Current Knowledge on Endocrine Disrupting Chemicals (EDCs) from Animal Biology to Humans, from Pregnancy to Adulthood: Highlights from a National Italian Meeting
Abstract
:1. Man-Made Environmental Endocrine Disrupting Contaminants: Impact on Wildlife and Human Health
1.1. Lesson from Wildlife
1.2. Animal Models: Evidence, Clinical and Epidemiological Studies
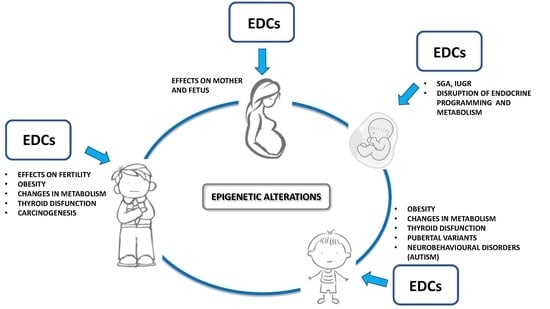
1.3. Transgenerational Effects, Epigenetics and Sustainability
2. Current Knowledge on Exposure to EDCs and Neurobehavioural Development: Lessons from Animals
2.1. Anxiety and Exploration
2.2. Learning and Memory
2.3. Socio-Sexual Behaviour
2.4. Maternal Behaviour
2.5. EDCs Effects Are Sex-Specific
3. EDCs and Neurodevelopmental Diseases in Humans: Focus on Autism
3.1. Hg
3.2. PCBs
3.3. Polycyclic Aromatic Hydrocarbons (PAHs)
3.4. Polybrominated Diphenyl Ethers (PBDEs)
3.5. Phthalates
3.6. BPA
3.7. Pesticides
4. EDCs and Metabolism
5. MDCs and Neuroendocrine Circuits Controlling Food Intake and Energy Metabolism
6. Effects of EDCs on Glucose Metabolism and Obesity
6.1. The Obesogenic Hypothesis
6.2. Diabetogenic Hypothesis
6.3. Trans-Generational Effects of EDCs and Metabolic Disturbances
6.4. Evidence in Humans
7. Effects of EDCs on Prenatal and Postnatal Growth
7.1. PBDEs
7.2. BPA
7.3. POPs
8. Effects of EDCs on the Thyroid Gland
8.1. Iodine Deficiency
8.2. Perchlorate and Thyocyanate
8.3. PCBs
9. Effects of EDCs on Puberty
9.1. Chlorinated Pesticides–DDT and DDE
9.2. PBDEs
9.3. Dioxins
9.4. Phthalates
9.5. BPA
10. Effects of EDCs on Fertility
11. EDCs and Carcinogenesis
12. Conclusions
Author Contributions
Conflict of Interest
Abbreviations
| ACTH | Adrenocorticotropic hormone (ACTH) |
| ADHD | Attention deficit hyperactivity disorder |
| AgRP | Agouti related neuropeptide |
| AOR | Adjusted odds ratio |
| ARC | Arcuate nucleus |
| As | Arsenic |
| ASD | Autism spectrum disorder |
| BCERP | Breast Cancer and the Environment Research Program |
| BPA | Bisphenol A |
| CART | Cocaine- and amphetamine-regulated transcript |
| Cd | Cadmium |
| CHARGE | Childhood Autism Risks from Genetics and the Environment |
| CRH | Corticotropin releasing hormone |
| DD | Developmental delay |
| DDE | Dichlorodiphenyl dichloroethylene |
| DDT | Dichlorodiphenyl trichloroethane |
| DEHP | Di-(2-ethylhexyl) phthalate |
| DES | Diethylstilbestrol |
| DOHaD | Developmental origins of health and disease |
| EDCs | Endocrine disrupting chemicals |
| GCK | Glucokinase |
| GESTE | GEStation Thyroid and Environment |
| GP | General population |
| Hg | Mercury |
| Hoxa10 | Homeobox A10 |
| Hoxa11 | Homeobox A11 |
| Hoxa9 | Homeobox A9 |
| HOXC6 | Homeobox C6 |
| HPA | Hypothalamus-hypophysis-adrenal axis |
| HPG | Hypothalamic-pituitary-gonadal axis |
| HPT | Hypothalamus–hypophysis–thyroid axis |
| IARC | International Agency for Research on Cancer |
| ICCIDD | Indian Coalition for Control of Iodine Deficiency Disorders |
| ID | Intellectual deficit |
| IGF | Insulin-like growth factor |
| IGFBP3 | IGF binding protein 3 |
| IQ | Intelligence level |
| IR | Insulin-resistance |
| LCGHR | Luteinizing hormone/choriogonadotropin receptor |
| MDC | Metabolism Disruptor Chemicals |
| MetS | Metabolic Syndrome |
| MOCEH | Mothers and Children’s Environmental Health (MOCEH) |
| MSH | Melanocyte-stimulating hormone |
| NHANES | National Health and Nutrition Examination Survey |
| Ni | Nickel |
| NIS | Sodium/iodide symporter |
| NO2 | Nitrogen dioxide |
| NOS | Nitric oxide synthase |
| NPY | Neuropeptide Y |
| NPY-Y1 | Neuropeptide Y receptor Y1 |
| OC | Organochlorine |
| OP | Organophosphate |
| OR | Odds ratio |
| PAHs | Polycyclic aromatic hydrocarbons |
| Pb | Lead |
| PBB | Polybrominated biphenyls |
| PBDEs | Polybrominated diphenyl ethers |
| PCBs | Polychlorinated biphenyls |
| PFASs | Perfluoroalkyl substances |
| PFHxS | Perfluorohexane sulfonate |
| PFNA | Perfluorononanoate |
| PFOA | Perfluorooctanoic acid |
| PFOS | Perfluorooctane Sulfonate |
| PM10 | Particulated matter with an aerodynamic diameter of 10 μm |
| PM2.5 | Particulate matter with an aerodynamic diameter of 2.5 μm |
| POMC | Pro-opio-melanocortin |
| POPs | Persistent organic pollutants |
| PPARγ | Peroxisome Proliferator Activated Receptor Gamma |
| PTESD | Premature Thelarche and Early Sexual Development |
| PVC | Polyvinyl chloride |
| PVN | Paraventricular nucleus |
| RXR | Retinoid X Receptor |
| SMT | Somatic mutation theory |
| T2D | Type 2 diabetes mellitus |
| T3 | Triiodothyronine |
| T4 | Thyroxine |
| TBT | Tributyltin |
| TCDD | 2,3,7,8-Tetrachlorodibenzo-p-dioxin |
| THs | Thyroid hormones |
| TRH | Thyrotropin-releasing hormone |
| TSH | Thyroid Stimulating Hormone |
| Wnt7a | Wnt Family Member 7A |
References
- Colborn, T.; vom Saal, F.S.; Soto, A.M. Developmental effects of endocrine-disrupting chemicals in wildlife and humans. Environ. Health Perspect. 1993, 101, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Myers, J.P.; Guillette, L.J., Jr.; Palanza, P.; Parmigiani, S.; Swan, S.H.; vom Saal, F.S. The emerging science of endocrine disruption. In International Seminars on Planetary Emergencies, 30th Session; Ragaini, R.C., Ed.; World Scientific Publishing: London, UK, 2004; pp. 105–121. [Google Scholar]
- Zoeller, R.T.; Brown, T.R.; Doan, L.L.; Gore, A.C.; Skakkebaek, N.E.; Soto, A.M.; Woodruff, T.J.; Vom Saal, F.S. Endocrine-disrupting chemicals and public health protection: A statement of principles from The Endocrine Society. Endocrinology 2012, 153, 4097–4110. [Google Scholar] [CrossRef] [PubMed]
- Heindel, J.J.; vom Saal, F.S.; Blumberg, B.; Bovolin, P.; Calamandrei, G.; Ceresini, G.; Cohn, B.A.; Fabbri, E.; Gioiosa, L.; Kassotis, C.; et al. Parma consensus statement on metabolic disruptors. Environ. Health 2015, 14, 54. [Google Scholar] [CrossRef] [PubMed]
- Guillette, L.J., Jr.; Edwards, T.M. Environmental influences on fertility: Can we learn lessons from studies of wildlife? Fertil. Steril. 2008, 89, e21–e24. [Google Scholar] [CrossRef] [PubMed]
- Guillette, L.J., Jr.; Gunderson, M.P. Alterations in development of reproductive and endocrine systems of wildlife populations exposed to endocrine-disrupting contaminants. Reproduction 2001, 122, 857–864. [Google Scholar] [CrossRef] [PubMed]
- Vos, J.G.; Dybing, E.; Greim, H.A.; Ladefoged, O.; Lambré, C.; Tarazona, J.V.; Brandt, I.; Vethaak, A.D. Health effects of endocrine-disrupting chemicals on wildlife, with special reference to the European situation. Crit. Rev. Toxicol. 2000, 30, 71–133. [Google Scholar] [CrossRef] [PubMed]
- Guillette, L.J., Jr.; Gross, T.S.; Masson, G.R.; Matter, J.M.; Percival, H.F.; Woodward, A.R. Developmental abnormalities of the gonad and abnormal sex hormone concentrations in juvenile alligators from contaminated and control lakes in Florida. Environ. Health Perspect. 1994, 102, 680–688. [Google Scholar] [CrossRef] [PubMed]
- Le Blanc, G. Steroid hormone-regulated processes in Invertebrates and their Susceptability to Environmental Endocrine disruption. In Environmental Endocrine Disruptors: An Evolutionary Perspective, 1st ed.; Crain, A., Guillette, L.J., Jr., Eds.; Taylor and Francis Publ.: London, UK, 1999; pp. 217–226. ISBN 9780203362808. [Google Scholar]
- Oehlmann, J.; Schulte-Oehlmann, U.; Kloas, W.; Jagnytsch, O.; Lutz, I.; Kusk, K.O.; Wollenberger, L.; Santos, E.M.; Paull, G.C.; Van Look, K.J.; et al. A critical analysis of the biological impacts of plasticizers on wildlife. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2009, 364, 2047–2062. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hutchinson, T.H. Reproductive and developmental effects of endocrine disrupters in invertebrates: In vitro and in vivo approaches. Toxicol. Lett. 2002, 131, 75–81. [Google Scholar] [CrossRef]
- Hamlin, H.; Guillette, L.J., Jr. Wildlife as sentinels of environmental impacts on reproductive health and fertility. In Environmental Impacts on Reproductive Health and Fertility; Woodruff, T., Janssen, S., Guillette, L.J., Jr., Giudice, L., Eds.; Cambridge University Press: Cambridge, UK, 2010. [Google Scholar] [CrossRef]
- Vom Saal, F.S.; Taylor, J.A.; Palanza, P.; Parmigiani, S. New Approaches to Risk Evaluation for Chemicals of Emerging Concern (CECs) that have Endocrine Disrupting Effects. In Proceedings of the International Seminar on Nuclear War and Planetary Emergencies 44th Session, Erice, Italy, 19–24 August 2011; Ragaini, R.C., Ed.; World Scientific Publishers: Hackensack, NJ, USA; London, UK; Singapore, 2011. [Google Scholar]
- Vom Saal, F.S. Triennial Reproduction Symposium: Environmental programming of reproduction during fetal life: Effects of intrauterine position and the endocrine disrupting chemical bisphenol A. J. Anim. Sci. 2016, 94, 2722–2736. [Google Scholar] [CrossRef] [PubMed]
- Parmigiani, S.; vom Saal, F.S.; Palanza, P.; Colborn, T. Exposure to very low doses of Endocrine disrupting chemicals (EDCs) during fetal life permanently alters brain development and behavior in animals and humans. In International Seminar on Nuclear War and Planetary Emergencies, 27th Session; Ragaini, R., Ed.; World Scientific Publishers: Hackensack, NJ, USA; London, UK; Singapore, 2003; pp. 293–308. ISBN 981-238-361-1. [Google Scholar]
- Vom Saal, F.S. The Intrauterine Position Phenomenon. Reference Module in Biomedical Sciences. 2018. Available online: https://doi.org/10.1016/B978-0-12-801238-3.64694-9 (accessed on 2 January 2018).
