Alpha-Mangostin Improves Insulin Secretion and Protects INS-1 Cells from Streptozotocin-Induced Damage
Abstract
:1. Introduction
2. Results
2.1. Effect of α-Mangostin on Glucose-Stimulated Secretion of Insulin (GSIS) in Cells
2.2. Effect of α-Mangostin on Viability of INS-1 Cells for Various Time Points with High Glucose
2.3. Effect of α-Mangostin on the Protein Expression and Intracellular Ca2+ Levels Involved in Insulin Signaling in INS-1 Cells with Hyperglycemia-Induced Insulin Resistance
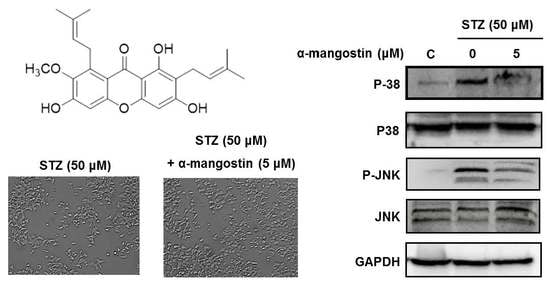
2.4. Effect of α-Mangostin on Streptozotocin (STZ)-Induced Damage in INS-1 Cells
2.5. Effect of α-Mangostin on Mitogen-Activated Protein Kinases (MAPKs), PI3K/Akt, and Cleaved Caspase-3 in INS-1 Cells with STZ-Induced Damage
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Cell Culture
4.3. Cell Viability Assay
4.4. Reactive Oxygen Species (ROS) Measurement
4.5. Insulin Secretion Assay
4.6. Western Blot Analysis
4.7. Staining for Intracellular Ca2+
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Olokoba, A.B.; Obateru, O.A.; Olokoba, L.B. Type 2 diabetes mellitus: A review of current trends. Oman Med. J. 2012, 27, 269–273. [Google Scholar] [CrossRef] [PubMed]
- Ryu, H.W.; Cho, J.K.; Curtis-Long, M.J.; Yuk, H.J.; Kim, Y.S.; Jung, S.; Kim, Y.S.; Lee, B.W.; Park, K.H. α-Glucosidase inhibition and antihyperglycemic activity of prenylated xanthones from Garcinia mangostana. Phytochemistry 2011, 72, 2148–2154. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Han, W.; Jiang, X.; Li, M.; Gao, L.; Zhao, J.J. Chronic leucine exposure results in reduced but reversible glucose-stimulated insulin secretion in INS-1 cells. Mol. Med. Rep. 2014, 9, 2554–2558. [Google Scholar] [CrossRef] [PubMed]
- Wilcox, G. Insulin and insulin resistance. Clin. Biochem. Rev. 2005, 26, 19–39. [Google Scholar] [PubMed]
- Reeds, D.N.; Stuart, C.A.; Perez, O.; Klein, S. Adipose tissue, hepatic, and skeletal muscle insulin sensitivity in extremely obese subjects with acanthosis nigricans. Metab. Clin. Exp. 2006, 55, 1658–1663. [Google Scholar] [CrossRef] [PubMed]
- Jariyapongskul, A.; Areebambud, C.; Suksamrarn, S.; Mekseepralard, C. α-mangostin attenuation of hyperglycemia-induced ocular hypoperfusion and blood retinal barrier leakage in the early stage of type 2 diabetes rats. BioMed Res. Int. 2015, 2015, 785826. [Google Scholar] [CrossRef] [PubMed]
- Sell, H.; Eckel, J.; Dietze-Schroeder, D. Pathways leading to muscle insulin resistance—The muscle—Fat connection. Arch. Physiol. Biochem. 2006, 112, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Accili, D. Lilly lecture 2003: The struggle for mastery in insulin action: From triumvirate to republic. Diabetes 2004, 53, 1633–1642. [Google Scholar] [CrossRef] [PubMed]
- Bugianesi, E.; McCullough, A.J.; Marchesini, G. Insulin resistance: A metabolic pathway to chronic liver disease. Hepatology 2005, 42, 987–1000. [Google Scholar] [CrossRef] [PubMed]
