar] [CrossRef] [PubMed]
- Delmotte, N.; Ahrens, C.H.; Knief, C.; Qeli, E.; Koch, M.; Fischer, H.-M.; Vorholt, J.A.; Hennecke, H.; Pessi, G. An integrated proteomics and transcriptomics reference data set provides new insights into the Bradyrhizobium japonicum bacteroid metabolism in soybean root nodules. Proteomics 2010, 10, 1391–1400. [Google Scholar] [CrossRef] [PubMed]
- Vercruysse, M.; Fauvart, M.; Beullens, S.; Braeken, K.; Cloots, L.; Engelen, K.; Marchal, K.; Michiels, J. A comparative transcriptome analysis of Rhizobium etli bacteroids: Specific gene expression during symbiotic nongrowth. Mol. Plant Microbe Interact. 2011, 24, 1553–1561. [Google Scholar] [CrossRef] [PubMed]
- Tian, C.F.; Zhou, Y.J.; Zhang, Y.M.; Li, Q.Q.; Zhang, Y.Z.; Li, D.F.; Wang, S.; Wang, J.; Gilbert, L.B.; Li, Y.R.; et al. Comparative genomics of rhizobia nodulating soybean suggests extensive recruitment of lineage-specific genes in adaptations. Proc. Natl. Acad. Sci. USA 2012, 109, 8629–8634. [Google Scholar] [CrossRef] [PubMed]
- Lardi, M.; Murset, V.; Fischer, H.-M.; Mesa, S.; Ahrens, C.H.; Zamboni, N.; Pessi, G. Metabolomic profiling of Bradyrhizobium diazoefficiens-induced root nodules reveals both host plant-specific and developmental signatures. Int. J. Mol. Sci. 2016, 17, 815. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Montaño, F.; del Cerro, P.; Jiménez-Guerrero, I.; López-Baena, F.J.; Cubo, M.T.; Hungria, M.; Megías, M.; Ollero, F.J. RNA-seq analysis of the Rhizobium tropici CIAT 899 transcriptome shows similarities in the activation patterns of symbiotic genes in the presence of apigenin and salt. BMC Genom. 2016, 17. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Li, R.; Chen, S.; Chen, H.; Zhang, C.; Chen, L.; Hao, Q.; Shan, Z.; Yang, Z.; Qiu, D.; et al. RNA-Seq analysis of differential gene expression responding to different rhizobium strains in soybean (Glycine max) roots. Front. Plant Sci. 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Guerrero, I.; Acosta-Jurado, S.; del Cerro, P.; Navarro-Gómez, P.; López-Baena, F.; Ollero, F.; Vinardell, J.; Pérez-Montaño, F. Transcriptomic studies of the effect of nod gene-inducing molecules in rhizobia: Different weapons, one purpose. Genes 2017, 9, 1. [Google Scholar] [CrossRef] [PubMed]
- Klonowska, A.; Melkonian, R.; Miché, L.; Tisseyre, P.; Moulin, L. Transcriptomic profiling of Burkholderia phymatum STM815, Cupriavidus taiwanensis LMG19424 and Rhizobium mesoamericanum STM3625 in response to Mimosa pudica root exudates illuminates the molecular basis of their nodulation competitiveness and symbiotic evolutionary history. BMC Genom. 2018, 19, 105. [Google Scholar] [CrossRef]
- Michiels, J.; Van Soom, T.; D’hooghe, I.; Dombrecht, B.; Benhassine, T.; de Wilde, P.; Vanderleyden, J. The Rhizobium etli rpoN locus: DNA sequence analysis and phenotypical characterization of rpoN, ptsN, and ptsA mutants. J. Bacteriol. 1998, 180, 1729–1740. [Google Scholar] [PubMed]
- Kullik, I.; Fritsche, S.; Knobel, H.; Sanjuan, J.; Hennecke, H.; Fischer, H.-M. Bradyrhizobium japonicum has two differentially regulated, functional homologs of the σ54 gene (rpoN). J. Bacteriol. 1991, 173, 1125–1138. [Google Scholar] [CrossRef] [PubMed]
- Lardi, M.; Liu, Y.; Purtschert, G.; Bolzan de Campos, S.; Pessi, G. Transcriptome analysis of Paraburkholderia phymatum under nitrogen starvation and during symbiosis with Phaseolus vulgaris. Genes 2017, 8, 389. [Google Scholar] [CrossRef]