- Parmigiani, S.; Palanza, P.; vom Saal, F.S. Ethotoxicology: An evolutionary approach to behavioral toxicology. In Environmental Endocrine Disruptors: An Evolutionary Perspective, 1st ed.; Crain, A., Guillette, L.J., Jr., Eds.; Taylor and Francis Publ.: London, UK, 1999; pp. 217–226. ISBN 9780203362808. [Google Scholar]
- Schug, T.T.; Johnson, A.F.; Birnbaum, L.S.; Colborn, T.; Guillette, L.J., Jr.; Crews, D.P.; Collins, T.; Soto, A.M.; Vom Saal, F.S.; McLachlan, J.A.; et al. Minireview: Endocrine Disruptors: Past Lessons and Future Directions. Mol. Endocrinol. 2016, 30, 833–847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vom Saal, F.S.; Parmigiani, S.; Palanza, P.L.; Everett, L.G.; Ragaini, R. The plastic world: Sources, amounts, ecological impacts and effects on development, reproduction, brain and behavior in aquatic and terrestrial animals and humans. Environ. Res. 2008, 108, 127–130. [Google Scholar] [CrossRef] [PubMed]
- Palanza, P.; Nagel, S.; Parmigiani, S.; vom Saal, F.S. Perinatal exposure to endocrine disruptors: Sex, timing and behavioral endpoints. Curr. Opin. Behav. Sci. 2016, 7, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Palanza, P.; Gioiosa, L.; vom Saal, F.S.; Parmigiani, S. Effects of developmental exposure to bisphenol A on brain and behavior in mice. Environ. Res. 2008, 108, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Braun, J.M. Early Life Exposure to Endocrine Disrupting Chemicals and Childhood Obesity and Neurodevelopment. Nat. Rev. Endocrinol. 2017, 13, 161–173. [Google Scholar] [CrossRef] [PubMed]
- Grandjean, P.; Landrigan, P.J. Neurobehavioural effects of developmental toxicity. Lancet Neurol. 2014, 13, 330–338. [Google Scholar] [CrossRef] [Green Version]
- Horan, T.S.; Marre, A.; Hassold, T.; Lawson, C.; Hunt, P.A. Germline and reproductive tract effects intensify in male mice with successive generations of estrogenic exposure. PLoS Genet. 2017, 13, e1006885. [Google Scholar] [CrossRef]
- Crews, D.; Gore, A.C.; Hsu, T.S.; Dangleben, N.L.; Spinetta, M.; Schallert, T.; Anway, M.D.; Skinner, M.K. Transgenerational epigenetic imprints on mate preference. Proc. Natl. Acad. Sci. USA 2007, 104, 5942–5946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heindel, J.J.; Blumberg, B.; Cave, M.; Machtinger, R.; Mantovani, A.; Mendez, M.A.; Nadal, A.; Palanza, P.; Panzica, G.; Sargis, R.; et al. Metabolism disrupting chemicals and metabolic disorders. Reprod. Toxicol. 2017, 68, 3–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolstenholme, J.T.; Edwards, M.; Shetty, S.R.; Gatewood, J.D.; Taylor, J.A.; Rissman, E.F.; Connelly, J.J. Gestational exposure to bisphenol a produces transgenerational changes in behaviors and gene expression. Endocrinology 2012, 153, 3828–3838. [Google Scholar] [CrossRef] [PubMed]
- Chin-Chan, M.; Navarro-Yepes, J.; Quintanilla-Vega, B. Environmental pollutants as risk factors for neurodegenerative disorders: Alzheimer and Parkinson diseases. Front. Cell. Neurosci. 2015, 10, 124. [Google Scholar] [CrossRef] [PubMed]
- Walker, D.M.; Gore, A.C. Epigenetic impacts of endocrine disruptors in the brain. Front. Neuroendocrinol. 2017, 44, 1–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gioiosa, L.; Parmigiani, S.; vom Saal, F.S.; Palanza, P. The Effects of Bisphenol A on Emotional Behavior Depend upon the Timing of Exposure, Age and Gender in Mice. Horm. Behav. 2013, 63, 598–605. [Google Scholar] [CrossRef] [PubMed]
- Kundakovic, M.; Gudsnuk, K.; Franks, B.; Madrid, J.; Miller, R.L.; Perera, F.P.; Champagne, F.A. Sex-specific epigenetic disruption and behavioral changes following low-dose in utero bisphenol A exposure. Proc. Natl. Acad. Sci. USA. 2013, 110, 9956–9961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsuda, S.; Matsuzawa, D.; Ishii, D.; Tomizawa, H.; Sutoh, C.; Nakazawa, K.; Amano, K.; Sajiki, J.; Shimizu, E. Effects of perinatal exposure to low dose of bisphenol A on anxiety like behavior and dopamine metabolites in brain. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2012, 39, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Hong, X.; Xie, L.; Li, T.; Yang, Y.; Zhang, Q.; Zhang, G.; Liu, X. Gestational and lactational exposure to bisphenol-A affects anxiety- and depression-like behaviors in mice. Horm. Behav. 2012, 62, 480–490. [Google Scholar] [CrossRef] [PubMed]
- Cox, K.H.; Gatewood, J.D.; Howeth, C.; Rissman, E.F. Gestational exposure to bisphenol A and cross-fostering affect behaviors in juvenile mice. Horm. Behav. 2010, 58, 754–761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gioiosa, L.; Fissore, E.; Ghirardelli, G.; Parmigiani, S.; Palanza, P. Developmental exposure to low-dose estrogenic endocrine disruptors alters sex differences in exploration and emotional responses in mice. Horm. Behav. 2007, 52, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Rubin, B.S.; Lenkowski, J.R.; Schaeberle, C.M.; Vandenberg, L.N.; Ronsheim, P.M.; Soto, A.M. Evidence of altered brain sexual differentiation in mice exposed perinatally to low, environmentally relevant levels of bisphenol A. Endocrinology 2006, 147, 3681–3691. [Google Scholar] [CrossRef] [PubMed]
- Patisaul, H.B.; Sullivan, A.W.; Radford, M.E.; Walker, D.M.; Adewale, H.B.; Winnik, B.; Coughlin, J.L.; Buckley, B.; Gore, A.C. Anxiogenic effects of developmental bisphenol A exposure are associated with gene expression changes in the juvenile rat amygdala and mitigated by soy. PLoS ONE 2012, 7, e43890. [Google Scholar] [CrossRef] [PubMed]
- Farabollini, F.; Porrini, S.; Dessi-Fulgheri, F. Perinatal exposure to the estrogenic pollutant bisphenol A affects behavior in male and female rats. Pharmacol. Biochem. Behav. 1999, 64, 687–694. [Google Scholar] [CrossRef]
- Jašarević, E.; Williams, S.A.; Vandas, G.M.; Ellersieck, M.R.; Liao, C.; Kannan, K.; Roberts, R.M.; Geary, D.C.; Rosenfeld, C.S. Sex and dose-dependent effects of developmental exposure to bisphenol A on anxiety and spatial learning in deer mice (Peromyscus maniculatus bairdii) offspring. Horm. Behav. 2013, 63, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Jašarević, E.; Sieli, P.T.; Twellman, E.E.; Welsh, T.H., Jr.; Schachtman, T.R.; Roberts, R.M.; Rosenfeld, C.S. Disruption of adult expression of sexually selected traits by developmental exposure to bisphenol A. Proc. Natl. Acad. Sci. USA 2011, 108, 11715–11720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evans, S.F.; Kobrosly, R.W.; Barrett, E.S.; Thurston, S.W.; Calafat, A.M.; Weiss, B.; Stahlhut, R.; Yolton, K.; Swan, S.H. Prenatal bisphenol A exposure and maternally reported behavior in boys and girls. Neurotoxicology 2014, 45, 91–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harley, K.G.; Gunier, R.B.; Kogut, K.; Johnson, C.; Bradman, A.; Calafat, A.M.; Eskenazi, B. Prenatal and early childhood bisphenol A concentrations and behaviour in school-aged children. Environ. Res. 2013, 126, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Perera, F.; Vishnevetsky, J.; Herbstman, J.B.; Calafat, A.M.; Xiong, W.; Rauh, V.; Wang, S. Prenatal bisphenol A exposure and child behavior in an inner-city cohort. Environ. Health Perspect. 2012, 120, 1190–1194. [Google Scholar] [CrossRef] [PubMed]
- Braun, J.M.; Kalkbrenner, A.E.; Calafat, A.M.; Yolton, K.; Ye, X.; Dietrich, K.N.; Lanphear, B.P. Impact of early-life bisphenol A exposure on behavior and executive function in children. Pediatrics 2011, 128, 873–882. [Google Scholar] [CrossRef] [PubMed]
- Kuwahara, R.; Kawaguchi, S.; Kohara, Y.; Cui, H.; Yamashita, K. Perinatal exposure to low-dose bisphenol A impairs spatial learning and memory in male rats. J. Pharmacol. 2013, 123, 132–139. [Google Scholar] [CrossRef]
- Kundakovic, M.; Gudsnuk, K.; Herbstman, J.B.; Tang, D.; Perera, F.P.; Champagne, F.A. DNA methylation of BDNF as a biomarker of early-life adversity. Proc. Natl. Acad. Sci. USA. 2015, 112, 6807–6813. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.B.; Hong, Y.C.; Kim, J.W.; Park, E.J.; Shin, M.S.; Kim, B.N.; Yoo, H.J.; Cho, I.H.; Bhang, S.Y.; Cho, S.C. Bisphenol A in relation to behavior and learning of school-age children. J. Child Psychol. Psychiatry 2013, 54, 890–899. [Google Scholar] [CrossRef] [PubMed]
- Maserejian, N.N.; Trachtenberg, F.L.; Hauser, R.; McKinlay, S.; Shrader, P.; Bellinger, D.C. Dental composite restorations and neuropsychological development in children: Treatment level analysis from a randomized clinical trial. Neurotoxicology 2012, 33, 1291–1297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Negishi, T.; Nakagami, A.; Kawasaki, K.; Nishida, Y.; Ihara, T.; Kuroda, Y.; Tashiro, T.; Koyama, T.; Yoshikawa, Y. Altered social interactions in male juvenile cynomolgus monkeys prenatally exposed to bisphenol A. Neurotoxicol. Teratol. 2014, 44, 46–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Porrini, S.; Belloni, V.; Della Seta, D.; Farabollini, F.; Giannelli, G.; Dessì-Fulgheri, F. Early exposure to a low dose of bisphenol A affects socio-sexual behavior of juvenile female rats. Brain Res. Bull. 2005, 65, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Jones, B.A.; Shimell, J.J.; Watson, N.V. Pre- and postnatal bisphenol A treatment results in persistent deficits in the sexual behavior of male rats, but not female rats, in adulthood. Horm. Behav. 2011, 59, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Kobrosly, R.W.; Evans, S.; Miodovnik, A.; Barrett, E.S.; Thurston, S.W.; Calafat, A.M.; Swan, S.H. Prenatal phthalate exposures and neurobehavioral development scores in boys and girls at 6–10 years of age. Environ. Health Perspect. 2014, 122, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Lien, Y.J.; Ku, H.Y.; Su, P.H.; Chen, S.J.; Chen, H.Y.; Liao, P.C.; Chen, W.J.; Wang, S.L. Prenatal exposure to phthalate esters and behavioral syndromes in children at eight years of age: Taiwan maternal and infant cohort study. Environ. Health Perspect. 2014, 123, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Palanza, P. The “Plastic” Mother. Endocrinology 2017, 158, 461–463. [Google Scholar] [CrossRef] [PubMed]
- Palanza, P.; Howdeshell, K.L.; Parmigiani, S.; vom Saal, F.S. Exposure to a low dose of bisphenol A during fetal life or in adulthood alters maternal behavior in mice. Environ. Health Perspect. 2002, 110, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Boudalia, S.; Berges, R.; Chabanet, C.; Folia, M.; Decocq, L.; Pasquis, B.; Abdennebi-Najar, L.; Canivenc-Lavier, M.C. A multi-generational study on low-dose BPA exposure in Wistar rats: Effects on maternal behavior, flavor intake and development. Neurotoxicol. Teratol. 2014, 41, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Della Seta, D.; Minder, I.; Dessì-Fulgheri, F.; Farabollini, F. Bisphenol-A exposure during pregnancy and lactation affects maternal behavior in rats. Brain Res. Bull. 2005, 65, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Catanese, M.C.; Vandenberg, LN. Bisphenol S (BPS) Alters Maternal Behavior and Brain in Mice Exposed During Pregnancy/Lactation and Their Daughters. Endocrinology 2017, 158, 516–530. [Google Scholar] [CrossRef] [PubMed]
- Engell, M.D.; Godwin, J.; Young, L.J.; Vandenbergh, J.G. Perinatal exposure to endocrine disrupting compounds alters behavior and brain in the female pine vole. Neurotoxicol. Teratol. 2006, 28, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.A.; Javurek, A.B.; Painter, M.S.; Peritore, M.P.; Ellersieck, M.R.; Roberts, R.M.; Rosenfeld, C.S. Disruption of parenting behaviors in california mice, a monogamous rodent species, by endocrine disrupting chemicals. PLoS ONE 2015, 10, e0126284. [Google Scholar] [CrossRef] [PubMed]
- Champagne, F.A.; Curley, J.P. Epigenetic mechanisms mediating the long-term effects of maternal care on development. Neurosci. Biobehav. Rev. 2009, 33, 593–600. [Google Scholar] [CrossRef] [PubMed]
- Panzica, G.C.; Viglietti-Panzica, C.; Mura, E.; Quinn, M.J., Jr.; Lavoie, E.; Palanza, P.; Ottinger, M.A. Effects of xenoestrogens on the differentiation of behaviorally-relevant neural circuits. Front. Neuroendocrinol. 2007, 28, 179–200. [Google Scholar] [CrossRef] [PubMed]
- Masuo, Y.; Ishido, M. Neurotoxicity of endocrine disruptors: Possible involvement in brain development and neurodegeneration. J. Toxicol. Environ. Health B Crit. Rev. 2011, 14, 346–369. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeld, C.S.; Trainor, B.C. Environmental Health Factors and Sexually Dimorphic Differences in Behavioral Disruptions. Curr. Environ. Health Rep. 2014, 1, 287–301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Naarden Braun, K.; Christensen, D.; Doernberg, N.; Schieve, L.; Rice, C.; Wiggins, L.; Schendel, D.; Yeargin-Allsopp, M. Trends in the prevalence of autism spectrum disorder, cerebral palsy, hearing loss, intellectual disability, and vision impairment, metropolitan atlanta, 1991–2010. PLoS ONE 2015, 10, e0124120. [Google Scholar] [CrossRef] [PubMed]