- Kawahito, S.; Kitahata, H.; Oshita, S. Problems associated with glucose toxicity: Role of hyperglycemia-induced oxidative stress. World J. Gastroenterol. 2009, 15, 4137–4142. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.H.; Guo, L.X.; Yin, F.; Zhang, Y.L.; Liu, Z.X.; Wang, Y.W. Geniposide regulates glucose-stimulated insulin secretion possibly through controlling glucose metabolism in INS-1 cells. PLoS ONE 2013, 8, e78315. [Google Scholar] [CrossRef] [PubMed]
- Abate, N.; Chandalia, M. Ethnicity and type 2 diabetes: Focus on Asian Indians. J. Diabetes Complicat. 2001, 15, 320–327. [Google Scholar] [CrossRef]
- Boucher, J.; Kleinridders, A.; Kahn, C.R. Insulin receptor signaling in normal and insulin-resistant states. Cold Spring Harb. Perspect. Biol. 2014, 6. [Google Scholar] [CrossRef] [PubMed]
- Pereira, S.; Park, E.; Moore, J.; Faubert, B.; Breen, D.M.; Oprescu, A.I.; Nahle, A.; Kwan, D.; Giacca, A.; Tsiani, E. Resveratrol prevents insulin resistance caused by short-term elevation of free fatty acids in vivo. Appl. Physiol. Nutr. Metab. 2015, 40, 1129–1136. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Wang, H.; Zhu, L.; Han, M.; Gao, Y.; Du, Y.; Wen, Y. Curcumin improves high glucose-induced INS-1 cell insulin resistance via activation of insulin signaling. Food Funct. 2015, 6, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Prabhakar, P.K.; Doble, M. Mechanism of action of natural products used in the treatment of diabetes mellitus. Chin. J. Integr. Med. 2011, 17, 563–574. [Google Scholar] [CrossRef] [PubMed]
- Rios, J.L.; Francini, F.; Schinella, G.R. Natural products for the treatment of type 2 diabetes mellitus. Planta Med. 2015, 81, 975–994. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.D.; Clinard, V.B. Natural products for the management of type 2 diabetes mellitus and comorbid conditions. J. Am. Pharm. Assoc. 2014, 54, e304–e318. [Google Scholar] [CrossRef] [PubMed]
- Mekseepralard, C.; Areebambud, C.; Suksamrarn, S.; Jariyapongskul, A. Effects of long-term α-mangostin supplementation on hyperglycemia and insulin resistance in type 2 diabetic rats induced by high fat diet and low dose streptozotocin. J. Med. Assoc. Thail. 2015, 98, S23–S30. [Google Scholar]
- Nelli, G.B.; Solomon, K.A.; Kilari, E.K. Antidiabetic effect of a-mangostin and its protective role in sexual dysfunction of streptozotocin induced diabetic male rats. Syst. Biol. Reproduct. Med. 2013, 59, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.J.; Sanderson, B.J.S.; Zhang, W. Significant anti-invasive activities of α-mangostin from the mangosteen pericarp on two human skin cancer cell lines. Anticancer Res. 2012, 32, 3805–3816. [Google Scholar] [PubMed]
- Lee, D.; Choi, Y.O.; Kim, K.H.; Chin, Y.W.; Namgung, H.; Yamabe, N.; Jung, K. Protective effect of α-mangostin against iodixanol-induced apoptotic damage in LLC-PK1 cells. Bioorg. Med. Chem. Lett. 2016, 26, 3806–3809. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.; Gilbert, E.R.; Liu, D. Regulation of insulin synthesis and secretion and pancreatic β-cell dysfunction in diabetes. Curr. Diabetes Rev. 2013, 9, 25–53. [Google Scholar] [CrossRef] [PubMed]