- Hauser, F.; Pessi, G.; Friberg, M.; Weber, C.; Rusca, N.; Lindemann, A.; Fischer, H.-M.; Hennecke, H. Dissection of the Bradyrhizobium japonicum NifA+σ54 regulon, and identification of a ferredoxin gene (fdxN) for symbiotic nitrogen fixation. Mol. Genet. Genom. 2007, 278, 255–271. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Liu, M.; Burgess, R.R. Promoter and regulon analysis of nitrogen assimilation factor, σ54, reveal alternative strategy for E. coli MG1655 flagellar biosynthesis. Nucleic Acids Res. 2010, 38, 1273–1283. [Google Scholar] [CrossRef] [PubMed]
- Van Heeswijk, W.C.; Westerhoff, H.V.; Boogerd, F.C. Nitrogen Assimilation in Escherichia coli: Putting molecular data into a systems perspective. Microbiol. Mol. Biol. Rev. 2013, 77, 628–695. [Google Scholar] [CrossRef] [PubMed]
- Lardi, M.; Aguilar, C.; Pedrioli, A.; Omasits, U.; Suppiger, A.; Cárcamo-Oyarce, G.; Schmid, N.; Ahrens, C.H.; Eberl, L.; Pessi, G. σ54-dependent response to nitrogen limitation and virulence in Burkholderia cenocepacia strain H111. Appl. Environ. Microbiol. 2015, 81, 4077–4089. [Google Scholar] [CrossRef] [PubMed]
- Hao, B.; Mo, Z.-L.; Xiao, P.; Pan, H.-J.; Lan, X.; Li, G.-Y. Role of alternative sigma factor 54 (RpoN) from Vibrio anguillarum M3 in protease secretion, exopolysaccharide production, biofilm formation, and virulence. Appl. Microbiol. Biotechnol. 2013, 97, 2575–2585. [Google Scholar] [CrossRef] [PubMed]
- Salazar, E.; Diaz-Mejia, J.J.; Moreno-Hagelsieb, G.; Martinez-Batallar, G.; Mora, Y.; Mora, J.; Encarnacion, S. Characterization of the NifA-RpoN regulon in Rhizobium etli in free life and in symbiosis with Phaseolus vulgaris. Appl. Environ. Microbiol. 2010, 76, 4510–4520. [Google Scholar] [CrossRef] [PubMed]
- Hayrapetyan, H.; Tempelaars, M.; Nierop Groot, M.; Abee, T. Bacillus cereus ATCC 14579 RpoN (Sigma 54) is a pleiotropic regulator of growth, carbohydrate metabolism, motility, biofilm formation and toxin production. PLoS ONE 2015, 10, e0134872. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Liu, Y.; Chen, Y.; Yam, J.; Chew, S.; Chua, S.; Wang, K.; Givskov, M.; Yang, L. RpoN regulates virulence factors of Pseudomonas aeruginosa via modulating the PqsR quorum sensing regulator. Int. J. Mol. Sci. 2015, 16, 28311–28319. [Google Scholar] [CrossRef] [PubMed]
- Dixon, R.; Kahn, D. Genetic regulation of biological nitrogen fixation. Nat. Rev. Microbiol. 2004, 2, 621–631. [Google Scholar] [CrossRef] [PubMed]
- Fuhrer, T.; Heer, D.; Begemann, B.; Zamboni, N. High-throughput, accurate mass metabolome profiling of cellular extracts by flow injection–time-of-flight mass spectrometry. Anal. Chem. 2011, 83, 7074–7080. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Pandey, A.K. Chemistry and biological activities of flavonoids: An overview. Sci. World J. 2013, 2013, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Mierziak, J.; Kostyn, K.; Kulma, A. Flavonoids as important molecules of plant interactions with the environment. Molecules 2014, 19, 16240–16265. [Google Scholar] [CrossRef] [PubMed]
- Hungria, M.; Joseph, C.M.; Phillips, D.A. Rhizobium nod gene inducers exuded naturally from roots of common bean (Phaseolus vulgaris L.). Plant Physiol. 1991, 97, 759–764. [Google Scholar] [CrossRef] [PubMed]
- Parniske, M.; Fischer, H.-M.; Hennecke, H.; Werner, D. Accumulation of the phytoalexin glyceollin I in soybean nodules infected by a Bradyrhizobium japonicum nifA mutant. Zeitschrift für Naturforschung C 1991, 46, 318–320. [Google Scholar]
- Falcone Ferreyra, M.L.; Rius, S.P.; Casati, P. Flavonoids: Biosynthesis, biological functions, and biotechnological applications. Front. Plant Sci. 2012, 3. [Google Scholar] [CrossRef] [PubMed]