- Boyle, C.A.; Decouflé, P.; Yeargin-Allsopp, M. Prevalence and health impact of developmental disabilities in US children. Pediatrics 1994, 93, 399–403. [Google Scholar] [PubMed]
- Lavelle, T.A.; Weinstein, M.C.; Newhouse, J.P.; Munir, K.; Kuhlthau, K.A.; Prosser, L.A. Economic burden of childhood autism spectrum disorders. Pediatrics 2014, 133, e520–e529. [Google Scholar] [CrossRef] [PubMed]
- Lai, M.C.; Lombardo, M.V.; Baron-Cohen, S. Autism. Lancet 2014, 383, 896–910. [Google Scholar] [CrossRef]
- Grandjean, P.; Landrigan, P.J. Developmental neurotoxicity of industrial chemicals. Lancet 2006, 368, 2167–2178. [Google Scholar] [CrossRef]
- Predki, P.F.; Sarkar, B. Metal replacement in “zinc finger” and its effect on DNA binding. Environ. Health Perspect. 1994, 102, 195–198. [Google Scholar] [CrossRef] [PubMed]
- Kern, J.K.; Geier, D.A.; Sykes, L.K.; Haley, B.E.; Geier, M.R. The relationship between mercury and autism: A comprehensive review and discussion. J. Trace Elem. Med. Biol. 2016, 37, 8–24. [Google Scholar] [CrossRef] [PubMed]
- Mostafa, G.A.; Bjørklund, G.; Urbina, M.A.; Al-Ayadhi, L.Y. The levels of blood mercury and inflammatory-related neuropeptides in the serum are correlated in children with autism spectrum disorder. Metab. Brain Dis. 2016, 31, 593–599. [Google Scholar] [CrossRef] [PubMed]
- Mostafa, G.A.; Al-Ayadhi, L.Y. The possible association between elevated levels of blood mercury and the increased frequency of serum anti-myelin basic protein auto-antibodies in autistic children. J. Clin. Cell. Immunol. 2015, 6, 2. [Google Scholar] [CrossRef]
- Sajdel-Sulkowska, E.M.; Lipinski, B.; Windom, H.; Audhya, T.; McGinnis, W. Oxidative stress in autism: Cerebellar 3 nitrotyrosine levels. Am. J. Biochem. Biotechnol. 2008, 4, 73–84. [Google Scholar]
- Mostafa, G.A.; Refai, T.M.K. Antineuronal antibodies in autistic children: Relation to blood mercury. Egypt. J. Pediatr. Allergy Immunol. 2007, 5, 21–30. [Google Scholar]
- Geier, D.A.; Kern, J.K.; King, P.G.; Sykes, L.K.; Geier, M.R. A case-control study evaluating the relationship between Thimerosal-containing Haemophilus influenzae Type b vaccine administration and the risk for a pervasive developmental disorder diagnosis in the United States. Biol. Trace Elem. Res. 2015, 163, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Geier, D.A.; Hooker, B.S.; Kern, J.K.; King, P.G.; Sykes, L.K.; Homme, K.G.; Geier, M.R. A dose-response relationship between organic mercury exposure from Thimerosal-containing vaccines and neurodevelopmental disorders. Int. J. Environ. Res. Public Health 2014, 11, 9156–9170. [Google Scholar] [CrossRef] [PubMed]
- Geier, D.A.; Kern, J.K.; King, P.G.; Sykes, L.K.; Homme, K.G.; Geier, M.R. The risk of neurodevelopmental disorders following a Thimerosal-preserved DTaP formulation in comparison to its Thimerosal-reduced formulation in the Vaccine Adverse Event Reporting System (VAERS). J. Biochem. Pharmacol. Res. 2014, 2, 64–73. [Google Scholar]
- Geier, D.A.; Hooker, B.S.; Kern, J.K.; King, P.G.; Sykes, L.K.; Geier, M.R. A two-phase cohort study of the relationship between Thimerosal-containing vaccine administration as a risk factor for an autism spectrum disorder diagnosis in the United States. Transl. Neurodegener. 2013, 2, 25. [Google Scholar] [CrossRef] [PubMed]
- Stamova, B.; Green, P.G.; Tian, Y.; Hertz-Picciotto, I.; Pessah, I.N.; Hansen, R.; Yang, X.; Teng, J.; Gregg, J.P.; Ashwood, P.; et al. Correlations between gene expression and mercury levels in blood of boys with and without autism. Neurotox. Res. 2011, 19, 31–48. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, A.; Audhya, T.; Chauhan, V. Brain region-specific glutathione redox imbalance in autism. Neurochem. Res. 2012, 37, 1681–1689. [Google Scholar] [CrossRef] [PubMed]
- Rose, S.; Wynne, R.; Frye, R.E.; Melnyk, S.; James, S.J. Increased susceptibility to ethylmercury-induced mitochondrial dysfunction in a subset of autism lymphoblastoid cell lines. J. Toxicol. 2015, 2015, 1–13. [Google Scholar] [CrossRef] [PubMed]
- James, S.J.; Melnyk, S.; Fuchs, G.; Reid, T.; Jernigan, S.; Pavliv, O.; Hubanks, A.; Gaylor, D.W. Efficacy of methylcobalamin and folinic acid treatment on glutathione redox status in children with autism. Am. J. Clin. Nutr. 2009, 89, 425–430. [Google Scholar] [CrossRef] [PubMed]
- James, S.J.; Melnyk, S.; Jernigan, S.; Cleves, M.A.; Halsted, C.H.; Wong, D.H.; Cutler, P.; Bock, K.; Boris, M.; Bradstreet, J.J.; et al. Metabolic endophenotype and related genotypes are associated with oxidative stress in children with autism. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2006, 141B, 947–956. [Google Scholar] [CrossRef] [PubMed]
- Frye, R.E.; Delatorre, R.; Taylor, H.; Slattery, J.; Melnyk, S.; Chowdhury, N.; James, S.J. Redox metabolism abnormalities in autistic children associated with mitochondrial disease. Transl. Psychiatry 2013, 3, e273. [Google Scholar] [CrossRef] [PubMed]
- Gu, F.; Chauhan, V.; Chauhan, A. Impaired synthesis and antioxidant defense of glutathione in the cerebellum of autistic subjects: Alterations in the activities and protein expression of glutathione-related enzymes. Free Radic. Biol. Med. 2013, 65, 488–496. [Google Scholar] [CrossRef] [PubMed]
- Rose, S.; Melnyk, S.; Pavliv, O.; Bai, S.; Nick, T.G.; Frye, R.E.; James, S.J. Evidence of oxidative damage and inflammation associated with low glutathione redox status in the autism brain. Transl. Psychiatry 2012, 10, e134. [Google Scholar] [CrossRef] [PubMed]
- Alabdali, A.; Al-Ayadhi, L.; El-Ansary, A. A key role for an impaired detoxification mechanism in the etiology and severity of autism spectrum disorders. Behav. Brain Funct. 2014, 10, 14. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.B.; Audhya, T.; McDonough-Means, S.; Rubin, R.A.; Quig, D.; Geis, E.; Gehn, E.; Loresto, M.; Mitchell, J.; Atwood, S.; et al. Toxicological status of children with autism vs. neurotypical children and the association with autism severity. Biol. Trace Elem. Res. 2013, 151, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Lakshmi Priya, M.D.; Geetha, A. Level of trace elements (copper, zinc, magnesium and selenium) and toxic elements (lead and mercury) in the hair and nail of children with autism. Biol. Trace Elem. Res. 2011, 142, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Woods, J.S.; Armel, S.E.; Fulton, D.I.; Allen, J.; Wessels, K.; Simmonds, P.L.; Granpeesheh, D.; Mumper, E.; Bradstreet, J.J.; Echeverria, D.; et al. Urinary porphyrin excretion in neurotypical and autistic children. Environ. Health Perspect. 2010, 118, 1450–1457. [Google Scholar] [CrossRef] [PubMed]
- Geier, D.A.; Kern, J.K.; Geier, M.R. A prospective blinded evaluation of urinary porphyrins verses the clinical severity of autism spectrum disorders. J. Toxicol. Environ. Health Part A 2009, 72, 1585–1591. [Google Scholar] [CrossRef] [PubMed]
- Blanchard, K.S.; Palmer, R.F.; Stein, Z. The value of ecologic studies: Mercury concentration in ambient air and the risk of autism. Rev. Environ. Health 2011, 26, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Dickerson, A.S.; Rahbar, M.H.; Han, I.; Bakian, A.V.; Bilder, D.A.; Harrington, R.A.; Pettygrove, S.; Durkin, M.; Kirby, R.S.; Wingate, M.S.; et al. Atism spectrum disorder prevalence and proximity to industrial facilities releasing arsenic, lead or mercury. Sci. Total Environ. 2015, 536, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Lyall, K.; Croen, L.A.; Sjödin, A.; Yoshida, C.K.; Zerbo, O.; Kharrazi, M.; Windham, G.C. Polychlorinated Biphenyl and Organochlorine Pesticide Concentrations in Maternal Mid-Pregnancy Serum Samples: Association with Autism Spectrum Disorder and Intellectual Disability. Environ. Health Perspect. 2017, 125, 474–480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Volk, H.E.; Lurmann, F.; Penfold, B.; Hertz-Picciotto, I.; McConnell, R. Traffic-related air pollution, particulate matter, and autism. JAMA Psychiatry 2013, 70, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Volk, H.E.; Hertz-Picciotto, I.; Delwiche, L.; Lurmann, F.; McConnell, R. Residential proximity to freeways and autism in the CHARGE study. Environ. Health Perspect. 2011, 119, 873–877. [Google Scholar] [CrossRef] [PubMed]
- Frederiksen, M.; Vorkamp, K.; Thomsen, M.; Knudsen, L.E. Human internal and external exposure to PBDEs—A review of levels and sources. Int. J. Hyg. Environ. Health 2009, 212, 109–134. [Google Scholar] [CrossRef] [PubMed]
- Vuong, A.M.; Yolton, K.; Dietrich, K.N.; Braun, J.M.; Lanphear, B.P.; Chen, A. Exposure to polybrominated diphenyl ethers (PBDEs) and child behavior: Current findings and future directions. Horm. Behav. 2017, 101, 94–104. [Google Scholar] [CrossRef] [PubMed]
- Jeddi, M.Z.; Janani, L.; Memari, A.H.; Akhondzadeh, S.; Yunesian, M. The role of phthalate esters in autism development: A systematic review. Environ. Res. 2016, 151, 493–504. [Google Scholar] [CrossRef] [PubMed]
- Stein, T.P.; Schluter, M.D.; Steer, R.A.; Guo, L.; Ming, X. Bisphenol A Exposure in Children with Autism Spectrum Disorders. Autism Res. 2015, 8, 272–283. [Google Scholar] [CrossRef] [PubMed]
- Shelton, J.F.; Geraghty, E.M.; Tancredi, D.J.; Delwiche, L.D.; Schmidt, R.J.; Ritz, B.; Hansen, R.L.; Hertz-Picciotto, I. Neurodevelopmental disorders and prenatal residential proximity to agricultural pesticides: The CHARGE study. Environ. Health Perspect. 2014, 122, 1103–1109. [Google Scholar] [CrossRef] [PubMed]
- Grün, F.; Blumberg, B. Environmental obesogens: Organotins and endocrine disruption via nuclear receptor signaling. Endocrinology 2006, 147, S50–S55. [Google Scholar] [CrossRef] [PubMed]
- Angle, B.M.; Do, R.P.; Ponzi, D.; Stahlhut, R.W.; Drury, B.E.; Nagel, S.C.; Welshons, W.V.; Besch-Williford, C.L.; Palanza, P.; Parmigiani, S.; et al. Metabolic disruption in male mice due to fetal exposure to low but not high doses of bisphenol A (BPA): Evidence for effects on body weight, food intake, adipocytes, leptin, adiponectin, insulin and glucose regulation. Reprod. Toxicol. 2013, 42, 256–268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palmer, B.F.; Clegg, D.J. The sexual dimorphism of obesity. Mol. Cell. Endocrinol. 2015, 402, 113–119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valassi, E.; Scacchi, M.; Cavagnini, F. Neuroendocrine control of food intake. Nutr. Metab. Cardiovasc. Dis. 2008, 18, 158–168. [Google Scholar] [CrossRef] [PubMed]
- Kalra, S.P.; Dube, M.G.; Pu, S.; Xu, B.; Horvath, T.L.; Kalra, P.S. Interacting appetite-regulating pathways in the hypothalamic regulation of body weight. Endocr. Rev. 1999, 20, 68–100. [Google Scholar] [CrossRef] [PubMed]
- Baskin, D.G.; Figlewicz Lattemann, D.; Seeley, R.J.; Woods, S.C.; Porte, D., Jr.; Schwartz, M.W. Insulin and leptin: Dual adiposity signals to the brain for the regulation of food intake and body weight. Brain Res. 1999, 848, 114–123. [Google Scholar] [CrossRef]
- Grill, H.J.; Kaplan, J.M. The neuroanatomical axis for control of energy balance. Front. Neuroendocrinol. 2002, 23, 2–40. [Google Scholar] [CrossRef] [PubMed]
- Joseph-Bravo, P.; Jaimes-Hoy, L.; Charli, J.L. Regulation of TRH neurons and energy homeostasis-related signals under stress. J. Endocrinol. 2015, 224, R139–R159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, H.; Strader, A.D.; Sorrell, J.E.; Chambers, J.B.; Woods, S.C.; Seeley, R.J. Sexually different actions of leptin in proopiomelanocortin neurons to regulate glucose homeostasis. Am. J. Physiol. Endocrinol. Metab. 2008, 294, E630–E639. [Google Scholar] [CrossRef] [PubMed]
- Bo, E.; Farinetti, A.; Marraudino, M.; Sterchele, D.; Eva, C.; Gotti, S.; Panzica, G.C. Adult exposure to tributyltin affects hypothalamic neuropeptide Y, Y1 receptor distribution, and circulating leptin in mice. Andrology 2016, 4, 723–734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martini, M.; Sica, M.; Gotti, S.; Eva, C.; Panzica, G.C. Effects of estrous cycle and sex on the expression of neuropeptide Y Y1 receptor in discrete hypothalamic and limbic nuclei of transgenic mice. Peptides 2011, 32, 1330–1334. [Google Scholar] [CrossRef] [PubMed]
- Clegg, D.J.; Brown, L.M.; Woods, S.C.; Benoit, S.C. Gonadal hormones determine sensitivity to central leptin and insulin. Diabetes 2006, 55, 978–987. [Google Scholar] [CrossRef] [PubMed]
- Walley, S.N.; Roepke, T.A. Perinatal exposure to endocrine disrupting compounds and the control of feeding behavior-An overview. Horm. Behav. 2017, 101, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Mackay, H.; Patterson, Z.; Khazall, R.; Patel, S.; Tsirlin, D.; Abizaid, A. Organizational effects of perinatal exposure to bisphenol-A and diethylstilbestrol on arcuate nucleus circuitry controlling food intake and energy expenditure in male and female CD-1 mice. Endocrinology 2013, 154, 1465–1475. [Google Scholar] [CrossRef] [PubMed]