- Puddu, A.; Sanguineti, R.; Mach, F.; Dallegri, F.; Viviani, G.L.; Montecucco, F. Update on the protective molecular pathways improving pancreatic β-cell dysfunction. Mediat. Inflamm. 2013, 2013, 750540. [Google Scholar] [CrossRef] [PubMed]
- Park, K.G.; Lee, K.M.; Seo, H.Y.; Suh, J.H.; Kim, H.S.; Wang, L.; Won, K.C.; Lee, H.W.; Park, J.Y.; Lee, K.U.; et al. Glucotoxicity in the INS-1 rat insulinoma cell line is mediated by the orphan nuclear receptor small heterodimer partner. Diabetes 2007, 56, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Rondinone, C.M.; Wang, L.-M.; Lonnroth, P.; Wesslau, C.; Pierce, J.H.; Smith, U. Insulin receptor substrate (IRS) 1 is reduced and IRS-2 is the main docking protein for phosphatidylinositol 3-kinase in adipocytes from subjects with non-insulin-dependent diabetes mellitus. Proc. Natl. Acad. Sci. USA 1997, 94, 4171–4175. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Soos, T.J.; Li, X.; Wu, J.; Degennaro, M.; Sun, X.; Littman, D.R.; Birnbaum, M.J.; Polakiewicz, R.D. Protein kinase C θ inhibits insulin signaling by phosphorylating IRS1 at Ser1101. J. Biol. Chem. 2004, 279, 45304–45307. [Google Scholar] [CrossRef] [PubMed]
- Dickson, L.M.; Rhodes, C.J. Pancreatic β-cell growth and survival in the onset of type 2 diabetes: A role for protein kinase B in the Akt? Am. J. Physiol. Endocrinol. Metab. 2004, 287, E192–E198. [Google Scholar] [CrossRef] [PubMed]
- Morales, N.B.; de Plata, C.A. Role of AKT/mTORC1 pathway in pancreatic β-cell proliferation. Colomb. Med. 2012, 43, 235–243. [Google Scholar]
- Humphrey, R.K.; Yu, S.M.; Flores, L.E.; Jhala, U.S. Glucose regulates steady-state levels of PDX1 via the reciprocal actions of GSK3 and AKT kinases. J. Biol. Chem. 2010, 285, 3406–3416. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Sinnett-Smith, J.; Liu, S.H.; Yu, J.; Wu, J.; Sanchez, R.; Pandol, S.J.; Abrol, R.; Nemunaitis, J.; Rozengurt, E.; et al. Down-regulation of pancreatic and duodenal homeobox-1 by somatostatin receptor subtype 5: A novel mechanism for inhibition of cellular proliferation and insulin secretion by somatostatin. Front. Physiol. 2014, 5, 226. [Google Scholar] [CrossRef] [PubMed]
- Rutter, G.A.; Tsuboi, T.; Ravier, M.A. Ca2+ microdomains and the control of insulin secretion. Cell Calcium 2006, 40, 539–551. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, T.; Kanemoto, N.; Ohbuchi, Y.; Okano, M.; Fukui, H.; Sudo, T. Characterization of STZ-induced type 2 diabetes using Zucker fatty rat. Exp. Anim. 2008, 57, 335–345. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.G.; Zhao, M.Q.; Ren, Y.N.; Wu, Y.J.; Yang, J.R. Sesamin suppresses STZ induced INS-1 cell apoptosis through inhibition of NF-κB activation and regulation of Bcl-2 family protein expression. Eur. J. Pharmacol. 2015, 750, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Sun, Z.J. Antiaging gene klotho attenuates pancreatic β-cell apoptosis in type 1 diabetes. Diabetes 2015, 64, 4298–4311. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Mei, H.; Shan, W.; Shi, L.; Chang, X.; Zhu, Y.; Chen, F.; Han, X. Lentinan protects pancreatic β cells from STZ-induced damage. J. Cell. Mol. Med. 2016, 20, 1803–1812. [Google Scholar] [CrossRef] [PubMed]
