Lithocholic Acid Is a Vitamin D Receptor Ligand That Acts Preferentially in the Ileum
Abstract
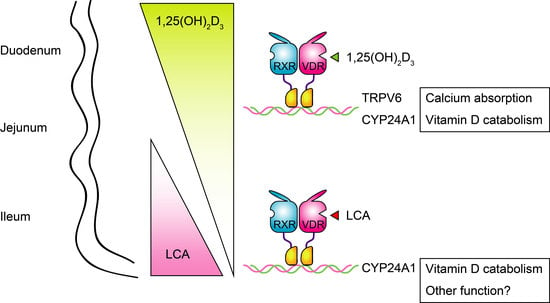
:1. Introduction
2. Results
2.1. LCA Induces CYP24A1 mRNA Expression in the Ileum but Not in the Duodemum or Jejunum
2.2. LCA Does Not Induce Intestinal Trpv6 Expression or Increase Plasma Calcium Levels
2.3. Effects of 1,25(OH)2D3 And LCA on Expression of VDR Target Genes in the Kidney
2.4. Ileal Cyp24a1 Induction by LCA Is Mediated by VDR Activation
3. Discussion
4. Materials and Methods
4.1. Animal Experiments
4.2. Reverse Transcrption and Real-Time Quantitative Polymerase Chain Reaction
4.3. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| VDR | vitamin D receptor |
| 1,25(OH)2D3 | 1α,25-dihydroxyvitamin D3 |
| RXR | retinoid X receptor |
| CYP | cytochrome P450 |
| TPPV | transient receptor potential vanilloid |
| LCA | lithocholic acid |
Appendix A

References
- Rosen, C.J.; Adams, J.S.; Bikle, D.D.; Black, D.M.; Demay, M.B.; Manson, J.E.; Murad, M.H.; Kovacs, C.S. The nonskeletal effects of vitamin d: An endocrine society scientific statement. Endocr. Rev. 2012, 33, 456–492. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. Vitamin d deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef] [PubMed]
- Plum, L.A.; DeLuca, H.F. Vitamin D, disease and therapeutic opportunities. Nat. Rev. Drug Discov. 2010, 9, 941–955. [Google Scholar] [CrossRef] [PubMed]
- Haussler, M.R.; Whitfield, G.K.; Kaneko, I.; Haussler, C.A.; Hsieh, D.; Hsieh, J.C.; Jurutka, P.W. Molecular mechanisms of vitamin d action. Calcif. Tissue Int. 2013, 92, 77–98. [Google Scholar] [CrossRef] [PubMed]
- Makishima, M.; Lu, T.T.; Xie, W.; Whitfield, G.K.; Domoto, H.; Evans, R.M.; Haussler, M.R.; Mangelsdorf, D.J. Vitamin d receptor as an intestinal bile acid sensor. Science 2002, 296, 1313–1316. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Radominska-Pandya, A.; Shi, Y.; Simon, C.M.; Nelson, M.C.; Ong, E.S.; Waxman, D.J.; Evans, R.M. An essential role for nuclear receptors sxr/pxr in detoxification of cholestatic bile acids. Proc. Natl. Acad. Sci. USA 2001, 98, 3375–3380. [Google Scholar] [CrossRef] [PubMed]
- Sawada, N.; Kusudo, T.; Sakaki, T.; Hatakeyama, S.; Hanada, M.; Abe, D.; Kamao, M.; Okano, T.; Ohta, M.; Inouye, K. Novel metabolism of 1α,25-dihydroxyvitamin D3 with C24-C25 bond cleavage catalyzed by human CYP24A1. Biochemistry 2004, 43, 4530–4537. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Hashizume, T.; Shuhart, M.C.; Davis, C.L.; Nelson, W.L.; Sakaki, T.; Kalhorn, T.F.; Watkins, P.B.; Schuetz, E.G.; Thummel, K.E. Intestinal and hepatic CYP3A4 catalyze hydroxylation of 1α,25-dihydroxyvitamin D3: Implications for drug-induced osteomalacia. Mol. Pharmacol. 2006, 69, 56–65. [Google Scholar] [PubMed]
- Adachi, R.; Honma, Y.; Masuno, H.; Kawana, K.; Shimomura, I.; Yamada, S.; Makishima, M. Selective activation of vitamin D receptor by lithocholic acid acetate, a bile acid derivative. J. Lipid Res. 2005, 46, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Gaspà, S.; Guañabens, N.; Enjuanes, A.; Peris, P.; Martinez-Ferrer, A.; Martinez de Osaba, M.J.; Gonzalez, B.; Alvarez, L.; Monegal, A.; Combalia, A.; et al. Lithocholic acid downregulates vitamin D effects in human osteoblasts. Eur. J. Clin. Investig. 2010, 40, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, A.F. Bile acids: Trying to understand their chemistry and biology with the hope of helping patients. Hepatology 2009, 49, 1403–1418. [Google Scholar] [CrossRef] [PubMed]