- Grun, F. The obesogen tributyltin. Vitam. Horm. 2014, 94, 277–325. [Google Scholar] [CrossRef] [PubMed]
- Decherf, S.; Demeneix, B.A. The obesogen hypothesis: A shift of focus from the periphery to the hypothalamus. J. Toxicol. Environ. Health B Crit. Rev. 2011, 14, 423–448. [Google Scholar] [CrossRef] [PubMed]
- Decherf, S.; Seugnet, I.; Fini, J.B.; Clerget-Froidevaux, M.S.; Demeneix, B.A. Disruption of thyroid hormone-dependent hypothalamic set-points by environmental contaminants. Mol. Cell. Endocrinol. 2010, 323, 172–182. [Google Scholar] [CrossRef] [PubMed]
- Bo, E.; Viglietti-Panzica, C.; Panzica, G.C. Acute exposure to tributyltin induces c-fos activation in the hypothalamic arcuate nucleus of adult male mice. Neurotoxicology 2011, 32, 277–280. [Google Scholar] [CrossRef] [PubMed]
- Farinetti, A.; Marraudino, M.; Ponti, G.; Gotti, S.; Panzica, G.C. Sexually dimorphic effect of chronic treatment with tributyltin on brain circuits involved in the food intake behavior in adult mice. In 9th International Meeting Steroids and Nervous System; Gotti, S., Panzica, G.C., Eds.; Fondazione Cavalieri Ottolenghi: Torino, Italy, 2017; p. 58. [Google Scholar]
- He, K.; Zhang, J.; Chen, Z. Effect of tributyltin on the food intake and brain neuropeptide expression in rats. Endokrynol. Polska 2014, 65, 485–490. [Google Scholar] [CrossRef] [PubMed]
- Merlo, E.; Podratz, P.L.; Sena, G.C.; de Araujo, J.F.; Lima, L.C.; Alves, I.S.; Gama-de-Souza, L.N.; Pelicao, R.; Rodrigues, L.C.; Brandao, P.A.; et al. The Environmental Pollutant Tributyltin Chloride Disrupts the Hypothalamic-Pituitary-Adrenal Axis at Different Levels in Female Rats. Endocrinology 2016, 157, 2978–2995. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarruf, D.A.; Yu, F.; Nguyen, H.T.; Williams, D.L.; Printz, R.L.; Niswender, K.D.; Schwartz, M.W. Expression of peroxisome proliferator-activated receptor-gamma in key neuronal subsets regulating glucose metabolism and energy homeostasis. Endocrinology 2009, 150, 707–712. [Google Scholar] [CrossRef] [PubMed]
- Tontonoz, P.; Spiegelman, B.M. Fat and beyond: The diverse biology of PPARgamma. Annu. Rev. Biochem. 2008, 77, 289–312. [Google Scholar] [CrossRef] [PubMed]
- Evans, R.M.; Barish, G.D.; Wang, Y.X. PPARs and the complex journey to obesity. Nat. Med. 2004, 10, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Tontonoz, P.; Hu, E.; Spiegelman, B.M. Stimulation of adipogenesis in fibroblasts by PPAR gamma 2, a lipid-activated transcription factor. Cell 1994, 79, 1147–1156. [Google Scholar] [CrossRef]
- Yin, L.; Yu, K.S.; Lu, K.; Yu, X. Benzyl butyl phthalate promotes adipogenesis in 3T3-L1 preadipocytes: A High Content Cellomics and metabolomic analysis. Toxicol. In Vitro 2016, 32, 297–309. [Google Scholar] [CrossRef] [PubMed]
- Pereira-Fernandes, A.; Demaegdt, H.; Vandermeiren, K.; Hectors, TL.; Jorens, PG.; Blust, R.; Vanparys, C. Evaluation of a screening system for obesogenic compounds: Screening of endocrine disrupting compounds and evaluation of the PPAR dependency of the effect. PLoS ONE 2013, 8, e77481. [Google Scholar] [CrossRef] [PubMed]
- Arsenescu, V.; Arsenescu, R.I.; King, V.; Swanson, H.; Cassis, L.A. Polychlorinated biphenyl-77 induces adipocyte differentiation and proinflammatory adipokines and promotes obesity and atherosclerosis. Environ. Health Perspect. 2008, 116, 761–768. [Google Scholar] [CrossRef] [PubMed]
- Rönn, M.; Lind, L.; Örberg, J.; Kullberg, J.; Söderberg, S.; Larsson, A.; Johansson, L.; Ahlström, H.; Lind, P.M. Bisphenol A is related to circulating levels of adiponectin, leptin and ghrelin, but not to fat mass or fat distribution in humans. Chemosphere 2014, 112, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Polyzos, S.A.; Kountouras, J.; Deretzi, G.; Zavos, C.; Mantzoros, C.S. The emerging role of endocrine disruptors in pathogenesis of insulin resistance: A concept implicating nonalcoholic fatty liver disease. Curr. Mol. Med. 2012, 12, 68–82. [Google Scholar] [CrossRef] [PubMed]
- O’Reilly, M.W.; House, P.J.; Tomlinson, J.W. Understanding androgen action in adipose tissue. J. Steroid. Biochem. Mol. Biol. 2014, 143, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Isidori, A.M.; Giannetta, E.; Greco, E.A.; Gianfrilli, D.; Bonifacio, V.; Isidori, A.; Lenzi, A.; Fabbri, A. Effects of testosterone on body composition, bone metabolism and serum lipid profile in middle-aged men: A meta-analysis. Clin. Endocrinol. 2005, 63, 280–293. [Google Scholar] [CrossRef] [PubMed]
- Darbre, P.D. Endocrine Disruptors and Obesity. Curr. Obes. Rep. 2017, 6, 18–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, G. Aromatase up-regulation, insulin and raised intracellular oestrogens in men, induce adiposity, metabolic syndrome and prostate disease, via aberrant ER-α and GPER signalling. Mol. Cell. Endocrinol. 2012, 351, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Lottrup, G.; Andersson, A.M.; Leffers, H.; Mortensen, G.K.; Toppari, J.; Skakkebaek, N.E.; Main, K.M. Possible impact of phthalates on infant reproductive health. Int. J. Androl. 2006, 29, 172–180. [Google Scholar] [CrossRef] [PubMed]
- McAninch, E.A.; Bianco, A.C. Thyroid hormone signaling in energy homeostasis and energy metabolism. Ann. N. Y. Acad. Sci. 2014, 1311, 77–87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santos-Silva, A.P.; Andrade, M.N.; Pereira-Rodrigues, P.; Paiva-Melo, F.D.; Soares, P.; Graceli, J.B.; Dias, G.R.M.; Ferreira, A.C.F.; de Carvalho, D.P.; Miranda-Alves, L. Frontiers in endocrine disruption: Impacts of organotin on the hypothalamus-pituitary-thyroid axis. Mol. Cell. Endocrinol. 2018, 460, 246–257. [Google Scholar] [CrossRef] [PubMed]
- Geens, T.; Dirtu, A.C.; Dirinck, E.; Malarvannan, G.; Van Gaal, L.; Jorens, P.G.; Covaci, A. Daily intake of bisphenol A and triclosan and their association with anthropometric data, thyroidhormones and weight loss in overweight and obese individuals. Environ. Int. 2015, 76, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Le Corre, L.; Besnard, P.; Chagnon, M.C. BPA, an energy balance disruptor. Crit. Rev. Food. Sci. Nutr. 2015, 55, 769–777. [Google Scholar] [CrossRef] [PubMed]
- Petrakis, D.; Vassilopoulou, L.; Mamoulakis, C.; Psycharakis, C.; Anifantaki, A.; Sifakis, S.; Docea, A.O.; Tsiaoussis, J.; Makrigiannakis, A.; Tsatsakis, A.M. Endocrine Disruptors Leading to Obesity and Related Diseases. Int. J. Environ. Res. Public Health 2017, 14, 1282. [Google Scholar] [CrossRef] [PubMed]
- Snedeker, S.M.; Hay, A.G. Do interactions between gut ecology and environmental chemicals contribute to obesity and diabetes? Environ. Health Perspect. 2012, 120, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Nichols, R.G.; Correll, J.; Murray, I.A.; Tanaka, N.; Smith, P.B.; Hubbard, T.D.; Sebastian, A.; Albert, I.; Hatzakis, E.; et al. Persistent Organic Pollutants Modify Gut Microbiota-Host Metabolic Homeostasis in Mice through Aryl Hydrocarbon Receptor Activation. Environ. Health Perspect. 2015, 123, 679–688. [Google Scholar] [CrossRef] [PubMed]
- Neel, B.A.; Sargis, R.M. The paradox of progress: Environmental disruption of metabolism and the diabetes epidemic. Diabetes 2011, 60, 1838–1848. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Z.; Wu, T.; Lin, M.; Zhang, S.; Yan, F.; Yang, Z.; Wang, Y.; Wang, C. Chronic exposure to tributyltin chloride induces pancreatic islet cell apoptosis and disrupts glucose homeostasis in male mice. Environ. Sci. Technol. 2014, 48, 5179–5186. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Wei, J.; Li, Y.; Chen, J.; Zhou, Z.; Song, L.; Wei, Z.; Lv, Z.; Chen, X.; Xia, W.; et al. Developmental exposure to di(2-ethylhexyl) phthalate impairs endocrine pancreas and leads to long-term adverse effects on glucose homeostasis in the rat. Am. J. Physiol. Endocrinol. Metab. 2011, 301, E527–E538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soriano, S.; Alonso-Magdalena, P.; García-Arévalo, M.; Novials, A.; Muhammed, S.J.; Salehi, A.; Gustafsson, J.A.; Quesada, I.; Nadal, A. Rapid insulinotropic action of low doses of bisphenol-A on mouse and human islets of Langerhans: Role of estrogen receptor β. PLoS ONE 2012, 7, e31109. [Google Scholar] [CrossRef] [PubMed]
- Perreault, L.; McCurdy, C.; Kerege, A.A.; Houck, J.; Færch, K.; Bergman, B.C. Bisphenol A impairs hepatic glucose sensing in C57BL/6 male mice. PLoS ONE 2013, 8, e69991. [Google Scholar] [CrossRef] [PubMed]
- Manikkam, M.; Tracey, R.; Guerrero-Bosagna, C.; Skinner, M.K. Plastics derived endocrine disruptors (BPA, DEHP and DBP) induce epigenetic transgenerational inheritance of obesity, reproductive disease and sperm epimutations. PLoS ONE 2013, 8, e55387. [Google Scholar] [CrossRef] [PubMed]
- Kirchner, S.; Kieu, T.; Chow, C.; Casey, S.; Blumberg, B. Prenatal exposure to the environmental obesogen tributyltin predisposes multipotent stem cells to become adipocytes. Mol. Endocrinol. 2010, 24, 526–539. [Google Scholar] [CrossRef] [PubMed]
- Skinner, M.K.; Manikkam, M.; Tracey, R.; Guerrero-Bosagna, C.; Haque, M.; Nilsson, E.E. Ancestral dichlorodiphenyltrichloroethane (DDT) exposure promotes epigenetic transgenerational inheritance of obesity. BMC Med. 2013, 11, 228. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Xia, W.; Wang, D.Q.; Wan, Y.J.; Xu, B.; Chen, X.; Li, Y.Y.; Xu, S.Q. Hepatic DNA methylation modifications in early development of rats resulting from perinatal BPA exposure contribute to insulin resistance in adulthood. Diabetologia 2013, 56, 2059–2067. [Google Scholar] [CrossRef] [PubMed]
- Le Magueresse-Battistoni, B.; Labaronne, E.; Vidal, H.; Naville, D. Endocrine disrupting chemicals in mixture and obesity, diabetes and related metabolic disorders. World J. Biol. Chem. 2017, 8, 108–119. [Google Scholar] [CrossRef] [PubMed]
- Michalek, J.E.; Pavuk, M. Diabetes and cancer in veterans of Operation Ranch Hand after adjustment for calendar period, days of spraying, and time spent in Southeast Asia. J. Occup. Environ. Med. 2008, 50, 330–340. [Google Scholar] [CrossRef] [PubMed]
- Pesatori, A.C.; Consonni, D.; Bachetti, S.; Zocchetti, C.; Bonzini, M.; Baccarelli, A.; Bertazzi, P.A. Short- and long-term morbidity and mortality in the population exposed to dioxin after the “Seveso accident”. Ind. Health 2003, 41, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Mustieles, V.; Fernández, M.F.; Martin-Olmedo, P.; González-Alzaga, B.; Fontalba-Navas, A.; Hauser, R.; Olea, N.; Arrebola, J.P. Human adipose tissue levels of persistent organic pollutants and metabolic syndrome components: Combining a cross-sectional with a 10-year longitudinal study using a multi-pollutant approach. Environ. Int. 2017, 104, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, M.S.; Rabasa-Lhoret, R.; Prud’homme, D.; Karelis, A.D.; Geng, D.; van Bavel, B.; Ruzzin, J. The metabolically healthy but obese phenotype is associated with lower plasma levels of persistent organic pollutants as compared to the metabolically abnormal obese phenotype. J. Clin. Endocrinol. Metab. 2014, 99, E1061–E1066. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Li, M.; Chen, B.; Xu, M.; Xu, Y.; Huang, Y.; Lu, J.; Chen, Y.; Wang, W.; Li, X.; et al. Urinary bisphenol A (BPA) concentration associates with obesity and insulin resistance. J. Clin. Endocrinol. Metab. 2012, 97, E223–E227. [Google Scholar] [CrossRef] [PubMed]
- Carwile, J.L.; Michels, K.B. Urinary bisphenol A and obesity: NHANES 2003–2006. Environ. Res. 2011, 111, 825–830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hatch, E.E.; Nelson, J.W.; Qureshi, M.M.; Weinberg, J.; Moore, L.L.; Singer, M.; Webster, T.F. Association of urinary phthalate metabolite concentrations with body mass index and waist circumference: A cross-sectional study of NHANES data, 1999–2002. Environ. Health 2008, 7, 27. [Google Scholar] [CrossRef] [PubMed]
- Stahlhut, R.W.; van Wijngaarden, E.; Dye, T.D.; Cook, S.; Swan, S.H. Concentrations of urinary phthalate metabolites are associated with increased waist circumference and insulin resistance in adult U.S. males. Environ. Health Perspect. 2007, 115, 876–882. [Google Scholar] [CrossRef] [PubMed]
- Smerieri, A.; Testa, C.; Lazzeroni, P.; Nuti, F.; Grossi, E.; Cesari, S.; Montanini, L.; Latini, G.; Bernasconi, S.; Papini, A.M.; et al. Di-(2-ethylhexyl) phthalate metabolites in urine show age-related changes and associations with adiposity and parameters of insulin sensitivity in childhood. PLoS ONE 2015, 10, e0117831. [Google Scholar] [CrossRef] [PubMed]
- Bergman, A.; Heindel, J.J.; Jobling, S. State of the Science of Endocrine Disrupting Chemicals—2012. WHO/UNEP, 2013. Available online: http://www.who.int/ceh/publications/endocrine/en (accessed on 15 February 2018).