- Anders, S.; Huber, W. Differential expression analysis for sequence count data. Genome Biol. 2010, 11, R106. [Google Scholar] [CrossRef] [PubMed]
- Powell, S.; Szklarczyk, D.; Trachana, K.; Roth, A.; Kuhn, M.; Muller, J.; Arnold, R.; Rattei, T.; Letunic, I.; Doerks, T.; et al. eggNOG v3.0: Orthologous groups covering 1133 organisms at 41 different taxonomic ranges. Nucleic Acids Res. 2012, 40, D284–D289. [Google Scholar] [CrossRef] [PubMed]
- Wadhams, G.H.; Armitage, J.P. Making sense of it all: Bacterial chemotaxis. Nat. Rev. Mol. Cell Biol. 2004, 5, 1024–1037. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.-A.; Moon, S.H.; Kim, K.-T.; Mendonca, A.F.; Paik, H.-D. Antimicrobial effects of various flavonoids on Escherichia coli O157:H7 cell growth and lipopolysaccharide production. Food Sci. Biotechnol. 2010, 19, 257–261. [Google Scholar] [CrossRef]
- Skiba, M.A.; Szendzielorz, K.; Mazur, B.; Król, W. The inhibitory effect of flavonoids on interleukin-8 release by human gastric adenocarcinoma (AGS) cells infected with cag PAI (+) Helicobacter pylori. Cent. Eur. J. Immunol. 2016, 41, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Ortega, C.; Olivares, J.; Martínez, J.L. RND multidrug efflux pumps: What are they good for? Front. Microbiol. 2013, 4. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lardi, M.; Pedrioli, A.; Eberl, L.; Pessi, G. NtrC-dependent control of exopolysaccharide synthesis and motility in Burkholderia cenocepacia H111. PLoS ONE 2017, 12, e0180362. [Google Scholar] [CrossRef] [PubMed]
- Čuklina, J.; Hahn, J.; Imakaev, M.; Omasits, U.; Förstner, K.U.; Ljubimov, N.; Goebel, M.; Pessi, G.; Fischer, H.-M.; Ahrens, C.H.; et al. Genome-wide transcription start site mapping of Bradyrhizobium japonicum grown free-living or in symbiosis—A rich resource to identify new transcripts, proteins and to study gene regulation. BMC Genom. 2016, 17. [Google Scholar] [CrossRef] [PubMed]
- LaPointe, G.; Viau, S.; LeBlanc, D.; Robert, N.; Morin, A. Cloning, sequencing, and expression in Escherichia coli of the D-hydantoinase gene from Pseudomonas putida and distribution of homologous genes in other microorganisms. Appl. Environ. Microbiol. 1994, 60, 888–895. [Google Scholar] [PubMed]
- Wu, D.; Kong, Y.; Han, C.; Chen, J.; Hu, L.; Jiang, H.; Shen, X. D-Alanine:D-alanine ligase as a new target for the flavonoids quercetin and apigenin. Int. J. Antimicrob. Agents 2008, 32, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Udvardi, M.; Poole, P.S. Transport and metabolism in legume-rhizobia symbioses. Annu. Rev. Plant Biol. 2013, 64, 781–805. [Google Scholar] [CrossRef] [PubMed]
- Hervás, A.B.; Canosa, I.; Santero, E. Transcriptome analysis of Pseudomonas putida in response to nitrogen availability. J. Bacteriol. 2008, 190, 416–420. [Google Scholar] [CrossRef] [PubMed]
- Reitzer, L. Nitrogen assimilation and global regulation in Escherichia coli. Annu. Rev. Microbiol. 2003, 57, 155–176. [Google Scholar] [CrossRef] [PubMed]
- Hervás, A.B.; Canosa, I.; Little, R.; Dixon, R.; Santero, E. NtrC-dependent regulatory network for nitrogen assimilation in Pseudomonas putida. J. Bacteriol. 2009, 191, 6123–6135. [Google Scholar] [CrossRef] [PubMed]
- Bertani, G. Studies on lysogenesis. I. The mode of phage liberation by lysogenic Escherichia coli. J. Bacteriol. 1951, 62, 293–300. [Google Scholar] [PubMed]
- Clark, D.J.; Maaløe, O. DNA replication and the division cycle in Escherichia coli. J. Mol. Biol. 1967, 23, 99–112. [Google Scholar] [CrossRef]
- Talbi, C.; Argandoña, M.; Salvador, M.; Alché, J.D.; Vargas, C.; Bedmar, E.J.; Delgado, M.J. Burkholderia phymatum improves salt tolerance of symbiotic nitrogen fixation in Phaseolus vulgaris. Plant Soil 2013, 367, 673–685. [Google Scholar] [CrossRef]
