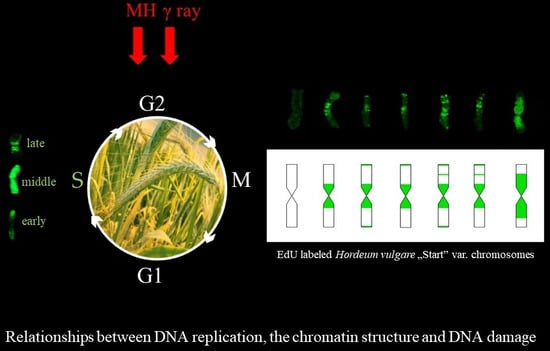
1. Introduction
Data regarding the effects of mutagens on plant nuclear genomes and DNA replication are of great importance. The spatiotemporal patterns of DNA replication in nuclei were recently characterized in detail in control cells [
1], as well as in relation to DNA damage and mutagenesis [
2] using a quantitative analysis. However, to date there is no similar data on the effects of mutagens on the pattern of DNA replication on chromosomes. Analyses of the distribution of the signals of DNA replication on the chromosomes can be more informative when exploring the relationships between DNA replication, the chromatin structure, and DNA damage than studies using non-dividing cells.
Until now, the localisation of replicated chromatin was only possible using bromodeoxyuridine (BrdU). One of the disadvantages of using BrdU is degradation of the chromatin structure during denaturation step, which is especially inconvenient in the context of an analysis of DNA damage during mutagenesis. The relatively large size of the detection sites caused by the need to use specific antibodies to detect BrdU is an unfavorable feature of an analysis of DNA replication sites, especially in the case of an analysis of the signals in chromosomes. Currently, the “click” reaction using 5-ethynyl-2′-deoxyuridine (EdU) [
3,
4] is commonly used. Its good preservation of chromatin and high resolution make this technique useful in a detailed analysis of the effects of mutagens on the S-phase [
2].
In this study, we present the distribution of the DNA replication pattern on chromosomes using pulse EdU labeling and analyze its relationship with the DNA damage that is induced by mutagenic treatment with maleic hydrazide (MH) and γ ray. To the best of our knowledge, this is the first example of a study of the effects of mutagens on the DNA replication pattern in chromosomes, as well as the first to use EdU labeling for these purposes.
We used barley (
Hordeum vulgare ‘Start’ variety, 2
n = 14) as the model plant species. Barley, which is characterized by relatively large chromosomes and a specific heterochromatin distribution [
5,
6], is a convenient species for an analysis of the distribution of the DNA replication pattern along the chromosomes, as well as in the context of mutagenesis. Barley is regarded to be a model species in analyses of the cytogenetic effects of mutagens, especially due to its chromosome size. Chromosome rearrangements [
7,
8], as well as disturbances of the cell cycle [
2] after mutagenic treatment, have previously been shown in barley cells. In our study, the duration of the cell cycle of the
Hordeum vulgare ‘Start’ variety was estimated, as well as the influence of MH and γ ray on it, by applying the EdU method.
3. Discussion
In present study we analyzed the distribution of a DNA replication pattern on the
H. vulgare ‘Start’ variety chromosomes, as well as its relationship to the DNA damage, using EdU method. Different replication patterns were observed in the chromosomes in the barley roots within individual metaphases. This may be due to differences in the DNA packing of individual chromosomes. It is well known that euchromatin and heterochromatin regions replicate at different times during the S-phase. Individual barley chromosomes are characterized by a different localisation of constitutive heterochromatin, as was demonstrated by the Giemsa C-banding technique [
9]. Therefore, the chromosomes belonging to one metaphase plate may have a different DNA replication pattern. Due to results, we can state that DNA replication in barley chromosomes begins in the terminal chromosome regions (early S-phase), after which its pattern is observed in whole chromosomes, and at the end—in the centromeric regions (late S-phase). These analyses indirectly provide information about the localisation of euchromatin and heterochromatin in the chromosomes of the
H. vulgare ‘Start’ variety. Since it is known that DNA replication starts in euchromatin and then continues in the heterochromatin regions, this implies the presence of transcriptionally active genes in the terminal chromosome regions and the inactive heterochromatin in the centromeric regions. A similar localisation of euchromatin and heterochromatin has previously been shown using the BrdU incorporation and detection methods, and fluorescence, in situ hybridisation with centromeric and telomeric probes [
10].
Thirteen types of replication patterns were distinguished in the control barley chromosomes with EdU incorporation and detection, whereas only five patterns had previously been observed using BrdU incorporation and detection [
11]. This is probably due to the possibility of discovering small signals with EdU method by using a small size detection azide and eliminating the denaturation step, which is necessary for detection of BrdU. For example, the chromosomes with just centromeric signals were observed using BrdU, whereas patterns with centromeric signals, as well as terminal, or terminal and interstitial, signals, were observed using the EdU method. The accuracy of this method allowed one to notice the differences between the occurrence times of individual replication patterns in control and treated cells. This can prove that EdU can potentially be applied to studying the effects of mutagens on cell cycle disturbances, especially in plant species that are characterized by small chromosomes.
Beside the typical replication signals comprising two chromatids, specific new chromosome replication patterns were observed in control at 10.5 h—with only one sister chromatid labeled within an individual chromosome. This labeling pattern was characteristic for the cells in which the DNA replication occurred twice—first, the DNA synthesis in the presence of EdU, and the second, without EdU. Other new patterns observed at this time were chromosomes with both chromatids labeled, although each of them in different localisation. This is characteristic of the sister chromatid exchange (SCE), which is commonly known in plants, as well as in animals and humans. The occurrence of SCEs was first demonstrated using autoradiography, and later a procedure that had a much greater resolution using BrdU was introduced [
12]. It is known that BrdU itself induces the SCEs due to its mutagenic effect. Until now, there is no data on the possible application of EdU in the SCE method and, consequently, on its effects on the induction of sister chromatid exchanges.