- Arbuckle, T.E.; Davis, K.; Marro, L.; Fisher, M.; Legrand, M.; LeBlanc, A.; Gaudreau, E.; Foster, W.G.; Choeurng, V.; Fraser, W.D.; et al. Phthalate and bisphenol A exposure among pregnant women in Canada—Results from the MIREC study. Environ. Int. 2014, 68, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Woodruff, T.J.; Zota, A.R.; Schwartz, J.M. Environmental chemicals in pregnant women in the United States: NHANES 2003–2004. Environ. Health Perspect. 2011, 119, 878–885. [Google Scholar] [CrossRef] [PubMed]
- Lenters, V.; Portengen, L.; Rignell-Hydbom, A.; Jönsson, B.A.; Lindh, C.H.; Piersma, A.H.; Toft, G.; Bonde, J.P.; Heederik, D.; Rylander, L.; Vermeulen, R. Prenatal phthalate, perfluoroalkyl acid, and organochlorine exposures and term birth weight in three birth cohorts: Multi-pollutant models based on elastic net regression. Environ. Health Perspect. 2016, 124, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Birks, L.; Casas, M.; Garcia, A.M.; Alexander, J.; Barros, H.; Bergström, A.; Bonde, J.P.; Burdorf, A.; Costet, N.; Danileviciute, A.; et al. Occupational exposure to endocrine-disrupting chemicals and birth weight and length of gestation: A European meta-analysis. Environ. Health Perspect. 2016, 124, 1785–1793. [Google Scholar] [CrossRef] [PubMed]
- Govarts, E.; Nieuwenhuijsen, M.; Schoeters, G.; Ballester, F.; Bloemen, K.; de Boer, M.; Chevrier, C.; Eggesbø, M.; Guxens, M.; Krämer, U.; et al. Birth weight and prenatal exposure to polychlorinated biphenyls (PCBs) and dichlorodiphenyldichloroethylene (DDE): A meta-analysis within 12 European birth cohorts. Environ. Health Perspect. 2012, 120, 162–170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Philippat, C.; Mortamais, M.; Chevrier, C.; Petit, C.; Calafat, A.M.; Ye, X.; Silva, M.J.; Brambilla, C.; Pin, I.; Charles, M.A.; et al. Exposure to phthalates and phenols during pregnancy and offspring size at birth. Environ. Health Perspect. 2012, 120, 464–470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Lin, L.; Cao, Y.; Chen, B.; Zheng, L.; Ge, R.S. Phthalate levels and low birth weight: A nested case-control study of Chinese newborns. J. Pediatr. 2009, 155, 500–504. [Google Scholar] [CrossRef] [PubMed]
- Wolff, M.S.; Engel, S.M.; Berkowitz, G.S.; Ye, X.; Silva, M.J.; Zhu, C.; Wetmur, J.; Calafat, A.M. Prenatal phenol and phthalate exposures and birth outcomes. Environ. Health Perspect. 2008, 116, 1092–1097. [Google Scholar] [CrossRef] [PubMed]
- Serme-Gbedo, Y.K.; Abdelouahab, N.; Pasquier, J.C.; Cohen, A.A.; Takser, L. Maternal levels of endocrine disruptors, polybrominated diphenyl ethers, in early pregnancy are not associated with lower birth weight in the Canadian birth cohort GESTE. Environ. Health 2016, 15, 49. [Google Scholar] [CrossRef] [PubMed]
- Lignell, S.; Aune, M.; Darnerud, P.O.; Hanberg, A.; Larsson, S.C.; Glynn, A. Prenatal exposure to polychlorinated biphenyls (PCBs) and polybrominated diphenyl ethers (PBDEs) may influence birth weight among infants in a Swedish cohort with background exposure: A cross-sectional study. Environ. Health 2013, 12, 44. [Google Scholar] [CrossRef] [PubMed]
- Alaee, M.; Arias, P.; Sjödin, A.; Bergman, A. An overview of commercially used brominated flame retardants, their applications, their use patterns in different countries/regions and possible modes of release. Environ. Int. 2003, 29, 683–689. [Google Scholar] [CrossRef]
- Sferruzzi-Perri, A.N.; Vaughan, O.R.; Forhead, A.J.; Fowden, A.L. Hormonal and nutritional drivers of intrauterine growth. Curr. Opin. Clin. Nutr. Metab. Care 2013, 16, 298–309. [Google Scholar] [CrossRef] [PubMed]
- Ceccatelli, R.; Faass, O.; Schlumpf, M.; Lichtensteiger, W. Gene expression and estrogen sensitivity in rat uterus after developmental exposure to the polybrominated diphenylether PBDE 99 and PCB. Toxicology 2006, 220, 104–116. [Google Scholar] [CrossRef] [PubMed]
- Shy, C.G.; Huang, H.L.; Chao, H.R.; Chang-Chien, G.P. Cord blood levels of thyroid hormones and IGF-1 weakly correlate with breast milk levels of PBDEs in Taiwan. Int. J. Hyg. Environ. Health 2012, 215, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Yekeen, T.A.; Xiao, Q.; Wang, Y.; Lu, F.; Huo, X. Placental IGF-1 and IGFBP-3 expression correlate with umbilical cord blood PAH and PBDE levels from prenatal exposure to electronic waste. Environ. Pollut. 2013, 182, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Espinosa, M.J.; Costa, O.; Vizcaino, E.; Murcia, M.; Fernandez-Somoano, A.; Iñiguez, C. Prenatal Exposure to Polybrominated Flame Retardants and Fetal Growth in the INMA Cohort (Spain). Environ. Sci. Technol. 2015, 49, 10108–10116. [Google Scholar] [CrossRef] [PubMed]
- Foster, W.G.; Gregorovich, S.; Morrison, K.M.; Atkinson, S.A.; Kubwabo, C.; Stewart, B. Human maternal and umbilical cord blood concentrations of polybrominated diphenyl ethers. Chemosphere 2011, 84, 1301–1309. [Google Scholar] [CrossRef] [PubMed]
- Harley, K.G.; Chevrier, J.; Aguilar Schall, R.; Sjödin, A.; Bradman, A.; Eskenazi, B. Association of prenatal exposure to polybrominated diphenyl ethers and infant birth weight. Am. J. Epidemiol. 2011, 174, 885–892. [Google Scholar] [CrossRef] [PubMed]
- Chao, H.R.; Wang, S.L.; Lee, W.J.; Wang, Y.F.; Päpke, O. Levels of polybrominated diphenyl ethers (PBDEs) in breast milk from central Taiwan and their relation to infant birth outcome and maternal menstruation effects. Environ. Int. 2007, 33, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wang, C.; Cui, C.; Ding, G.; Zhou, Y.; Jin, J.; Gao, Y.; Tian, Y. Prenatal exposure to polybrominated diphenyl ethers and birth outcomes. Environ. Pollut. 2015, 206, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.H.; Zhang, J.; Wang, Y.M.; Ye, Y.P.; Luo, Q.Q. Perinatal exposure to bisphenol-A impairs learning-memory by concomitant down-regulation of N-methyl-d-aspartate receptors of hippocampus in male offspring mice. Horm. Behav. 2010, 58, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Chou, W.C.; Chen, J.L.; Lin, C.F.; Chen, Y.C.; Shih, F.C.; Chuang, C.Y. Biomonitoring of bisphenol A concentrations in maternal and umbilical cord blood in regard to birth outcomes and adipokine expression: A birth cohort study in Taiwan. Environ. Health 2011, 10, 94. [Google Scholar] [CrossRef] [PubMed]
- Veiga-Lopez, A.; Kannan, K.; Liao, C.; Ye, W.; Domino, S.E.; Padmanabhan, V. Gender-Specific Effects on Gestational Length and Birth Weight by Early Pregnancy BPA Exposure. J. Clin. Endocrinol. Metab. 2015, 100, E1394–E1403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, Y.M.; Hong, Y.C.; Ha, M.; Kim, Y.; Park, H.; Kim, H.S.; Ha, E.H. Prenatal Bisphenol-A exposure affects fetal length growth by maternal glutathione transferase polymorphisms, and neonatal exposure affects child volume growth by sex: From multiregional prospective birth cohort MOCEH study. Sci. Total Environ. 2018, 612, 1433–1441. [Google Scholar] [CrossRef] [PubMed]
- Bach, C.C.; Bech, B.H.; Brix, N.; Nohr, E.A.; Bonde, J.P.; Henriksen, T.B. Perfluoroalkyl and polyfluoroalkyl substances and human fetal growth: A systematic review. Crit. Rev. Toxicol. 2015, 45, 53–67. [Google Scholar] [CrossRef] [PubMed]
- Manzano-Salgadoa, C.B.; Casasa, M.; Lopez-Espinosa, M.J.; Ballester, F.; Iñiguez, C.; Martineza, D.; Costad, O.; Santa-Marina, L.; Pereda-Pereda, E.; Schettgenh, T.; et al. Prenatal exposure to perfluoroalkyl substances and birth outcomes in a Spanish birth cohort. Environ. Int. 2017, 108, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Lauritzen, H.B.; Larose, T.L.; Øien, T.; Sandanger, T.M.; Odland, J.O.; van de Bor, M.; Jacobsen, G.W. Maternal serum levels of perfluoroalkyl substances and organochlorines and indices of fetal growth: A Scandinavian case–cohort study. Pediatr. Res. 2017, 81, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Gore, A.C.; Chappell, V.A.; Fenton, S.E.; Flaws, J.A.; Nadal, A.; Prins, G.S.; Toppari, J.; Zoeller, R.T. EDC-2: The Endocrine Society’s second scientific statement on Endocrine-Disrupting Chemicals. Endocr. Rev. 2015, 36, E1–E150. [Google Scholar] [CrossRef] [PubMed]
- Hartoft-Nielsen, M.L.; Boas, M.; Bliddal, S.; Rasmussen, A.K.; Main, K.; Feldt-Rasmussen, U. Do thyroid disrupting chemicals influence foetal development during pregnancy? J. Thyr. Res. 2011, 2011, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Preau, L.; Fini, J.B.; Morvan-Dubois, G.; Demeneix, B. Thyroid hormone signaling during early neurogenesis and its significance as a vulnerable window for endocrine disruption. Biochim. Biophys. Acta 2015, 1849, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, M.E.; Rovet, J.; Chen, Z.; Koibuchi, N. Developmental thyroid hormone disruption: Prevalence, environmental contaminants and neurodevelopmental consequences. Neurotoxicology. 2012, 33, 842–852. [Google Scholar] [CrossRef] [PubMed]
- Blount, B.C.; Pirkle, J.L.; Osterloh, J.D.; Valentin-Blasini, L.; Caldwell, K.L. Urinary perchlorate and thyroid hormone levels in adolescent and adult men and women living in the United States. Environ Health Perspect. 2006, 114, 1865–1871. [Google Scholar] [CrossRef] [PubMed]
- Steinmaus, C.; Miller, M.D.; Howd, R. Impact of smoking and thiocyanate on perchlorate and thyroid hormone associations in the 2001–2002 National Health and Nutrition Examinatio Survey. Environ. Health Perspect. 2007, 115, 1333–1338. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.T.; Wu, C.F.; Chen, B.H.; Chen, E.K.; Chen, Y.L.; Shiea, J.; Lee, W.T.; Chao, M.C.; Wu, J.R. Intake of phthalate-tainted foods alters thyroid functions in Taiwanese children. PLoS ONE 2013, 8, e55005. [Google Scholar] [CrossRef] [PubMed]
- El Majidi, N.; Bouchard, M.; Carrier, G. Systematic analysis of the relationship between standardized biological levels of polychlorinated biphenyls and thyroid function in pregnant women and newborns. Chemosphere 2014, 98, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Gentilcore, D.; Porreca, I.; Rizzo, F.; Ganbaatar, E.; Carchia, E.; Mallardo, M.; De Felice, M.; Ambrosino, C. Bisphenol A interferes with thyroid specific gene expression. Toxicology 2013, 304, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Kuriyama, S.N.; Wanner, A.; Fidalgo-Neto, A.A.; Talsness, C.E.; Koerner, W.; Chahoud, I. Developmental exposure to low-dose PBDE-99: Tissue distribution and thyroid hormone levels. Toxicology 2007, 242, 80–90. [Google Scholar] [CrossRef] [PubMed]
- Ibhazehiebo, K.; Koibuchi, N. Thyroid hormone receptor-mediated transcription is suppressed by low dose phthalate. Niger. J. Physiol. Sci. 2011, 26, 143–149. [Google Scholar] [PubMed]
- Giera, S.; Bansal, R.; Ortiz-Toro, T.M.; Taub, D.G.; Zoeller, R.T. Individual polychlorinated biphenyl (PCB) congeners produce tissue- and gene-specific effects on thyroid hormone signaling during development. Endocrinology 2011, 152, 2909–2919. [Google Scholar] [CrossRef] [PubMed]
- Hallgren, S.; Sinjari, T.; Håkansson, H.; Darnerud, P.O. Effects of polybrominated diphenyl ethers (PBDEs) and polychlorinated biphenyls (PCBs) on thyroid hormone and vitamin A levels in rats and mice. Arch. Toxicol. 2001, 75, 200–208. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, J.C.; Frame, S.R.; Ladics, G.S. Evaluation of a 15-day screening assay using intact male rats for identifying antiandrogens. Toxicol. Sci. 2002, 69, 92–108. [Google Scholar] [CrossRef] [PubMed]
- Chevrier, J.; Warner, M.; Gunier, R.B.; Brambilla, P.; Eskenazi, B.; Mocarelli, P. Serum Dioxin Concentrations and Thyroid Hormone Levels in the Seveso Women’s Health Study. Am. J. Epidemiol. 2014, 180, 490–498. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, R.; Yamaguchi, M.; Uramaru, N.; Kuroki, H.; Ohta, S.; Kitamura, S.; Sugihara, K. Structure-activity relationships of 44 halogenated compounds for iodotyrosine deiodinase-inhibitory activity. Toxicology 2013, 314, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Moriyama, K.; Tagami, T.; Akamizu, T.; Usui, T.; Saijo, M.; Kanamoto, N.; Hataya, Y.; Shimatsu, A.; Kuzuya, H.; Nakao, K. Thyroid hormone action is disrupted by bisphenol A as an antagonist. J. Clin. Endocrinol. Metab. 2002, 87, 5185–5190. [Google Scholar] [CrossRef] [PubMed]
- Zoeller, R.T.; Dowling, A.L.; Vas, A.A. Developmental exposure to polychlorinated biphenyls exerts thyroid hormone-like effects on the expression of RC3/neurogranin and myelin basic protein messenger ribonucleic acids in the developing rat brain. Endocrinology 2000, 141, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Londoño, M.; Shimokawa, N.; Miyazaki, W.; Iwasaki, T.; Koibuchi, N. Hydroxylated PCB induces Ca2+ oscillations and alterations of membrane potential in cultured cortical cells. J. Appl. Toxicol. 2010, 30, 334–342. [Google Scholar] [CrossRef] [PubMed]
