Melatonin Protects against Lung Fibrosis by Regulating the Hippo/YAP Pathway
Abstract
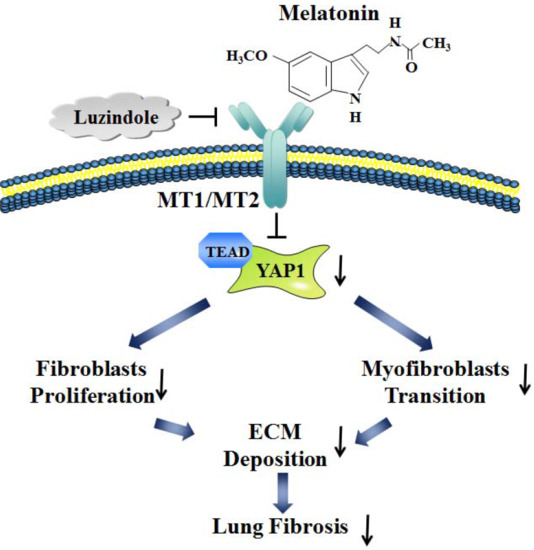
:1. Introduction
2. Results
2.1. Protective Role of Melatonin in BLM-Induced Lung Fibrosis in Mice
2.2. Melatonin Attenuates Pulmonary Fibrosis by Interacting with Its Specific Receptors
2.3. Hippo/YAP1 Pathway Contributes to the Inhibitory Function of Melatonin during Pulmonary Fibrosis
2.4. Overexpression of YAP1 Abates the Anti-Fibrotic Effect of Melatonin in Lung Fibroblasts
3. Discussion
4. Materials and Methods
4.1. Experimental Pulmonary Fibrosis Model and Treatment
4.2. Isolation of Neonatal Mouse Lung Fibroblasts
4.3. Procedures for Cell Transfection
4.4. Western Blot Analysis
4.5. Masson’s Trichrome Staining
4.6. Immunohistochemistry Staining
4.7. SircolTM Soluble Collagen Assay
4.8. Immunofluorescence Staining
4.9. Scratch Wound-Healing Assay
4.10. EdU Fluorescence Staining
4.11. Quantitative RT-PCR
4.12. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| IPF | Idiopathic pulmonary fibrosis |
| Mel | Melatonin |
| Luz | Luzindole |
| GPCR | G-protein-coupled receptor |
| BLM | Bleomycin |
References
- Martinez, F.J.; Collard, H.R.; Pardo, A.; Raghu, G.; Richeldi, L.; Selman, M.; Swigris, J.J.; Taniguchi, H.; Wells, A.U. Idiopathic pulmonary fibrosis. Nat. Rev. Dis. Prim. 2017, 3, 17074. [Google Scholar] [CrossRef] [PubMed]
- Kolb, M.; Bonella, F.; Wollin, L. Therapeutic targets in idiopathic pulmonary fibrosis. Resp. Med. 2017, 131, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, X.; Bai, X.; Lin, Y.; Li, Z.; Fu, J.; Li, M.; Zhao, T.; Yang, H.; Xu, R.; et al. Melatonin prevents endothelial cell pyroptosis via regulation of long noncoding RNA MEG3/miR-223/NLRP3 axis. J. Pineal Res. 2017, 64. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Ma, Z.; Jiang, S.; Fan, C.; Deng, C.; Yan, X.; Di, S.; Lv, J.; Reiter, R.J.; Yang, Y. Melatonin: The dawning of a treatment for fibrosis? J. Pineal Res. 2016, 60, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Yang, L.; Li, Y.; Yan, G.; Feng, C.; Liu, T.; Gong, R.; Yuan, Y.; Wang, N.; Idiiatullina, E.; et al. Melatonin protects bone marrow mesenchymal stem cells against iron overload-induced aberrant differentiation and senescence. J. Pineal Res. 2017, 63. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Won, J.; Lee, Y.; Lee, S.; Park, K.; Chang, K.T. Melatonin treatment induces interplay of apoptosis, autophagy, and senescence in human colorectal cancer cells. J. Pineal Res. 2014, 56, 264–274. [Google Scholar] [CrossRef] [PubMed]
- Jehan, S.; Jean-Louis, G.; Zizi, F.; Auguste, E.; Pandi-Perumal, S.R.; Gupta, R.; Attarian, H.; McFarlane, S.I.; Hardeland, R.; Brzezinski, A. Sleep, Melatonin, and the Menopausal Transition: What Are the Links? Sleep Sci. 2017, 10, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Calvo, J.R.; Gonzalez-Yanes, C.; Maldonado, M.D. The role of melatonin in the cells of the innate immunity: A review. J. Pineal Res. 2013, 55, 103–120. [Google Scholar] [CrossRef] [PubMed]
- Sinha, B.; Wu, Q.; Li, W.; Tu, Y.; Sirianni, A.C.; Chen, Y.; Jiang, J.; Zhang, X.; Chen, W.; Zhou, S.; et al. Protection of melatonin in experimental models of newborn hypoxic-ischemic brain injury through MT1 receptor. J. Pineal Res. 2017, 64, e12443. [Google Scholar] [CrossRef] [PubMed]