- Abdel Aziz, M.T.; El-Asmar, M.F.; Rezq, A.M.; Mahfouz, S.M.; Wassef, M.A.; Fouad, H.H.; Ahmed, H.H.; Taha, F.M. The effect of a novel curcumin derivative on pancreatic islet regeneration in experimental type-1 diabetes in rats (long term study). Diabetol. Metab. Syndr. 2013, 5, 75. [Google Scholar] [CrossRef] [PubMed]
- Coskun, O.; Ocakci, A.; Bayraktaroglu, T.; Kanter, M. Exercise training prevents and protects streptozotocin-induced oxidative stress and β-cell damage in rat pancreas. Tohoku J. Exp. Med. 2004, 203, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.L.; Goldfine, I.D.; Maddux, B.A.; Grodsky, G.M. Are oxidative stress-activated signaling pathways mediators of insulin resistance and β-cell dysfunction? Diabetes 2003, 52, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Kim, Y.R.; Song, I.G.; Ha, S.J.; Kim, Y.E.; Baek, N.-I.; Hong, E.K. Cyanidin-3-glucoside isolated from mulberry fruit protects pancreatic β-cells against oxidative stress-induced apoptosis. Int. J. Mol. Med. 2015, 35, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Vetere, A.; Choudhary, A.; Burns, S.M.; Wagner, B.K. Targeting the pancreatic β-cell to treat diabetes. Nat. Rev. Drug Discov. 2014, 13, 278–289. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.L.; Kang, K.S. Protective effect of ginsenoside Rh3 against anticancer drug-induced apoptosis in LLC-PK1 kidney cells. J. Ginseng Res. 2017, 41, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Kim, K.H.; Lee, J.; Hwang, G.S.; Lee, H.L.; Hahm, D.H.; Huh, C.K.; Lee, S.C.; Lee, S.; Kang, K.S. Protective effect of cirsimaritin against streptozotocin-induced apoptosis in pancreatic β cells. J. Pharm. Pharmacol. 2017, 69, 875–883. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.; Kim, H.; Nam, K.; Shin, I. Glut1 promotes cell proliferation, migration and invasion by regulating epidermal growth factor receptor and integrin signaling in triple-negative breast cancer cells. BMB Rep. 2017, 50, 132–137. [Google Scholar] [CrossRef] [PubMed]





© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, D.; Kim, Y.-M.; Jung, K.; Chin, Y.-W.; Kang, K.S. Alpha-Mangostin Improves Insulin Secretion and Protects INS-1 Cells from Streptozotocin-Induced Damage. Int. J. Mol. Sci. 2018, 19, 1484. https://doi.org/10.3390/ijms19051484
Lee D, Kim Y-M, Jung K, Chin Y-W, Kang KS. Alpha-Mangostin Improves Insulin Secretion and Protects INS-1 Cells from Streptozotocin-Induced Damage. International Journal of Molecular Sciences. 2018; 19(5):1484. https://doi.org/10.3390/ijms19051484
Chicago/Turabian StyleLee, Dahae, Young-Mi Kim, Kiwon Jung, Young-Won Chin, and Ki Sung Kang. 2018. "Alpha-Mangostin Improves Insulin Secretion and Protects INS-1 Cells from Streptozotocin-Induced Damage" International Journal of Molecular Sciences 19, no. 5: 1484. https://doi.org/10.3390/ijms19051484
APA StyleLee, D., Kim, Y. -M., Jung, K., Chin, Y. -W., & Kang, K. S. (2018). Alpha-Mangostin Improves Insulin Secretion and Protects INS-1 Cells from Streptozotocin-Induced Damage. International Journal of Molecular Sciences, 19(5), 1484. https://doi.org/10.3390/ijms19051484