- Bansal, R.; Tighe, D.; Danai, A.; Rawn, D.F.; Gaertner, D.W.; Arnold, D.L.; Gilbert, M.E.; Zoeller, R.T. Polybrominated diphenyl ether (DE-71) interferes with thyroid hormone action independent of effects on circulating levels of thyroid hormone in male rats. Endocrinology 2014, 155, 4104–4112. [Google Scholar] [CrossRef] [PubMed]
- Schmutzler, C.; Gotthardt, I.; Hofmann, P.J.; Radovic, B.; Kovacs, G.; Stemmler, L.; Nobis, I.; Bacinski, A.; Mentrup, B.; Ambrugger, P.; et al. Endocrine Disruptors and the thyroid gland—A combined in vitro and in vivo analysis of potential new biomarkers. Environ. Health Perspect. 2007, 115, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Charatcharoenwitthaya, N.; Ongphiphadhanakul, B.; Pearce, E.N.; Somprasit, C.; Chanthasenanont, A.; He, X.; Chailurkit, L.; Braverman, L.E. The association between perchlorate and thiocyanate exposure and thyroid function in first-trimester pregnant Thai women. J. Clin. Endocrinol. Metab. 2014, 99, 2365–2371. [Google Scholar] [CrossRef] [PubMed]
- Taylor, P.N.; Okosieme, O.E.; Murphy, R.; Hales, C.; Chiusano, E.; Maina, A.; Joomun, M.; Bestwick, J.P.; Smyth, P.; Paradice, R.; et al. Maternal perchlorate levels in women with borderline thyroid function during pregnancy and the cognitive development of their offspring: Data from the controlled antenatal thyroid study. J. Clin. Endocrinol. Metab. 2014, 99, 4291–4298. [Google Scholar] [CrossRef] [PubMed]
- Schantz, S.L.; Widholm, J.J.; Rice, D.C. Effects of PCB exposure on neuropsychological function in children. Environ. Health Perspect. 2003, 111, 357–376. [Google Scholar] [CrossRef] [PubMed]
- Zoeller, R.T.; Dowling, A.L.S.; Herzig, C.T.A.; Iannacone, E.A.; Gauger, K.J.; Bansal, R. Thyroid hormone, brain development, and the environment. Environ. Health Perspect. 2002, 110, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, J.L.; Jacobson, S.W. Intellectual impairment in children exposed to polychlorinated biphenyls in utero. N. Engl. J. Med. 1996, 335, 783–789. [Google Scholar] [CrossRef] [PubMed]
- Parent, A.S.; Franssen, D.; Fudvoye, J.; Gerard, A.; Bourguignon, J.P. Developmental variations in environmental influences including endocrine disruptors on pubertal timing and neuroendocrine control: Revision of human observations and mechanistic insight from rodents. Front. Neuroendocrinol. 2015, 38, 12–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aksglaede, L.; Sorensen, K.; Petersen, J.H.; Skakkebaek, N.E.; Juul, A. Recent decline in age at breast development: The Copenhagen Puberty Study. Pediatrics 2009, 123, e932–e939. [Google Scholar] [CrossRef] [PubMed]
- Herman-Giddens, M.E.; Slora, E.J.; Wasserman, R.C.; Bourdony, C.J.; Bhapkar, M.V.; Koch, G.G.; Hasemeier, C.M. Secondary sexual characteristics and menses in young girls seen in office practice: A study from the Pediatric Research in Office Settings network. Pediatrics 1997, 99, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Liwnicz, B.H.; Liwnicz, R.G. On endocrine function. In Clinical Chemistry: Theory, Analysis and Correlation, 2nd ed.; Kaplan, L.A., Pesce, A.J., Eds.; CV Mosby Company: St. Lewis, MO, USA, 1989; pp. 607–619. ISBN 0801627044. [Google Scholar]
- Buck Louis, G.M.; Gray, L.E., Jr.; Marcus, M.; Ojeda, S.R.; Pescovitz, O.H.; Witchel, S.F.; Sippell, W.; Abbott, D.H.; Soto, A.; Tyl, R.W.; et al. Environmental factors and puberty timing: Expert panel research needs. Pediatrics 2008, 121, S192–S207. [Google Scholar] [CrossRef] [PubMed]
- Bourguignon, J.P.; Juul, A.; Franssen, D.; Fudvoye, J.; Pinson, A.; Parent, A.S. Contribution of the endocrine perspective in the evaluation of endocrine disrupting chemical effects: The case study of pubertal timing. Horm. Res. Paediatr. 2016, 86, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Scippo, M.L.; Argiris, C.; Van De Weerdt, C.; Muller, M.; Willemsen, P.; Martial, J.; Maghuin-Rogister, G. Recombinant human estrogen, androgen and progesterone receptors for detection of potential endocrine disruptors. Anal. Bioanal. Chem. 2004, 378, 664–669. [Google Scholar] [CrossRef] [PubMed]
- Caserta, D.; Maranghi, L.; Mantovani, A.; Marci, R.; Maranghi, F.; Moscarini, M. Impact of endocrine disruptor chemicals in gynaecology. Hum. Reprod. Update 2008, 14, 59–72. [Google Scholar] [CrossRef] [PubMed]
- Rasier, G.; Toppari, J.; Parent, A.S.; Bourguignon, J.P. Female sexual maturation and reproduction after prepubertal exposure to estrogens and endocrine disrupting chemicals: A review of rodent and human data. Mol. Cell. Endocrinol. 2006, 254–255, 187–201. [Google Scholar] [CrossRef] [PubMed]
- Diamanti-Kandarakis, E.; Bourguignon, J.P.; Giudice, L.C.; Hauser, R.; Prins, G.S.; Soto, A.M.; Zoeller, R.T.; Gore, A.C. Endocrine-disrupting chemicals: An Endocrine Society scientific statement. Endocr. Rev. 2009, 30, 293–342. [Google Scholar] [CrossRef] [PubMed]
- Krstevska-Konstantinova, M.; Charlier, C.; Craen, M.; Du Caju, M.; Heinrichs, C.; de Beaufort, C.; Plomteux, G.; Bourguignon, J.P. Sexual precocity after immigration from developing countries to Belgium: Evidence of previous exposure to organochlorine pesticides. Hum. Reprod. 2001, 16, 1020–1026. [Google Scholar] [CrossRef] [PubMed]
- Parent, A.S.; Teilmann, G.; Juul, A.; Skakkebaek, N.E.; Toppari, J.; Bourguignon, J.P. The timing of normal puberty and the age limits of sexual precocity: Variations around the world, secular trends, and changes after migration. Endocr. Rev. 2001, 24, 668–693. [Google Scholar] [CrossRef] [PubMed]
- Vasiliu, O.; Muttineni, J.; Karmaus, W. In utero exposure to organochlorines and age at menarche. Hum. Reprod. 2004, 19, 1506–1512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gladen, B.C.; Ragan, N.B.; Rogan, W.J. Pubertal growth and development and prenatal and lactational exposure to polychlorinated biphenyls and dichlorodiphenyl dichloroethene. J. Pediatr. 2000, 136, 490–496. [Google Scholar] [CrossRef]
- Ouyang, F.; Perry, M.J.; Venners, S.A.; Chen, C.; Wang, B.; Yang, F.; Fang, Z.; Zang, T.; Wang, L.; Xu, X.; et al. Serum DDT, age at menarche, and abnormal menstrual cycle length. Occup. Environ. Med. 2005, 62, 878–884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, A.; Chung, E.; DeFranco, E.A.; Pinney, S.M.; Dietrich, K.N. Serum PBDEs and age at menarche in adolescent girls: Analysis of the National Health and Nutrition Examination Survey 2003–2004. Environ. Res. 2011, 111, 831–837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tassinari, R.; Mancini, F.R.; Mantovani, A.; Busani, L.; Maranghi, F. Pilot study on the dietary habits and lifestyles of girls with idiopathic precocious puberty from the city of Rome: Potential impact of exposure to flame retardant polybrominated diphenyl ethers. J. Pediatr. Endocrinol. Metab. 2015, 28, 1369–1372. [Google Scholar] [CrossRef] [PubMed]
- Link, B.; Gabrio, T.; Mann, V.; Schilling, B.; Maisner, V.; König, M.; Flicker-Klein, A.; Zöllner, I.; Fischer, G. Polybrominated diphenyl ethers (PBDE) in blood of children in Baden-Württemberg between 2002/03 and 2008/09. Int. J. Hyg. Environ. Health 2012, 215, 224–228. [Google Scholar] [CrossRef] [PubMed]
- Deodati, A.; Sallemi, A.; Maranghi, F.; Germani, D.; Puglianiello, A.; Baldari, F.; Busani, L.; Mancini, F.R.; Tassinari, R.; Mantovani, A.; et al. Serum levels of polybrominated diphenyl ethers in girls with premature thelarche. Horm. Res. Paediatr. 2016, 86, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Blanck, H.M.; Marcus, M.; Tolbert, P.E.; Rubin, C.; Henderson, A.K.; Hertzberg, V.S.; Zhang, R.H.; Cameron, L. Age at menarche and tanner stage in girls exposed in utero and postnatally to polybrominated biphenyl. Epidemiology 2000, 11, 641–647. [Google Scholar] [CrossRef] [PubMed]
- Harley, K.G.; Rauch, S.A.; Chevrier, J.; Kogut, K.; Parra, K.L.; Trujillo, C.; Lustig, R.H.; Greenspan, L.C.; Sjödin, A.; Bradman, A.; et al. Association of prenatal and childhood PBDE exposure with timing of puberty in boys and girls. Environ. Int. 2017, 100, 132–138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wormke, M.; Stoner, M.; Saville, B.; Walker, K.; Abdelrahim, M.; Burghardt, R.; Safe, S. The aryl hydrocarbon receptor mediates degradation of estrogen receptor alpha through activation of proteasomes. Mol. Cell. Biol. 2003, 23, 1843–1855. [Google Scholar] [CrossRef] [PubMed]
- Leijs, M.M.; Koppe, J.G.; Olie, K.; van Aalderen, W.M.; Voogt, P.; Vulsma, T.; Westra, M.; ten Tusscher, G.W. Delayed initiation of breast development in girls with higher prenatal dioxin exposure: A longitudinal cohort study. Chemosphere 2008, 73, 999–1004. [Google Scholar] [CrossRef] [PubMed]
- Den Hond, E.; Roels, H.A.; Hoppenbrouwers, K.; Nawrot, T.; Thijs, L.; Vandermeulen, C.; Winneke, G.; Vanderschueren, D.; Staessen, J.A. Sexual maturation in relation to polychlorinated aromatic hydrocarbons: Sharpe and Skakkebaek’s hypothesis revisited. Environ. Health Perspect. 2002, 110, 771–776. [Google Scholar] [CrossRef]
- Warner, M.; Samuels, S.; Mocarelli, P.; Gerthoux, P.M.; Needham, L.; Patterson, D.G., Jr.; Eskenazi, B. Serum dioxin concentrations and age at menarche. Environ. Health Perspect. 2004, 112, 1289–1292. [Google Scholar] [CrossRef] [PubMed]
- Bongiovanni, A.M. An epidemic of premature thelarche in Puerto Rico. J. Pediatr. 1983, 103, 245–246. [Google Scholar] [CrossRef]
- Bourdony, C.J. Premature Thelarche and Early Sexual Development Registry; Annual Report; Department of Health: San Juan, Puerto Rico, 1998. [Google Scholar]
- Colon, I.; Caro, D.; Bourdony, C.J.; Rosario, O. Identification of phthalate esters in the serum of young Puerto Rican girls with premature breast development. Environ. Health Perspect. 2000, 108, 895–900. [Google Scholar] [CrossRef] [PubMed]
- Frederiksen, H.; Sorensen, K.; Mouritsen, A.; Aksglaede, L.; Hagen, C.P.; Petersen, J.H.; Skakkebaek, N.E.; Andersson, A.M.; Juul, A. High urinary phthalate concentration associated with delayed pubarche in girls. Int. J. Androl. 2012, 35, 216–226. [Google Scholar] [CrossRef] [PubMed]
- Mouritsen, A.; Frederiksen, H.; Sorensen, K.; Aksglaede, L.; Hagen, C.; Skakkebaek, N.E.; Main, K.M.; Andersson, A.M.; Juul, A. Urinary phthalates from 168 girls and boys measured twice a year during a 5-year period: Associations with adrenal androgen levels and puberty. J. Clin. Endocrinol. Metab. 2013, 98, 3755–3764. [Google Scholar] [CrossRef] [PubMed]
- Srilanchakon, K.; Thadsri, T.; Jantarat, C.; Thengyai, S.; Nosoognoen, W.; Supornsilchai, V. Higher phthalate concentrations are associated with precocious puberty in normal weight Thai girls. J. Pediatr. Endocrinol. Metab. 2017, 30, 1293–1298. [Google Scholar] [CrossRef] [PubMed]
- Buluş, A.D.; Aşci, A.; Erkekoglu, P.; Balci, A.; Andiran, N.; Koçer-Gümüşel, B. The evaluation of possible role of endocrine disruptors in central and peripheral precocious puberty. Toxicol. Mech. Methods 2016, 26, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Wolff, M.S.; Pajak, A.; Pinney, S.M.; Windham, G.C.; Galvez, M.; Rybak, M.; Silva, M.J.; Ye, X.; Calafat, A.M.; Kushi, L.H.; et al. Associations of urinary phthalate and phenol biomarkers with menarche in a multiethnic cohort of young girls. Reprod. Toxicol. 2017, 67, 56–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leonardi, A.; Cofini, M.; Rigante, D.; Lucchetti, L.; Cipolla, C.; Penta, L.; Esposito, S. The effect of bisphenol A on puberty: A critical review of the medical literature. Int. J. Environ. Res. Public Health 2017, 14, 1044. [Google Scholar] [CrossRef] [PubMed]
- Supornsilchai, V.; Jantarat, C.; Nosoognoen, W.; Pornkunwilai, S.; Wacharasindhu, S.; Soder, O. Increased levels of bisphenol A (BPA) in Thai girls with precocious puberty. J. Pediatr. Endocrinol. Metab. 2016, 29, 1233–1239. [Google Scholar] [CrossRef] [PubMed]
- Durmaz, E.; Aşçı, A.; Erkekoğlu, P.; Akçurin, S.; Gümüşel, B.K.; Bircan, I. Urinary bisphenol a levels in girls with idiopathic central precocious puberty. J. Clin. Res. Pediatr. Endocrinol. 2014, 6, 16–21. [Google Scholar] [CrossRef] [PubMed]
- McGuinn, L.A.; Ghazarian, A.A.; Joseph Su, L.; Ellison, G.L. Urinary bisphenol A and age at menarche among adolescent girls: Evidence from NHANES 2003–2010. Environ. Res. 2015, 136, 381–386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miao, M.; Wang, Z.; Liu, X.; Liang, H.; Zhou, Z.; Tan, H.; Yuan, W.; Li, D.K. Urinary bisphenol A and pubertal development in Chinese school-aged girls: A cross-sectional study. Environ. Health 2017, 16, 80. [Google Scholar] [CrossRef] [PubMed]
- Wolff, M.S.; Teitelbaum, S.L.; Pinney, S.M.; Windham, G.; Liao, L.; Biro, F.; Kushi, L.H.; Erdmann, C.; Hiatt, R.A.; Rybak, M.E.; et al. Investigation of relationships between urinary biomarkers of phytoestrogens, phthalates and phenols and pubertal stages in girls. Environ. Health Perspect. 2010, 118, 1039–1046. [Google Scholar] [CrossRef] [PubMed]