- Sharan, K.; Lewis, K.; Furukawa, T.; Yadav, V.K. Regulation of bone mass through pineal-derived melatonin-MT2 receptor pathway. J. Pineal Res. 2017, 63, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Martinez, E.; Jurado-Lopez, R.; Valero-Munoz, M.; Bartolome, M.V.; Ballesteros, S.; Luaces, M.; Briones, A.M.; Lopez-Andres, N.; Miana, M.; Cachofeiro, V. Leptin induces cardiac fibrosis through galectin-3, mTOR and oxidative stress: Potential role in obesity. J. Hypertens. 2014, 32, 1104–1114. [Google Scholar] [CrossRef] [PubMed]
- Das, N.; Mandala, A.; Naaz, S.; Giri, S.; Jain, M.; Bandyopadhyay, D.; Reiter, R.J.; Roy, S.S. Melatonin protects against lipid-induced mitochondrial dysfunction in hepatocytes and inhibits stellate cell activation during hepatic fibrosis in mice. J. Pineal Res. 2017, 62. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, Z.; Kotuk, M.; Erdogan, H.; Iraz, M.; Yagmurca, M.; Kuku, I.; Fadillioglu, E. Preventive effect of melatonin on bleomycin-induced lung fibrosis in rats. J. Pineal Res. 2006, 40, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Richardson, H.E.; Portela, M. Tissue growth and tumorigenesis in Drosophila: Cell polarity and the Hippo pathway. Curr. Opin. Cell Biol. 2017, 48, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Pfleger, C.M. The Hippo Pathway: A Master Regulatory Network Important in Development and Dysregulated in Disease. Curr. Top. Dev. Biol. 2017, 123, 181–228. [Google Scholar] [PubMed]
- Zhang, C.; Bian, M.; Chen, X.; Jin, H.; Zhao, S.; Yang, X.; Shao, J.; Chen, A.; Guo, Q.; Zhang, F.; et al. Oroxylin A prevents angiogenesis of LSECs in liver fibrosis via inhibition of YAP/HIF-1alpha signaling. J. Cell. Biochem. 2017. [Google Scholar] [CrossRef]
- Lo Sardo, F.; Muti, P.; Blandino, G.; Strano, S. Melatonin and Hippo Pathway: Is There Existing Cross-Talk? Int. J. Mol. Sci. 2017, 18. [Google Scholar] [CrossRef] [PubMed]
- Moroishi, T.; Hansen, C.G.; Guan, K.L. The emerging roles of YAP and TAZ in cancer. Nat. Rev. Cancer 2015, 15, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Xu, C.; Pan, Z.; Zhang, Y.; Xu, Z.; Chen, Y.; Li, T.; Li, X.; Liu, Y.; Huangfu, L.; et al. The antifibrotic effects and mechanisms of microRNA-26a action in idiopathic pulmonary fibrosis. Mol. Ther. 2014, 22, 1122–1133. [Google Scholar] [CrossRef] [PubMed]
- Athwal, V.S.; Pritchett, J.; Llewellyn, J.; Martin, K.; Camacho, E.; Raza, S.M.; Phythian-Adams, A.; Birchall, L.J.; Mullan, A.F.; Su, K.; et al. SOX9 predicts progression toward cirrhosis in patients while its loss protects against liver fibrosis. EMBO Mol. Med. 2017, 9, 1696–1710. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Li, P.X.; Wu, J.; Gao, Y.J.; Yin, M.X.; Lin, Y.; Yang, M.; Chen, D.P.; Sun, H.P.; Liu, Z.B.; et al. Involvement of the Hippo pathway in regeneration and fibrogenesis after ischaemic acute kidney injury: YAP is the key effector. Clin. Sci. 2016, 130, 349–363. [Google Scholar] [CrossRef] [PubMed]