- Storey, J.D.; Tibshirani, R. Statistical significance for genomewide studies. Proc. Natl. Acad. Sci. USA 2003, 100, 9440–9445. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S.; et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 2005, 102, 15545–15550. [Google Scholar] [CrossRef] [PubMed]
- Pessi, G.; Braunwalder, R.; Grunau, A.; Omasits, U.; Ahrens, C.H.; Eberl, L. Response of Burkholderia cenocepacia H111 to micro-oxia. PLoS ONE 2013, 8, e72939. [Google Scholar] [CrossRef] [PubMed]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef] [PubMed]
- Tan, G.; Lenhard, B. TFBSTools: An R/bioconductor package for transcription factor binding site analysis. Bioinformatics 2016, 32, 1555–1556. [Google Scholar] [CrossRef] [PubMed]
- Datsenko, K.A.; Wanner, B.L. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 2000, 97, 6640–6645. [Google Scholar] [CrossRef] [PubMed]
- Shastri, S.; Spiewak, H.L.; Sofoluwe, A.; Eidsvaag, V.A.; Asghar, A.H.; Pereira, T.; Bull, E.H.; Butt, A.T.; Thomas, M.S. An efficient system for the generation of marked genetic mutants in members of the genus Burkholderia. Plasmid 2017, 89, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Lardi, M.; Bolzan de Campos, S.; Purtschert, G.; Eberl, L.; Pessi, G. Competition experiments for legume infection Identify Burkholderia phymatum as a highly competitive β-Rhizobium. Front. Microbiol. 2017, 8, 1527. [Google Scholar] [CrossRef] [PubMed]
- Agnoli, K.; Schwager, S.; Uehlinger, S.; Vergunst, A.; Viteri, D.F.; Nguyen, D.T.; Sokol, P.A.; Carlier, A.; Eberl, L. Exposing the third chromosome of Burkholderia cepacia complex strains as a virulence plasmid. Mol. Microbiol. 2012, 83, 362–378. [Google Scholar] [CrossRef] [PubMed]
- Colebatch, G.; Desbrosses, G.; Ott, T.; Krusell, L.; Montanari, O.; Kloska, S.; Kopka, J.; Udvardi, M.K. Global changes in transcription orchestrate metabolic differentiation during symbiotic nitrogen fixation in Lotus japonicus. Plant J. 2004, 39, 487–512. [Google Scholar] [CrossRef] [PubMed]
- Desbrosses, G.G. Lotus japonicus metabolic profiling. Development of gas chromatography-mass spectrometry resources for the study of plant-microbe interactions. Plant Physiol. 2005, 137, 1302–1318. [Google Scholar] [CrossRef] [PubMed]
- Barsch, A.; Tellström, V.; Patschkowski, T.; Küster, H.; Niehaus, K. Metabolite profiles of nodulated alfalfa plants indicate that distinct stages of nodule organogenesis are accompanied by global physiological adaptations. Mol. Plant Microbe Interact. 2006, 19, 998–1013. [Google Scholar] [CrossRef] [PubMed]
- Vauclare, P.; Bligny, R.; Gout, E.; Widmer, F. An overview of the metabolic differences between Bradyrhizobium japonicum 110 bacteria and differentiated bacteroids from soybean (Glycine max) root nodules: An in vitro 13C- and 31P-nuclear magnetic resonance spectroscopy study. FEMS Microbiol. Lett. 2013, 343, 49–56. [Google Scholar] [CrossRef] [PubMed]






| Enriched KEGG Pathway 1 | rpoN mt nod > wt nod | wt nod > rpoN mt nod |
|---|---|---|
| q-value 2 | q-value 3 | |
| Plant KEGG database | ||
| Flavonoid biosynthesis | 1.44 × 10−8 | |
| Citrate cycle (TCA cycle) | 1.88 × 10−4 | |
| Glyoxylate and dicarboxylate metabolism | 1.72 × 10−3 | |
| Isoflavonoid biosynthesis | 2.13 × 10−3 | |
| Ascorbate and aldarate metabolism | 3.15 × 10−3 | |
| Brassinosteroid biosynthesis | 3.15 × 10−3 | |
| Ubiquinone and other terpenoid-quinone biosynthesis | 9.51 × 10−3 | |
| Fatty acid elongation | 4.12 × 10−4 | |