- Virtanen, H.E.; Jørgensen, N.; Toppari, J. Semen quality in the 21st century. Nat. Rev. Urol. 2017, 14, 120–130. [Google Scholar] [CrossRef] [PubMed]
- Centola, G.M.; Blanchard, A.; Demick, J.; Li, S.; Eisenberg, M.L. Decline in sperm count and motility in young adult men from 2003 to 2013: Observations from a U.S. sperm bank. Andrology 2016, 4, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Carlsen, E.; Giwercman, A.; Keiding, N.; Skakkebaek, N.E. Evidence for decreasing quality of semen during past 50 years. BMJ 1992, 305, 609–613. [Google Scholar] [CrossRef] [PubMed]
- Nordkap, L.; Joensen, U.N.; Blomberg, J.M.; Jørgensen, N. Regional differences and temporal trends in male reproductive health disorders: Semen quality may be a sensitive marker of environmental exposures. Mol. Cell. Endocrinol. 2012, 355, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Sifakis, S.; Androutsopoulos, V.P.; Tsatsakis, A.M.; Spandidos, D.A. Human exposure to endocrine disrupting chemicals: Effects on the male and female reproductive systems. Environ. Toxicol. Pharmacol. 2017, 51, 56–70. [Google Scholar] [CrossRef] [PubMed]
- Patiño-García, D.; Cruz-Fernandes, L.; Buñay, J.; Palomino, J.; Moreno, R.D. Reproductive Alterations in Chronically Exposed Female Mice to Environmentally Relevant Doses of a Mixture of Phthalates and Alkylphenols. Endocrinology 2018, 159, 1050–1061. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Gao, L.; Flaws, J.A. Prenatal exposure to an environmentally relevant phthalate mixture disrupts reproduction in F1 female mice. Toxicol. Appl. Pharmacol. 2017, 318, 49–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Latchney, S.E.; Fields, A.M.; Susiarjo, M. Linking inter-individual variability to endocrine disruptors: Insights for epigenetic inheritance. Mamm. Genome 2018, 29, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Durmaz, E.; Asci, A.; Erkekoglu, P.; Balcı, A.; Bircan, I.; Koçer-Gumusel, B. Urinary bisphenol A levels in Turkish girls with premature thelarche. Hum. Exp. Toxicol. 2018, 1, 960327118756720. [Google Scholar] [CrossRef] [PubMed]
- Watkins, D.J.; Sánchez, B.N.; Téllez-Rojo, M.M.; Lee, J.M.; Mercado-García, A.; Blank-Goldenberg, C.; Peterson, K.E.; Meeker, J.D. Phthalate and bisphenol A exposure during in utero windows of susceptibility in relation to reproductive hormones and pubertal development in girls. Environ. Res. 2017, 159, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Fang, F.; Zhu, W.; Chen, Z.J.; Du, Y.; Zhang, J. Bisphenol A and Ovarian Reserve among Infertile Women with Polycystic Ovarian Syndrome. Int. J. Environ. Res. Public Health 2016, 14, 18. [Google Scholar] [CrossRef] [PubMed]
- Mínguez-Alarcón, L.; Gaskins, A.J.; Chiu, Y.H.; Williams, P.L.; Ehrlich, S.; Chavarro, J.E.; Petrozza, J.C.; Ford, J.B.; Calafat, A.M.; Hauser, R.; et al. Urinary bisphenol A concentrations and association with in vitro fertilization outcomes among women from a fertility clinic. Hum. Reprod. 2015, 30, 2120–2128. [Google Scholar] [CrossRef] [PubMed]
- Upson, K.; Sathyanarayana, S.; De Roos, A.J.; Koch, H.M.; Scholes, D.; Holt, V.L. A population-based case-control study of urinary bisphenol A concentrations and risk of endometriosis. Hum. Reprod. 2014, 29, 2457–2464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jukic, A.M.; Calafat, A.M.; McConnaughey, D.R.; Longnecker, M.P.; Hoppin, J.A.; Weinberg, C.R.; Wilcox, A.J.; Baird, D.D. Urinary Concentrations of Phthalate Metabolites and Bisphenol A and Associations with Follicular-Phase Length, Luteal-Phase Length, Fecundability, and Early Pregnancy Loss. Environ. Health Perspect. 2016, 124, 321–328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomsen, A.M.; Riis, A.H.; Olsen, J.; Jönsson, B.A.; Lindh, C.H.; Hjollund, N.H.; Jensen, T.K.; Bonde, J.P.; Toft, G. Female exposure to phthalates and time to pregnancy: A first pregnancy planner study. Hum. Reprod. 2017, 32, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Mu, D.; Gao, F.; Fan, Z.; Shen, H.; Peng, H.; Hu, J. Levels of Phthalate Metabolites in Urine of Pregnant Women and Risk of Clinical Pregnancy Loss. Environ. Sci. Technol. 2015, 49, 10651–10657. [Google Scholar] [CrossRef] [PubMed]
- Al-Hussaini, T.K.; Abdelaleem, A.A.; Elnashar, I.; Shabaan, O.M.; Mostafa, R.; El-Baz, M.A.H.; El-Deek, S.E.M.; Farghaly, T.A. The effect of follicullar fluid pesticides and polychlorinated biphenyls concentrations on intracytoplasmic sperm injection (ICSI) embryological and clinical outcome. Eur. J. Obstet. Gynecol. Reprod. Biol. 2018, 220, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Ploteau, S.; Cano-Sancho, G.; Volteau, C.; Legrand, A.; Vénisseau, A.; Vacher, V.; Marchand, P.; Le Bizec, B.; Antignac, J.P. Associations between internal exposure levels of persistent organic pollutants in adipose tissue and deep infiltrating endometriosis with or without concurrent ovarian endometrioma. Environ. Int. 2017, 108, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Hart, R.J.; Doherty, D.A.; Keelan, J.A.; Minaee, N.S.; Thorstensen, E.B.; Dickinson, J.E.; Pennell, C.E.; Newnham, J.P.; McLachlan, R.; Norman, R.J.; et al. The impact of antenatal Bisphenol A exposure on male reproductive function at 20–22 years of age. Reprod. Biomed. Online 2018, 36, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Adoamnei, E.; Mendiola, J.; Vela-Soria, F.; Fernández, M.F.; Olea, N.; Jørgensen, N.; Swan, S.H.; Torres-Cantero, A.M. Urinary bisphenol A concentrations are associated with reproductive parameters in young men. Environ. Res. 2018, 161, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Minatoya, M.; Sasaki, S.; Araki, A.; Miyashita, C.; Itoh, S.; Yamamoto, J.; Matsumura, T.; Mitsui, T.; Moriya, K.; Cho, K.; et al. Cord Blood Bisphenol A Levels and Reproductive and Thyroid Hormone Levels of Neonates: The Hokkaido Study on Environment and Children’s Health. Epidemiology 2017, 28, S3–S9. [Google Scholar] [CrossRef] [PubMed]
- Vitku, J.; Heracek, J.; Sosvorova, L.; Hampl, R.; Chlupacova, T.; Hill, M.; Sobotka, V.; Bicikova, M.; Starka, L. Associations of bisphenol A and polychlorinated biphenyls with spermatogenesis and steroidogenesis in two biological fluids from men attending an infertility clinic. Environ. Int. 2016, 89–90, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Fernández, M.F.; Arrebola, J.P.; Jiménez-Díaz, I.; Sáenz, J.M.; Molina-Molina, J.M.; Ballesteros, O.; Kortenkamp, A.; Olea, N. Bisphenol A and other phenols in human placenta from children with cryptorchidism or hypospadias. Reprod. Toxicol. 2016, 59, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Yang, H.; Zhou, N.; Sun, L.; Bao, H.; Tan, L.; Chen, H.; Ling, X.; Zhang, G.; Huang, L.; et al. Phthalate exposure, even below US EPA reference doses, was associated with semen quality and reproductive hormones: Prospective MARHCS study in general population. Environ. Int. 2017, 104, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Axelsson, J.; Rylander, L.; Rignell-Hydbom, A.; Lindh, C.H.; Jönsson, B.A.; Giwercman, A. Prenatal phthalate exposure and reproductive function in young men. Environ. Res. 2015, 138, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Bloom, M.S.; Whitcomb, B.W.; Chen, Z.; Ye, A.; Kannan, K.; Buck Louis, G.M. Associations between urinary phthalate concentrations and semen quality parameters in a general population. Hum. Reprod. 2015, 30, 2645–2657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiu, Y.H.; Gaskins, A.J.; Williams, P.L.; Mendiola, J.; Jørgensen, N.; Levine, H.; Hauser, R.; Swan, S.H.; Chavarro, J.E. Intake of Fruits and Vegetables with Low-to-Moderate Pesticide Residues Is Positively Associated with Semen-Quality Parameters among Young Healthy Men. J. Nutr. 2016, 146, 1084–1092. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García, J.; Ventura, M.I.; Requena, M.; Hernández, A.F.; Parrón, T.; Alarcón, R. Association of reproductive disorders and male congenital anomalies with environmental exposure to endocrine active pesticides. Reprod. Toxicol. 2017, 71, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Winston, J.J.; Emch, M.; Meyer, R.E.; Langlois, P.; Weyer, P.; Mosley, B.; Olshan, A.F.; Band, L.E.; Luben, T.J.; National Birth Defects Prevention Study. Hypospadias and maternal exposure to atrazine via drinking water in the National Birth Defects Prevention study. Environ. Health 2016, 15, 76. [Google Scholar] [CrossRef] [PubMed]
- Daoud, S.; Sellami, A.; Bouassida, M.; Kebaili, S.; Ammar Keskes, L.; Rebai, T.; Chakroun Feki, N. Routine assessment of occupational exposure and its relation to semen quality in infertile men: A cross-sectional study. Turk. J. Med. Sci. 2017, 47, 902–907. [Google Scholar] [CrossRef] [PubMed]
- Cremonese, C.; Piccoli, C.; Pasqualotto, F.; Clapauch, R.; Koifman, R.J.; Koifman, S.; Freire, C. Occupational exposure to pesticides, reproductive hormone levels and sperm quality in young Brazilian men. Reprod. Toxicol. 2017, 67, 174–185. [Google Scholar] [CrossRef] [PubMed]
- Koskenniemi, J.J.; Virtanen, H.E.; Kiviranta, H.; Damgaard, I.N.; Matomäki, J.; Thorup, J.M.; Hurme, T.; Skakkebaek, N.E.; Main, K.M.; Toppari, J. Association between levels of persistent organic pollutants in adipose tissue and cryptorchidismin early childhood: A case-control study. Environ. Health 2015, 14, 78. [Google Scholar] [CrossRef] [PubMed]
- Soto, A.M.; Sonnenschein, C. The somatic mutation theory of cancer: Growing problems with the paradigm? Bioessays 2004, 26, 1097–1107. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. The hallmarks of cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef]
- Rey, O.; Danchin, E.; Mirouze, M.; Loot, C.; Blanchet, S. Adaptation to Global Change: A Transposable Element-Epigenetics Perspective. Trends Ecol. Evol. 2016, 31, 514–526. [Google Scholar] [CrossRef] [PubMed]
- Knudson, A. Mutation and cancer: Statistical study of retinoblastoma. Proc. Natl. Acad. Sci. USA 1971, 68, 820–823. [Google Scholar] [CrossRef] [PubMed]
- Hanson, M.A.; Gluckman, P.D. Early Developmental Conditioning of Later Health and Disease: Physiology or Pathophysiology? Physiol. Rev. 2014, 94, 1027–1076. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soto, A.M.; Maffini, M.V.; Sonnenschein, C. Neoplasia as development gone awry: The role of endocrine disruptors. Int. J. Androl. 2008, 31, 288–293. [Google Scholar] [CrossRef] [PubMed]
- Melnick, R.L.; Huff, J. Lorenzo Tomatis and primary prevention of environmental cancer. Environ. Health 2011, 10, S14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomatis, L. Prenatal exposure to chemical carcinogens and its effect on subsequent generations. Natl. Cancer Inst. Monogr. 1979, 51, 159–184. [Google Scholar]
- Huo, D.; Anderson, D.; Palmer, J.R.; Herbst, A.L. Incidence rates and risks of diethylstilbestrol-related clear-cell adenocarcinoma of the vagina and cervix: Update after 40-year follow-up. Gynecol. Oncol. 2017, 146, 566–571. [Google Scholar] [CrossRef] [PubMed]
- Herbst, A.L.; Ulfelder, H.; Poskanzer, D.C. Adenocarcinoma of the vagina. Association of maternal stilbestrol therapy with tumor appearance in young women. N. Engl. J. Med. 1971, 284, 878–881. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, H.; Loktionov, A.; Tomatis, L. Perinatal and multigenerational effect of carcinogens: Possible contribution to determination of cancer susceptibility. Environ. Health Perspect. 1992, 98, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Miller, C.; Degenhardt, K.; Sassoon, D.A. Fetal exposure to DES results in de-regulation of Wnt7a during uterine morphogenesis. Nat. Genet. 1998, 20, 228–230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Block, K.; Kardana, A.; Igarashi, P.; Taylor, H.S. In utero diethylstilbestrol (DES) exposure alters Hox gene expression in the developing mullerian system. FASEB J. 2000, 14, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Hussain, I.; Bhan, A.; Ansari, I.K.; Deba, P.; Bobzeanb, S.A.M.; Perrotti, L.I.; Mandal, S.S. Bisphenol-A induces expression of HOXC6, an estrogen-regulated homeobox-containing gene associated with breast cancer. Biochim. Biophys. Acta 2015, 1849, 697–708. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cook, J.D.; Davis, B.J.; Cai, S.L.; Barrett, J.C.; Conti, C.J.; Walker, C.L. Interaction between genetic susceptibility and early-life environmental exposure determines tumor-suppressor-gene penetrance. Proc. Natl. Acad. Sci. USA 2005, 102, 8644–8649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Padmanabhan, R.; Hendry, I.R.; Knapp, J.R.; Shuai, B.; Hendry, W.J. Altered microRNA expression patterns during the initiation and promotion stages of neonatal diethylstilbestrol-induced dysplasia/neoplasia in the hamster (Mesocricetus auratus) uterus. Cell. Biol. Toxicol. 2017, 33, 483–500. [Google Scholar] [CrossRef] [PubMed]