- Mannaerts, I.; Leite, S.B.; Verhulst, S.; Claerhout, S.; Eysackers, N.; Thoen, L.F.; Hoorens, A.; Reynaert, H.; Halder, G.; van Grunsven, L.A. The Hippo pathway effector YAP controls mouse hepatic stellate cell activation. J. Hepatol. 2015, 63, 679–688. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Guo, S.; Zhang, Y.; Yin, J.; Yin, W.; Tao, S.; Wang, Y.; Zhang, C. Proton-sensing GPCR-YAP Signalling Promotes Cancer-associated Fibroblast Activation of Mesenchymal Stem Cells. Int. J. Biol. Sci. 2016, 12, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Cai, B.; Ma, W.; Bi, C.; Yang, F.; Zhang, L.; Han, Z.; Huang, Q.; Ding, F.; Li, Y.; Yan, G.; et al. Long noncoding RNA H19 mediates melatonin inhibition of premature senescence of c-kit(+) cardiac progenitor cells by promoting miR-675. J. Pineal Res. 2016, 61, 82–95. [Google Scholar] [CrossRef] [PubMed]
- Karimfar, M.H.; Rostami, S.; Haghani, K.; Bakhtiyari, S.; Noori-Zadeh, A. Melatonin Alleviates Bleomycin-Induced Pulmonary Fibrosis in Mice. J. Biol. Regul. Homeost. Agents 2015, 29, 327–334. [Google Scholar] [PubMed]
- Arslan, S.O.; Zerin, M.; Vural, H.; Coskun, A. The effect of melatonin on bleomycin-induced pulmonary fibrosis in rats. J. Pineal Res. 2002, 32, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Wu, Q.Q.; Cao, L.F.; Qing, H.Y.; Zhang, C.; Chen, Y.H.; Wang, H.; Liu, R.Y.; Xu, D.X. Melatonin inhibits endoplasmic reticulum stress and epithelial-mesenchymal transition during bleomycin-induced pulmonary fibrosis in mice. PLoS ONE 2014, 9, e97266. [Google Scholar] [CrossRef] [PubMed]
- Shajari, S.; Laliena, A.; Heegsma, J.; Tunon, M.J.; Moshage, H.; Faber, K.N. Melatonin suppresses activation of hepatic stellate cells through RORalpha-mediated inhibition of 5-lipoxygenase. J. Pineal Res. 2015, 59, 391–401. [Google Scholar] [CrossRef] [PubMed]
- Tocharus, C.; Puriboriboon, Y.; Junmanee, T.; Tocharus, J.; Ekthuwapranee, K.; Govitrapong, P. Melatonin enhances adult rat hippocampal progenitor cell proliferation via ERK signaling pathway through melatonin receptor. Neuroscience 2014, 275, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Zhang, J.; Luo, X.; Li, M.; Yue, Y.; Laudon, M.; Jia, Z.; Zhang, R. Neu-P11, a novel MT1/MT2 agonist, reverses diabetes by suppressing the hypothalamic-pituitary-adrenal axis in rats. Eur. J. Pharmacol. 2017, 812, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Lockley, S.W.; Dressman, M.A.; Licamele, L.; Xiao, C.; Fisher, D.M.; Flynn-Evans, E.E.; Hull, J.T.; Torres, R.; Lavedan, C.; Polymeropoulos, M.H. Tasimelteon for non-24-hour sleep-wake disorder in totally blind people (SET and RESET): Two multicentre, randomised, double-masked, placebo-controlled phase 3 trials. Lancet 2015, 386, 1754–1764. [Google Scholar] [CrossRef]
- Du, K.; Hyun, J.; Premont, R.T.; Choi, S.S.; Michelotti, G.A.; Swiderska-Syn, M.; Dalton, G.D.; Thelen, E.; Rizi, B.S.; Jung, Y.; et al. Hedgehog-YAP Signaling Pathway Regulates Glutaminolysis to Control Hepatic Stellate Cell Activation. Gastroenterology 2018. [Google Scholar] [CrossRef] [PubMed]
- Nishio, M.; Sugimachi, K.; Goto, H.; Wang, J.; Morikawa, T.; Miyachi, Y.; Takano, Y.; Hikasa, H.; Itoh, T.; Suzuki, S.O.; et al. Dysregulated YAP1/TAZ and TGF-beta signaling mediate hepatocarcinogenesis in Mob1a/1b-deficient mice. Proc. Natl. Acad. Sci. USA 2016, 113, E71–E80. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.X.; Zheng, B.B.; Sun, N.N.; Zheng, Z.M.; Yang, Q.J.; Meng, Y. Role of Yes-associated protein 1 in angiotensin-induced pulmonary fibrosis in rats. Zhonghua Yi Xue Za Zhi 2017, 97, 2208–2214. [Google Scholar] [PubMed]