| Lysine degradation | 4.89 × 10−4 | |
| Fatty acid degradation | 1.56 × 10−3 | |
| Lysine biosynthesis | 1.81 × 10−3 | |
| Glycine, serine and threonine metabolism | 1.84 × 10−3 | |
| Butanoate metabolism | 1.27 × 10−2 | |
| Cyanoamino acid metabolism | 1.59 × 10−2 | |
| Glycerophospholipid metabolism | 1.59 × 10−2 | |
| Ether lipid metabolism | 1.59 × 10−2 | |
| Bacteria KEGG database | ||
| Citrate cycle (TCA cycle) | 2.10 × 10−2 | |
| Fatty acid metabolism | 1.96 × 10−3 | |
| Microbial metabolism in diverse environments | 1.12 × 10−2 | |
| Lysine degradation | 1.12 × 10−2 | |
| Glycine, serine and threonine metabolism | 1.19 × 10−2 | |
| ABC transporters | 1.19 × 10−2 | |
| Lysine biosynthesis | 1.19 × 10−2 | |
| Butanoate metabolism | 1.47 × 10−2 | |
| Caprolactam degradation | 1.47 × 10−2 | |
| Glycerophospholipid metabolism | 1.50 × 10−2 | |
| Valine, leucine and isoleucine biosynthesis | 1.61 × 10−2 | |
| Aminoacyl-tRNA biosynthesis | 1.61 × 10−2 | |
| Aminobenzoate degradation | 1.78 × 10−2 | |
| Biosynthesis of secondary metabolites | 1.78 × 10−2 | |
| Histidine metabolism | 1.78 × 10−2 | |
| Cyanoamino acid metabolism | 1.78 × 10−2 | |
| Arginine and proline metabolism | 1.78 × 10−2 | |
| Sulfur metabolism | 1.95 × 10−2 | |
| Valine, leucine and isoleucine degradation | 1.95 × 10−2 | |
| Vitamin B6 metabolism | 2.16 × 10−2 | |
| Metabolites 1 | ID 1 | log2FC (rpoN mt nod vs. wt nod) 2 |
|---|---|---|
| More abundant in nodules induced by the rpoN mutant | ||
| Naringenin | C00509 | 3.0 |
| 2-C-Methyl-d-erythritol 2,4-cyclodiphosphate | C11453 | 2.4 |
| 2-C-Methyl-d-erythritol 4-phosphate | C11434 | 2.3 |
| Homoeriodictyol chalcone | C16405 | 2.1 |
| 1-Nitronaphthalene-5,6-oxide | C14800 | 2.0 |
| C22:0 | C08281 | 2.0 |
| Parathion | C06604 | 1.8 |
| 2′,7-Dihydroxy-4′,5′-methylenedioxyisoflavone | C16226 | 1.7 |
| 6-Thiourate | C16613 | 1.5 |
| Luteolin | C01514 | 1.5 |
| Apigenin | C01477 | 1.5 |
| Oxalic acid | C00209 | 1.5 |
| 3-Dehydroteasterone | C15792 | 1.5 |
| N-Acetylneuraminate | C00270 | 1.4 |
| Phenyl acetate | C01454 | 1.3 |
| 3β-Hydroxy-4β-methyl-5α-cholest-7-ene-4α-carboxylate | C04840 | 1.3 |
| Afzelechin | C09320 | 1.3 |
| (−)Vestitone | C00786 | 1.3 |
| Histidine | C00135 | 1.2 |
| Phosphoenolpyruvate | C00074 | 1.2 |
| Cinnamate | C00423 | 1.2 |
| Thymidine | C00214 | 1.2 |
| N-Acetyl-d-glucosamine | C00140 | 1.1 |
| Quinate | C00296 | 1.1 |
| 3,9-Dihydroxypterocarpan | C04271 | 1.1 |
| Furoic acid | C01546 | 1.1 |
| Aconitate | C00417 | 1.1 |
| (Iso)Citrate | C00158 | 1.1 |
| Propanoyl phosphate | C02876 | 1.0 |
| Phosphoaspartate | C03082 | 1.0 |
| Formamidopyrimidine nucleoside triphosphate | C05922 | 1.0 |
| 6-Deoxoteasterone | C15799 | 1.0 |
| O-Succinyl-l-homoserine | C01118 | 1.0 |
| Aspartate | C00049 | 0.9 |
| UDP-6-sulfoquinovose | C11521 | 0.9 |
| γ-Tocopherol | C02483 | 0.9 |
| sn-Glycerol 3-phosphate | C00093 | 0.9 |
| Teasterone | C15791 | 0.8 |
| ITP | C00081 | 0.8 |
| 3-Methyl-cis,cis-hexadienedioate | C04112 | 0.8 |
| 2-Oxo-3-hydroxy-4-phosphobutanoate | C06054 | 0.8 |
| Leukotriene B4 | C02165 | 0.8 |
| Glutamate | C00025 | 0.8 |
| 4-Maleylacetoacetate | C01036 | 0.8 |
| 1-O-Sinapoyl-β-d-glucose | C01175 | 0.7 |
| Gallate | C01424 | 0.7 |
| 2-Dehydropantoate | C00966 | 0.7 |
| Chlorogenate | C00852 | 0.7 |
| 5-Hydroxyferulic acid methyl ester | C05619 | 0.7 |
| 3-Methoxyapigenin | C05902 | 0.7 |
| Indole-3-acetate | C00954 | 0.7 |
| Biotin | C00120 | 0.7 |
| Naphthalene-1,2-diol | C03012 | 0.7 |
| 3′,5′-cyclic di-GMP | C16463 | 0.7 |
| Serine | C00065 | 0.7 |
| 5-l-Glutamyltaurine | C05844 | 0.6 |