- Russell, D.W. Fifty years of advances in bile acid synthesis and metabolism. J. Lipid Res. 2009, 50, S120–S125. [Google Scholar] [CrossRef] [PubMed]
- Ridlon, J.M.; Kang, D.J.; Hylemon, P.B. Bile salt biotransformations by human intestinal bacteria. J. Lipid Res. 2006, 47, 241–259. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Chiang, J.Y. Bile acid signaling in metabolic disease and drug therapy. Pharmacol. Rev. 2014, 66, 948–983. [Google Scholar] [CrossRef] [PubMed]
- Makishima, M.; Yamada, S. Targeting the vitamin D receptor: Advances in drug discovery. Expert Opin. Ther. Pat. 2005, 15, 1133–1145. [Google Scholar] [CrossRef]
- Ishizawa, M.; Matsunawa, M.; Adachi, R.; Uno, S.; Ikeda, K.; Masuno, H.; Shimizu, M.; Iwasaki, K.; Yamada, S.; Makishima, M. Lithocholic acid derivatives act as selective vitamin D receptor modulators without inducing hypercalcemia. J. Lipid Res. 2008, 49, 763–772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamada, S.; Makishima, M. Structure-activity relationship of nonsecosteroidal vitamin d receptor modulators. Trends Pharmacol. Sci. 2014, 35, 324–337. [Google Scholar] [CrossRef] [PubMed]
- Ozeki, J.; Choi, M.; Endo-Umeda, K.; Sakurai, K.; Amano, S.; Makishima, M. Enhanced transcription of pancreatic peptide yy by 1α-hydroxyvitamin D3 administration in streptozotocin-induced diabetic mice. Neuropeptides 2013, 47, 329–332. [Google Scholar] [CrossRef] [PubMed]
- Benn, B.S.; Ajibade, D.; Porta, A.; Dhawan, P.; Hediger, M.; Peng, J.B.; Jiang, Y.; Oh, G.T.; Jeung, E.B.; Lieben, L.; et al. Active intestinal calcium transport in the absence of transient receptor potential vanilloid type 6 and calbindin-d9k. Endocrinology 2008, 149, 3196–3205. [Google Scholar] [CrossRef] [PubMed]
- Meyer, M.B.; Zella, L.A.; Nerenz, R.D.; Pike, J.W. Characterizing early events associated with the activation of target genes by 1,25-dihydroxyvitamin D3 in mouse kidney and intestine in vivo. J. Biol. Chem. 2007, 282, 22344–22352. [Google Scholar] [CrossRef] [PubMed]
- Nijenhuis, T.; Hoenderop, J.G.; Bindels, R.J. Trpv5 and trpv6 in Ca2+ (re)absorption: Regulating Ca2+ entry at the gate. Pflugers Arch. 2005, 451, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Ishizawa, M.; Iwasaki, K.; Kato, S.; Makishima, M. Hypergravity modulates vitamin D receptor target gene mrna expression in mice. Am. J. Physiol. Endocrinol. Metab. 2009, 297, E728–E734. [Google Scholar] [CrossRef] [PubMed]
- Makishima, M.; Okamoto, A.Y.; Repa, J.J.; Tu, H.; Learned, R.M.; Luk, A.; Hull, M.V.; Lustig, K.D.; Mangelsdorf, D.J.; Shan, B. Identification of a nuclear receptor for bile acids. Science 1999, 284, 1362–1365. [Google Scholar] [CrossRef] [PubMed]
- Staudinger, J.L.; Goodwin, B.; Jones, S.A.; Hawkins-Brown, D.; MacKenzie, K.I.; LaTour, A.; Liu, Y.; Klaassen, C.D.; Brown, K.K.; Reinhard, J.; et al. The nuclear receptor PXR is a lithocholic acid sensor that protects against liver toxicity. Proc. Natl. Acad. Sci. USA 2001, 98, 3369–3374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshizawa, T.; Handa, Y.; Uematsu, Y.; Takeda, S.; Sekine, K.; Yoshihara, Y.; Kawakami, T.; Arioka, K.; Sato, H.; Uchiyama, Y.; et al. Mice lacking the vitamin D receptor exhibit impaired bone formation, uterine hypoplasia and growth retardation after weaning. Nat. Genet. 