Additionally to the main aim, the results of this study provided new data about the duration of the G2-phase and the cell cycle of the
H. vulgare ‘Start’ variety cells. The first labeled metaphases in the control roots were observed at 4 h after the incorporation of EdU, while at 2 h after the incorporation of EdU no labeling has been observed within the chromosomes. This indicates that the duration of the G2-phase is between 2 h and 4 h. The replication pattern with labeling in only one chromatid of the chromosome was observed at 10.5 h. This proves that during 10.5 h, in addition to the G2-phase, there was also a complete next cell cycle without the presence of thymidine analogs. Considering that the length of the G2 phase is more than 2 h, it could be concluded that the duration of the cell cycle in
Hordeum vulgare ‘Start’ variety is at most 8.5 h. It should also be emphasized that the pattern of replication with labeling in only one chromatid within the chromosome could also be observed in the earlier hours after incorporation of EdU—between 7.5 h and 10.5 h. It is also possible that the duration of the G2 phase may be longer than 2 h. In both cases, this would mean that the duration of the cell cycle in this variety of barley can be even shorter than 8.5 h. The experiments for the ‘Start’ variety were planned according to the mean duration of the cell cycle of other varieties of barley, e.g., ‘Brage’—10.4 h [
13], ‘Sultan’—12.4 h, ‘Maris Otter’—12 h [
14], and ‘Amethyst’—9.2 h [
15]. The results of this study indirectly prove that the duration of the cell cycle of the
H. vulgare ‘Start’ variety is definitely shorter than in the previously described varieties of this species.
The analyses showed the differences in the chromosome replication pattern for a particular experimental group and at specific time points. After treatment with maleic hydrazide, only six replication patterns were observed. First chromosomes with replication patterns in the MH-treated roots were observed only at 10.5 h. This may be due to the mechanism of MH action, namely, its influence on the synthesis of the nucleic acids and enzymes that are involved in the mitotic spindle [
16,
17]. The mitotic activity can even be totally stopped after MH treatment [
18]. The patterns of replication that were observed after MH treatment were characteristic of the regions that have been replicated in the early and middle S-phase, even though the patterns for the late S-phase should be observed first. This may indicate that MH led to a complete cell cycle arrest in the cells that were in the late S-phase during the incorporation of EdU. After treatment with MH, the observed patterns differed considerably from patterns that were observed at the same time point in the control cells. No replication patterns with signals involving one sister chromatid have been observed, thus excluding a second DNA synthesis without the presence of EdU. MH is a clastogenic and mutagenic agent that may cause the S-phase to be extended. We found that the first labeled metaphases in the MH-treated cells were observed at 10.5 h while in the control cells already at 4 h. This may be due to both the extension of the S-phase or a delayed G2/M transition. For the first time, comparing the time of appearance of the first labeled metaphases in control and treated material, we can precisely evaluate that after MH treatment the duration of cell transitions from the S-phase of the cell cycle to mitosis was extended for about 6.5 h.
Differences at specific time points in the control and γ ray-treated roots were also observed. The labeling of whole chromosomes in the control was observed at 6 h, whereas in the γ ray-treated roots, it was already observed at 4 h. Similarly, the chromosomes with labeling in the terminal regions of a chromosome were observed at 6 h in the γ ray-treated roots, whereas in the control, they were observed at 7.5 h. It is known that γ ray acts during the G1, S, and G2 cell cycle phases. We conclude that γ ray can lead to a shortening of the S-phase or acceleration of the G2/M transition, which is judged by the presence of the replication patterns that are characteristic for the early and middle S-phase earlier than in the control cells. This physical mutagen may also have the opposite effect—extending the S-phase of the cell cycle or G2/M transition, as was evidenced by the presence of replication patterns that are characteristic for the late S-phase longer after the end of the EdU incorporation than in the control cells.
Summarizing, differences in the temporal distribution of the replication patterns between the control, γ ray-, and MH-treated roots were found. Slight differences were observed regarding the replication patterns in the control and γ ray-treated roots, while differences in replication patterns in the control and MH-treated cells were more significant. The results obtained in this work are consistent with those previously obtained by Kwasniewska et al. [
2] during an analysis of the replication process in the barley nuclei. MH has a stronger effect on DNA replication than γ radiation. It was demonstrated that treatment with MH and γ ray did not change the characteristic S-phase patterns in the nuclei; however, the frequencies of the S-phase labeled cells after mutagenic treatment were different than in the control cells. The results of this study on the pattern of replication in barley chromosomes also confirm that no new replication patterns are observed after mutagenic treatment. However, differences were found in the temporal distribution of the replication patterns between the control, γ ray-, and MH-treated roots, as well as differences in the frequency of the labeled metaphases. Moreover, previous studies on the replication patterns in barley cells have also shown that the frequencies of EdU-labeled cell nuclei in the early, middle, and late S-phase were different in the control cells and cells that had been treated with mutagens. After treatment with MH, a significant increase has been observed in the frequency of labeled cells in the middle S-phase. This may indicate an extension of the S-phase of the cell cycle after treatment with this chemical mutagen, as is also confirmed by the data presented in this paper. After treatment with γ ray, in turn, a significant increase in the frequency of labeled cells in the late S-phase has been observed before, which confirms that γ ray can extend the late S-phase in barley cells. This is followed by the observation that the pattern of replication in chromosomes that are characteristic for late S-phase phase occurs longer after the incorporation of EdU than in the control cells.