- Liu, F.; Lagares, D.; Choi, K.M.; Stopfer, L.; Marinkovic, A.; Vrbanac, V.; Probst, C.K.; Hiemer, S.E.; Sisson, T.H.; Horowitz, J.C.; et al. Mechanosignaling through YAP and TAZ drives fibroblast activation and fibrosis. Am. J. Physiol. Lung Cell. Mol. Physiol. 2015, 308, L344–L357. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.X.; Zhao, B.; Panupinthu, N.; Jewell, J.L.; Lian, I.; Wang, L.H.; Zhao, J.; Yuan, H.; Tumaneng, K.; Li, H.; et al. Regulation of the Hippo-YAP pathway by G-protein-coupled receptor signaling. Cell 2012, 150, 780–791. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.X.; Luo, J.; Mo, J.S.; Liu, G.; Kim, Y.C.; Meng, Z.; Zhao, L.; Peyman, G.; Ouyang, H.; Jiang, W.; et al. Mutant Gq/11 promote uveal melanoma tumorigenesis by activating YAP. Cancer Cell 2014, 25, 822–830. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Wang, S.; Wang, Z.; Feng, X.; Liu, P.; Lv, X.B.; Li, F.; Yu, F.X.; Sun, Y.; Yuan, H.; et al. Estrogen regulates Hippo signaling via GPER in breast cancer. J. Clin. Investig. 2015, 125, 2123–2135. [Google Scholar] [CrossRef] [PubMed]
- Wennmann, D.O.; Vollenbroker, B.; Eckart, A.K.; Bonse, J.; Erdmann, F.; Wolters, D.A.; Schenk, L.K.; Schulze, U.; Kremerskothen, J.; Weide, T.; et al. The Hippo pathway is controlled by Angiotensin II signaling and its reactivation induces apoptosis in podocytes. Cell Death Dis. 2014, 5, e1519. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Liu, S.; Chen, Y.; Bai, X.; Liu, L.; Dong, Y.; Hu, M.; Su, X.; Huangfu, L.; Li, X.; et al. miR-26a suppresses EMT by disrupting the Lin28B/let-7d axis: Potential cross-talks among miRNAs in IPF. J. Mol. Med. 2016, 94, 655–665. [Google Scholar] [CrossRef] [PubMed]
- Henri, O.; Pouehe, C.; Houssari, M.; Galas, L.; Nicol, L.; Edwards-Levy, F.; Henry, J.P.; Dumesnil, A.; Boukhalfa, I.; Banquet, S.; et al. Selective Stimulation of Cardiac Lymphangiogenesis Reduces Myocardial Edema and Fibrosis Leading to Improved Cardiac Function Following Myocardial Infarction. Circulation 2016, 133, 1484–1497; discussion 1497. [Google Scholar] [CrossRef] [PubMed]






© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, X.; Sun, J.; Su, W.; Shan, H.; Zhang, B.; Wang, Y.; Shabanova, A.; Shan, H.; Liang, H. Melatonin Protects against Lung Fibrosis by Regulating the Hippo/YAP Pathway. Int. J. Mol. Sci. 2018, 19, 1118. https://doi.org/10.3390/ijms19041118
Zhao X, Sun J, Su W, Shan H, Zhang B, Wang Y, Shabanova A, Shan H, Liang H. Melatonin Protects against Lung Fibrosis by Regulating the Hippo/YAP Pathway. International Journal of Molecular Sciences. 2018; 19(4):1118. https://doi.org/10.3390/ijms19041118
Chicago/Turabian StyleZhao, Xiaoguang, Jian Sun, Wei Su, Huitong Shan, Bowen Zhang, Yining Wang, Azaliia Shabanova, Hongli Shan, and Haihai Liang. 2018. "Melatonin Protects against Lung Fibrosis by Regulating the Hippo/YAP Pathway" International Journal of Molecular Sciences 19, no. 4: 1118. https://doi.org/10.3390/ijms19041118
APA StyleZhao, X., Sun, J., Su, W., Shan, H., Zhang, B., Wang, Y., Shabanova, A., Shan, H., & Liang, H. (2018). Melatonin Protects against Lung Fibrosis by Regulating the Hippo/YAP Pathway. International Journal of Molecular Sciences, 19(4), 1118. https://doi.org/10.3390/ijms19041118