| Tartaric acid | C00552 | 0.6 |
| 5-Hydroxyindoleacetate | C05635 | 0.6 |
| Tryptophan | C00078 | 0.6 |
| 7-Methyluric acid | C16355 | 0.6 |
| 3,4-Dihydroxyphenylethyleneglycol | C05576 | 0.6 |
| Cathasterone | C15790 | 0.6 |
| UDP-deoxyhexose | C02199 | 0.6 |
| 22-Hydroxydocosanoate | C19623 | 0.6 |
| 5-Amino-6-(5′-phospho-d-ribitylamino)uracil | C04454 | 0.6 |
| 1-Phospho-α-d-galacturonate | C04037 | 0.6 |
| Itaconate | C00433 | 0.6 |
| UTP | C00075 | 0.6 |
| Inosine | C00294 | 0.5 |
| Aminobutanoic acid (ABA) | C00334 | 0.5 |
| AMP | C00020 | 0.5 |
| Glyoxylic acid | C00048 | 0.5 |
| (8Z,11Z,14Z)-Icosatrienoic acid | C03242 | 0.5 |
| GDP | C00035 | 0.5 |
| Pseudobaptigenin | C10522 | 0.5 |
| Succinic aldehyde | C00741 | 0.5 |
| (6Z,9Z,12Z)-Octadecatrienoic acid | C06426 | 0.5 |
| Less abundant in nodules induced by the rpoN mutant | ||
| Oxobutanoic acid | C00109 | −0.5 |
| N-Acetylmuramate | C02713 | −0.5 |
| 7,8-Diaminononanoate | C01037 | −0.5 |
| (R)-3-((R)-3-Hydroxybutanoyloxy)butanoate | C04546 | −0.5 |
| Orcinol | C02923 | −0.6 |
| 3-Hydroxy-5-methylhex-4-enoyl-CoA | C16469 | −0.6 |
| FMN (ox) | C00061 | −0.6 |
| 3-O-Methylquercetin | C04443 | −0.6 |
| Sinapoyl aldehyde | C05610 | −0.6 |
| Octanoyl-CoA | C01944 | −0.6 |
| Allantoate | C00499 | −0.6 |
| Homoserine lactone | C01234 | −0.6 |
| C4:0 (Butyric acid) | C00246 | −0.6 |
| 5-Aminolevulinic acid | C00430 | −0.6 |
| Gibberellin A1 | C00859 | −0.6 |
| 2-Hydroxy-2,4-pentadienoic acid | C07091 | −0.7 |
| Coproporphyrinogen III | C03263 | −0.7 |
| UDP-3-O-(3-hydroxytetradecanoyl)-d-glucosamine | C06022 | −0.7 |
| ADP-ribose | C00301 | −0.7 |
| Tetradecanoyl-CoA | C02593 | −0.7 |
| Estrone 3-sulfate | C02538 | −0.7 |
| (9Z)-Hexadecenoic acid | C08362 | −0.7 |
| 2-Deoxy-d-ribose 1-phosphate | C00672 | −0.8 |
| (S)-3-Hydroxytetradecanoyl-CoA | C05260 | −0.8 |
| N-Acetyl-l-glutamate | C00624 | −0.8 |
| Pipecolate | C00408 | −0.9 |
| Ala-Ala | C00993 | −0.9 |
| Lysine | C00047 | −0.9 |
| S-(Formylmethyl)glutathione | C14871 | −0.9 |
| 5-Amino-4-imidazolecarboxyamide | C04051 | −1.0 |
| Pentose | C00121 | −1.0 |
| 5-Hydroxyisourate | C11821 | −1.0 |
| 5,7,24(28)-Ergostatrienol | C15778 | −1.1 |
| Acrolein | C05986 | −1.1 |
| Lipoamide | C00248 | −1.2 |
| Sphingosine 1-phosphate | C06124 | −1.2 |
| 5-Hydroxyectoine | C16432 | −1.2 |
| 3-Carbamoyl-2-phenylpropionaldehyde | C16587 | −1.2 |
| Ketovaline | C00141 | −1.2 |
| 3-Propylmalate | C02504 | −1.2 |
| Dihydrothymine | C05715 | −1.3 |
| Acetyl-Glu-semialdehyde | C01250 | −1.3 |
| Propenoic acid C3:1 | C00511 | −1.3 |
| Leukotriene A4 | C00909 | −1.4 |
| d-2-Hydroxyisocaproate | C06103 | −1.4 |
| Nicotinate d-ribonucleotide | C01185 | −1.4 |
| Threonine | C00188 | −1.4 |
| Ectoine | C06231 | −1.4 |
| sn-Glycero-3-phosphocholine | C00670 | −1.4 |
| (2R)-2-Hydroxy-2-methylbutanenitrile | C18796 | −1.5 |
| 4,4-Dimethyl-5α-cholesta-8,14,24-trien-3β-ol | C11455 | −1.5 |
| Coniferyl aldehyde | C02666 | −1.6 |
| Histidinol | C00860 | −1.6 |
| 2-Hydroxycyclohexan-1-one | C01147 | −1.7 |
| 3-Phosphonooxypyruvate | C03232 | −1.7 |
| 3-Methyl-2-butenal | C07330 | −1.7 |
| Glyphosate | C11638 | −1.8 |
| Oxoglutarate | C00026 | −1.8 |
| Isopropylmaleate | C02631 | −1.9 |
| Aminoadipate | C00956 | −2.2 |
| Cyclohexanone | C00414 | −2.3 |
| Butynol | C20701 | −2.4 |
| Pyridoxamine phosphate | C00647 | −2.5 |
| Alanine | C00041 | −2.9 |
| Glutamine | C00064 | −2.9 |
| 10-Formyl-THF | C00234 | −3.0 |
| Diaminopimelate | C00666 | −3.2 |
| Ornithine | C00077 | −3.2 |
| Arginine | C00062 | −3.3 |
| Chorismate | C00251 | −4.7 |
| Locus ID 1 | Description 1 | Gene Name | log2FC (rpoN mt nod vs. wt nod) 2 |