- Vorderstrasse, B.A.; Fenton, S.E.; Bohn, A.A.; Cundiff, J.A.; Lawrence, B.P. A novel effect of dioxin: Exposure during pregnancy severely impairs mammary gland differentiation. Toxicol. Sci. 2004, 78, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Steenland, K.; Bertazzi, P.; Baccarelli, A.; Kogevinas, M. Dioxin Revisited: Developments since the 1997 IARC Classification of Dioxin as a Human Carcinogen. Environ. Health Perspect. 2004, 112, 1265–1268. [Google Scholar] [CrossRef] [PubMed]
- Moral, R.; Wang, R.; Russo, I.H.; Lamartiniere, C.A.; Pereira, J.; Russo, J. Effect of prenatal exposure to the endocrine disruptor bisphenol a on mammary gland morphology and gene expression signature. J. Endocrinol. 2008, 196, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Gray, J.M.; Rasanayagam, S.; Engel, C.; Rizzo, J. State of the evidence 2017: An update on the connection between breast cancer and the environment. Environ. Health 2017, 16, 94. [Google Scholar] [CrossRef] [PubMed]
- Giulivo, M.; Lopez de Alda, M.; Capri, E.; Barceló, D. Human exposure to endocrine disrupting compounds: Their role in reproductive systems, metabolic syndrome and breast cancer. A review. Environ. Res. 2016, 151, 251–264. [Google Scholar] [CrossRef] [PubMed]
- Prins, G.S.; Tang, W.Y.; Belmonte, J.; Ho, S.M. Perinatal exposure to oestradiol and bisphenol A alters the prostate epigenome and increases susceptibility to carcinogenesis. Basic Clin. Pharmacol. Toxicol. 2008, 102, 134–138. [Google Scholar] [CrossRef] [PubMed]
- Doherty, L.F.; Bromer, J.G.; Zhou, Y.; Aldad, T.S.; Taylor, H.S. In utero exposure to diethylstilbestrol (DES) or bisphenol-A (BPA) increases EZH2 expression in the mammary gland: An epigenetic mechanism linking endocrine disruptors to breast cancer. Horm. Cancer 2010, 1, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Di Donato, M.; Cernera, G.; Giovannelli, P.; Galasso, G.; Bilancio, A.; Migliaccio, A.; Castoria, G. Recent advances on bisphenol-A and endocrine disruptor effects on human prostate cancer. Mol. Cell. Endocrinol. 2017, 457, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Santangeli, S.; Maradonna, F.; Olivotto, I.; Piccinetti, C.C.; Gioacchini, G.; Carnevali, O. Effects of BPA on female reproductive function: The involvement of epigenetic mechanism. Gen. Comp. Endocrinol. 2017, 245, 122–126. [Google Scholar] [CrossRef] [PubMed]
- Baccarelli, A.; Hirt, C.; Pesatori, A.C.; Consonni, D.; Patterson, D.G., Jr.; Bertazzi, P.A.; Dölken, G.; Landi, M.T. t(14;18) translocations in lymphocytes of healthy dioxin-exposed individuals from Seveso, Italy. Carcinogenesis 2006, 27, 2001–2007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agopian, J.; Navarro, J.M.; Gac, A.C.; Lecluse, Y.; Briand, M.; Grenot, P.; Gauduchon, P.; Ruminy, P.; Lebailly, P.; Nadel, B.; et al. Agricultural pesticide exposure and the molecular connection to lymphomagenesis. J. Exp. Med. 2009, 206, 1473–1483. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Chemical | Metabolite | Site of Action |
|---|---|---|
| Phthalates | DBP, BP, DHEP | Steroid receptors (anti androgen), PPARs, RXR [128,137] |
| Phenolic compounds | BPA | Steroid receptors (xeno-estrogen), PPARs, RXR [131,140,141] |
| Pharmaceutical compounds | DES | Estrogen receptor [136] |
| Organotin compounds | TBT | PPARs, RXR [129,139] |
| Dioxins | TCDD | Aryl hydrocarbon receptor [144] |
| PCBs and POPs | PCB 153-170-187 | Aryl hydrocarbon receptor [130,144] |
| Pesticides | DDT | Steroid receptors [133,134] |
| Flame retardants | Penta-DBE | Steroid receptors [133,134] |
| Alkylphenols | NP | Steroid receptors [133,134] |
| EDC | Population | Endpoint |
|---|---|---|
| TCDD (Dioxin) | U.S. Ranch Hand Veterans (Adults) | Increased risk of T2DM [155] |
| TCDD (Dioxin) | North Italy (Seveso incident, adults) | Increased risk of T2DM (Female) [156] |
| Persistent Organic Pollutants | Spain (Adults) | Increased risk of metabolic syndrome [157] |
| Persistent Organic Pollutants | Canada (Adults) | Increased risk of metabolic syndrome [158] |
| BPA | China (Adults) | Increased BMI, waist circumference and decreased insulin sensitivity [159] |
| BPA | NHANES (U.S., adults) | Increased BMI and waist circumference [160] |
| Phthalates | NHANES (U.S. Adults and children) | Increased BMI [161] |
| Phthalates | NHANES (U.S. Adults and children) | Increased waist circumference, decreased insulin sensitivity (adult males) [162] |
| Phthalates | Italy (Children) | Increased waist circumference, decreased insulin sensitivity [163] |
| Contaminant | Substrate | Cohort | Results |
|---|---|---|---|
| BPA | Urine | 25 Turkish prepubertal girls with premature thelarche (PT), 25 healthy prepubertal girls | The median urinary concentrations of BPA were found to be significantly higher in the PT group compared to the healthy control group, weak positive correlation between uterus volume, estradiol and luteinizing hormone [265] |
| BPA, phthalates | Urine from mothers during first, second, and third trimesters of pregnancy. | 120 female prepubertal subjects | Phthalate metabolites were associated with higher serum testosterone concentrations in prepuberty while a number of metabolites measured in the third trimester were associated with higher DHEA-S. MEHP levels across pregnancy were associated with lower odds of having a Tanner Stage >1 for breast development, while MEHP in the third trimester was associated with higher odds of having a Tanner Stage for pubic hair development >1 [266] |
| BPA | Urine | 268 infertile women diagnosed with PCOS | BPA was detected in all women. Increased BPA correlated with decreased antral follicle count and was negatively associated with AMH and day-3 FSH levels, but neither of these reached statistical significance [267] |
| BPA | Urine | 256 women | No associations between urinary BPA concentrations and IVF outcome [268] |
| BPA | Urine | 143 patients with endometriosis, 287 healthy women | No associations between BPA concentrations and endometriosis. Positive association with non-ovarian pelvic endometriosis [269] |
| Phthalate metabolites, BPA | Urine | 221 women | BPA and MCOP (or its precursors) were associated with shorter luteal phase. DEHP metabolites were associated with reduced early pregnancy loss [270] |
| Phthalate metabolites | Urine | 229 women | No significant association with MBP, MBzP and MEHP. Urinary concentration of MEP was associated with a decreased fecundity [271] |
| Phthalate metabolites | Urine | 128 women | Pregnancy loss was increased in women with urinary increase in MEHP [272] |
| Pesticides | Follicular fluid | 94 women of infertile couples | Higher concentrations of any studied PCBs and pesticides are associated with thinner endometrial thickness and affected embryological ICSI outcomes [273] |
| Dioxins, PCBs, PBDEs, PBBs, HBCDs, OC pesticides | Adipose tissue and serum samples | 55 patients and 44 healthy women | Significant associations between deep infiltrating endometriosis and adipose tissue levels of PCB, PBDE, PBB, benzenes and organochlorine pesticides [274] |
| Contaminant | Substrate | Cohort | Results |
|---|---|---|---|
| BPA | Semen and serum | 365 semen samples. Maternal serum collected at 18 and 34 weeks’ gestation | Sperm concentration and motility were significantly correlated with maternal serum BPA levels [275] |
| BPA | Semen and urine | 215 healthy young men (18–23 years) | BPA levels were significantly and negatively correlated with sperm concentration. No significant associations were found among BPA and other semen quality parameters or reproductive hormone levels [276] |
| BPA | Cord blood | 283 neonates | Positive association of BPA levels with testosterone, estradiol, and progesterone levels in boys [277] |
| BPA, phthalates | Urine from 1st, 2nd, 3rd trimesters of pregnancy | 109 boys | Exposure to phthalates during the 3rd trimester associated with lower odds of having Pubic Hair Tanner stage >1 for and higher peripubertal SHBG levels [266] |
| BPA, PCBs | Plasma and semen | 191 men | Seminal BPA, but not plasma BPA, was negatively associated with sperm concentration and morphology. PCB was negatively associated with testosterone, free testosterone, free androgen index and DHT in plasma [278] |
| BPA | Placenta | 28 cases and 51 healthy controls in newborns | Increase of BPA levels are associated with of cryptorchidism and hypospadias [279] |
| Phthalate | Urine, semen and blood | 796 healthy man | Association with low semen quality and alteration of reproductive hormones even with a dose below the reference doses [280] |
| Phthalate | Serum | 112 adolescents | Highest exposure of one DiNP metabolites associated with lower total testicular volume, higher levels of FSH and lower semen volume. Men in the highest exposure of one DEHP metabolite show lower semen volume [281] |
| Phthalate metabolites | Urine and semen | 501 healthy man | Association between urinary metabolites and lower total sperm counts and concentrations, larger sperm head sizes, higher proportions of megalo head sperm morphology. MEHP was significantly associated with higher sperm motility [282] |
| Pesticides | Blood | 189 healthy young men | The total intake of fruit and vegetables was unrelated to semen quality. Intake with low-to-moderate pesticide residues was associated with a higher total sperm count and sperm concentration [283] |
| Organochlorine Pesticides | Environmental level | 963 cryptorchid men; 678 hypospadias; 65 micropenis; 587,142 controls | Prevalence rates for cryptorchidism, hypospadias and micropenis were significantly greater in areas of high environmental exposure to pesticides in relation to those with low exposure [284] |
| Pesticides (atrazine) | Drinking water | 343 cases with hypospadias and 1422 male controls | No association between hypospadias and daily maternal atrazine exposure during the critical window of genitourinary development [285] |
| Pesticides | Semen | 2122 men who underwent andrological investigation for couple infertility | Exposure to pesticides was associated with a significantly higher risk of asthenozoospermia and necrozoospermia [286] |
| Pesticides | Serum and semen | 99 rural and 36 urban men (18–23 years) | Rural men had poorer sperm morphology, higher sperm count, and lower LH levels than urban subjects. Maternal farming during pregnancy was associated with larger anogenital distance and testis volume [287] |
| PCBs, PCDD/Fs, and PBDEs | Subcutaneous adipose tissue biopsies | 44 cryptorchid cases, and 38 controls | Prenatal exposure to PCDD/Fs and PCDD/F-like PCBs may be associated with increased risk for cryptorchidism [288] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Street, M.E.; Angelini, S.; Bernasconi, S.; Burgio, E.; Cassio, A.; Catellani, C.; Cirillo, F.; Deodati, A.; Fabbrizi, E.; Fanos, V.; et al. Current Knowledge on Endocrine Disrupting Chemicals (EDCs) from Animal Biology to Humans, from Pregnancy to Adulthood: Highlights from a National Italian Meeting. Int. J. Mol. Sci. 2018, 19, 1647. https://doi.org/10.3390/ijms19061647
Street ME, Angelini S, Bernasconi S, Burgio E, Cassio A, Catellani C, Cirillo F, Deodati A, Fabbrizi E, Fanos V, et al. Current Knowledge on Endocrine Disrupting Chemicals (EDCs) from Animal Biology to Humans, from Pregnancy to Adulthood: Highlights from a National Italian Meeting. International Journal of Molecular Sciences. 2018; 19(6):1647. https://doi.org/10.3390/ijms19061647
Chicago/Turabian StyleStreet, Maria Elisabeth, Sabrina Angelini, Sergio Bernasconi, Ernesto Burgio, Alessandra Cassio, Cecilia Catellani, Francesca Cirillo, Annalisa Deodati, Enrica Fabbrizi, Vassilios Fanos, and et al. 2018. "Current Knowledge on Endocrine Disrupting Chemicals (EDCs) from Animal Biology to Humans, from Pregnancy to Adulthood: Highlights from a National Italian Meeting" International Journal of Molecular Sciences 19, no. 6: 1647. https://doi.org/10.3390/ijms19061647
APA StyleStreet, M. E., Angelini, S., Bernasconi, S., Burgio, E., Cassio, A., Catellani, C., Cirillo, F., Deodati, A., Fabbrizi, E., Fanos, V., Gargano, G., Grossi, E., Iughetti, L., Lazzeroni, P., Mantovani, A., Migliore, L., Palanza, P., Panzica, G., Papini, A. M., ... Amarri, S. (2018). Current Knowledge on Endocrine Disrupting Chemicals (EDCs) from Animal Biology to Humans, from Pregnancy to Adulthood: Highlights from a National Italian Meeting. International Journal of Molecular Sciences, 19(6), 1647. https://doi.org/10.3390/ijms19061647