|---|---|---|---|
| Cell wall/membrane/envelope biogenesis | |||
| Bphy_0649 | RND efflux system outer membrane lipoprotein | −5.0 | |
| Bphy_0919 | NLP/P60 protein | −1.6 | |
| Bphy_1282 | OmpW family protein | −2.6 | |
| Bphy_1546 | phospholipase C | −2.1 | |
| Bphy_1681 | group 1 glycosyl transferase | −2.2 | |
| Bphy_1689 | exopolysaccharide transport protein family | −3.2 | |
| Bphy_1690 | polysaccharide export protein | −4.3 | |
| Bphy_1691 | exopolysaccharide biosynthesis polyprenyl glycosylphosphotransferase | −3.4 | |
| Bphy_2283 | mannose-1-phosphate guanylyltransferase | −2.9 | |
| Bphy_2316 | dTDP-4-dehydrorhamnose reductase | −2.3 | |
| Bphy_2460 | group 1 glycosyl transferase | −2.5 | |
| Bphy_2464 | group 1 glycosyl transferase | −3.1 | |
| Bphy_2468 | putative glycosyl transferase | −2.1 | |
| Bphy_2469 | group 1 glycosyl transferase | −3.1 | |
| Bphy_2470 | NAD-dependent epimerase/dehydratase | −3.2 | |
| Bphy_2471 | GDP-mannose 4,6-dehydratase | −3.8 | |
| Bphy_2472 | exopolysaccharide transport protein family | −3.7 | |
| Bphy_2473 | polysaccharide export protein | −2.7 | |
| Bphy_2474 | undecaprenyl-phosphate glucose phosphotransferase | −3.8 | |
| Bphy_2475 | mannose-1-phosphate guanylyltransferase | −3.2 | |
| Bphy_2670 | polypeptide-transport-associated domain-containing protein | −1.5 | |
| Bphy_2671 | d-alanine:d-alanine ligase | ddl | −3.0 |
| Bphy_2672 | UDP-N-acetylmuramate-l-alanine ligase | murC | −1.8 |
| Bphy_2678 | UDP-N-acetylmuramoylalanyl-d-glutamate-2 | murE | −1.6 |
| Bphy_3069 | lytic transglycosylase | −1.9 | |
| Bphy_3557 | glycosyl transferase family protein | −2.3 | |
| Bphy_4515 | porin | −2.0 | |
| Bphy_5347 | NAD-dependent epimerase/dehydratase | −4.7 | |
| Bphy_7633 | d-alanine:d-alanine ligase | −3.7 | |
| Bphy_7707 | glycosyl transferase family protein | −3.6 | |
| Bphy_7819 | porin | −2.9 | |
| Energy production and conversion | |||
| Bphy_1284 | aldehyde dehydrogenase | −1.8 | |
| Bphy_1649 | alkanesulfonate monooxygenase | −3.2 | |
| Bphy_2012 | PIG3 family NAD(P)H quinone oxidoreductase | −1.9 | |
| Bphy_2272 | FAD linked oxidase domain-containing protein | −2.8 | |
| Bphy_3029 | F0F1 ATP synthase subunit α | −1.5 | |
| Bphy_3031 | F0F1 ATP synthase subunit B | −1.6 | |
| Bphy_3032 | F0F1 ATP synthase subunit C | −2.6 | |
| Bphy_3646 | cytochrome o ubiquinol oxidase subunit IV | cyoD | −3.9 |
| Bphy_3647 | cytochrome o ubiquinol oxidase, subunit III | cyoC | −5.1 |
| Bphy_3648 | cytochrome o ubiquinol oxidase, subunit I | cyoB | −4.0 |
| Bphy_3649 | ubiquinol oxidase, subunit II | cyoA | −4.2 |
| Bphy_3759 | transketolase central region | −2.3 | |
| Bphy_3760 | pyruvate dehydrogenase (acetyl-transferring) | −2.5 | |
| Bphy_4125 | acylphosphatase | −2.8 | |
| Bphy_4520 | formate dehydrogenase, γ subunit | −1.3 | |
| Bphy_5148 | AraC family transcriptional regulator | −1.8 | |
| Bphy_5156 | l-lactate dehydrogenase (cytochrome) | −1.9 | |
| Bphy_5235 | alkanesulfonate monooxygenase | −3.5 | |
| Bphy_5641 | glycolate oxidase iron-sulfur subunit | glcF | −2.5 |
| Bphy_6505 | formylmethanofuran dehydrogenase subunit A | −3.5 | |
| Bphy_7231 | cytochrome c class I | −2.6 | |
| Bphy_7232 | xenobiotic (desulfurization)monooxygenase subunit A | −2.9 | |
| Bphy_7263 | Ni/Fe-hydrogenase, b-type cytochrome subunit | −2.8 | |
| Bphy_7264 | nickel-dependent hydrogenase large subunit | −4.2 | |
| Bphy_7265 | hydrogenase (NiFe) small subunit | hydA | −4.3 |
| Bphy_7406 | aldehyde dehydrogenase | −5.6 | |
| Bphy_7729 | nitrogenase MoFe cofactor biosynthesis protein | nifE | −4.6 |
| Bphy_7730 | nitrogenase molybdenum-cofactor biosynthesis protein | nifN | −2.9 |
| Bphy_7733 | ferredoxin III, nif-specific | −5.0 | |
| Bphy_7737 | electron-transferring-flavoprotein dehydrogenase | −4.2 | |
| Bphy_7738 | electron transfer flavoprotein α/β-subunit | −4.5 | |
| Bphy_7739 | electron transfer flavoprotein α/β-subunit | −5.2 | |
| Bphy_7754 | nitrogenase molybdenum-iron protein α chain | nifD | −4.3 |
| Bphy_7755 | nitrogenase molybdenum-iron protein β chain | nifK | −4.0 |
| Bphy_7803 | electron transfer flavoprotein α subunit | −3.5 | |
| Bphy_7804 | electron transfer flavoprotein α/β-subunit | −3.1 | |
| Inorganic ion transport and metabolism | |||
| Bphy_0141 | CutC family protein | −3.2 | |
| Bphy_0257 | ammonium transporter | amtB | −2.1 |
| Bphy_1627 | sulfate ABC transporter inner membrane subunit | cysW | −1.7 |
| Bphy_1629 | sulfate ABC transporter periplasmic sulfate-binding protein | −1.7 | |
| Bphy_1647 | ABC transporter-like protein | −2.9 | |
| Bphy_1648 | transport systems inner membrane component | −2.7 | |
| Bphy_2231 | sulfate adenylyltransferase large subunit | −2.2 | |
| Bphy_2235 | sulfite reductase | −2.0 | |
| Bphy_3602 | ABC transporter related | −1.7 | |
| Bphy_3603 | ABC transporter periplasmic ligand-binding protein | −2.4 | |
| Bphy_5040 | NlpA lipoprotein | −4.0 | |
| Bphy_5227 | ABC-type glycine betaine transport system | −2.8 | |
| Bphy_5229 | aliphatic sulfonate ABC transporter periplasmic protein | −3.7 | |
| Bphy_5232 | rhodanese domain-containing protein | −4.0 | |
| Bphy_5473 | Dyp-type peroxidase family protein | −1.9 | |
| Bphy_5555 | sulfatase | −1.6 | |
| Bphy_6080 | taurine ABC transporter, periplasmic binding protein | −4.6 | |
| Bphy_7233 | ABC transporter related | −3.2 | |
| Bphy_7234 | transport systems inner membrane component | −3.7 | |
| Bphy_7235 | transport systems inner membrane component | −3.0 | |
| Bphy_7236 | ABC sulfate ester transporter, periplasmic protein | −2.5 | |
| Bphy_7645 | transport systems inner membrane component | −2.6 | |
| Bphy_7646 | transport systems inner membrane component | −3.1 | |
| Bphy_7647 | ABC transporter related | −3.1 | |
| Bphy_7753 | nitrogenase reductase | nifH1 | −5.2 |
| Bphy_7808 | nitrogenase reductase | nifH2 | −5.2 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lardi, M.; Liu, Y.; Giudice, G.; Ahrens, C.H.; Zamboni, N.; Pessi, G. Metabolomics and Transcriptomics Identify Multiple Downstream Targets of Paraburkholderia phymatum σ54 During Symbiosis with Phaseolus vulgaris. Int. J. Mol. Sci. 2018, 19, 1049. https://doi.org/10.3390/ijms19041049
Lardi M, Liu Y, Giudice G, Ahrens CH, Zamboni N, Pessi G. Metabolomics and Transcriptomics Identify Multiple Downstream Targets of Paraburkholderia phymatum σ54 During Symbiosis with Phaseolus vulgaris. International Journal of Molecular Sciences. 2018; 19(4):1049. https://doi.org/10.3390/ijms19041049
Chicago/Turabian StyleLardi, Martina, Yilei Liu, Gaetano Giudice, Christian H. Ahrens, Nicola Zamboni, and Gabriella Pessi. 2018. "Metabolomics and Transcriptomics Identify Multiple Downstream Targets of Paraburkholderia phymatum σ54 During Symbiosis with Phaseolus vulgaris" International Journal of Molecular Sciences 19, no. 4: 1049. https://doi.org/10.3390/ijms19041049
APA StyleLardi, M., Liu, Y., Giudice, G., Ahrens, C. H., Zamboni, N., & Pessi, G. (2018). Metabolomics and Transcriptomics Identify Multiple Downstream Targets of Paraburkholderia phymatum σ54 During Symbiosis with Phaseolus vulgaris. International Journal of Molecular Sciences, 19(4), 1049. https://doi.org/10.3390/ijms19041049