1997, 16, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Ishizawa, M.; Akagi, D.; Yamamoto, J.; Makishima, M. 1α,25-Dihydroxyvitamin D3 enhances TRPV6 transcription through p38 mapk activation and GADD45 expression. J. Steroid Biochem. Mol. Biol. 2017, 172, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Bouillon, R.; Van Cromphaut, S.; Carmeliet, G. Intestinal calcium absorption: Molecular vitamin D mediated mechanisms. J. Cell. Biochem. 2003, 88, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Hollander, D.; Muralidhara, K.S.; Zimmerman, A. Vitamin D-3 intestinal absorption in vivo: Influence of fatty acids, bile salts, and perfusate ph on absorption. Gut 1978, 19, 267. [Google Scholar] [CrossRef] [PubMed]
- Emmanuelle, R.; Aurélie, G.; Christine, C.; Romain, B.; Marion, N.; Jean-François, L.; Dominique, J.R.; Claire, D.; Xavier, C.; Patrick, B. Vitamin D intestinal absorption is not a simple passive diffusion: Evidences for involvement of cholesterol transporters. Mol. Nutr. Food Res. 2011, 55, 691–702. [Google Scholar]
- Goff, J.P.; Koszewski, N.J.; Haynes, J.S.; Horst, R.L. Targeted delivery of vitamin d to the colon using β-glucuronides of vitamin D: Therapeutic effects in a murine model of inflammatory bowel disease. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 302, G460–G469. [Google Scholar] [CrossRef] [PubMed]
- Nishida, S.; Ozeki, J.; Makishima, M. Modulation of bile acid metabolism by 1α-hydroxyvitamin D3 administration in mice. Drug Metab. Dispos. 2009, 37, 2037–2044. [Google Scholar] [CrossRef] [PubMed]
- Nehring, J.A.; Zierold, C.; DeLuca, H.F. Lithocholic acid can carry out in vivo functions of vitamin D. Proc. Natl. Acad. Sci. USA 2007, 104, 10006–10009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maruyama, T.; Miyamoto, Y.; Nakamura, T.; Tamai, Y.; Okada, H.; Sugiyama, E.; Nakamura, T.; Itadani, H.; Tanaka, K. Identification of membrane-type receptor for bile acids (M-BAR). Biochem. Biophys. Res. Commun. 2002, 298, 714–719. [Google Scholar] [CrossRef]
- Kawamata, Y.; Fujii, R.; Hosoya, M.; Harada, M.; Yoshida, H.; Miwa, M.; Fukusumi, S.; Habata, Y.; Itoh, T.; Shintani, Y.; et al. A G protein-coupled receptor responsive to bile acids. J. Biol. Chem. 2003, 278, 9435–9440. [Google Scholar] [CrossRef] [PubMed]
- Matsubara, T.; Yoshinari, K.; Aoyama, K.; Sugawara, M.; Sekiya, Y.; Nagata, K.; Yamazoe, Y. Role of vitamin D receptor in the lithocholic acid-mediated CYP3A induction in vitro and in vivo. Drug Metab. Dispos. 2008, 36, 2058–2063. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Zhang, Y.G.; Lu, R.; Xia, Y.; Zhou, D.; Petrof, E.O.; Claud, E.C.; Chen, D.; Chang, E.B.; Carmeliet, G.; et al. Intestinal epithelial vitamin D receptor deletion leads to defective autophagy in colitis. Gut 2015, 64, 1082–1094. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.T.; Stenger, S.; Li, H.; Wenzel, L.; Tan, B.H.; Krutzik, S.R.; Ochoa, M.T.; Schauber, J.; Wu, K.; Meinken, C.; et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science 2006, 311, 1770–1773. [Google Scholar] [CrossRef] [PubMed]
- Bakke, D.; Sun, J. Ancient nuclear receptor VDR with new functions: Microbiome and inflammation. Inflamm. Bowel Dis. 2018, 24, 1149–1154. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Glowacki, J. Chronic kidney disease and vitamin D metabolism in human bone marrow-derived mscs. Ann. N. Y. Acad. Sci. 2017, 1402, 43–55. [Google Scholar] [CrossRef] [PubMed]
- Basoli, V.; Santaniello, S.; Cruciani, S.; Ginesu, G.C.; Cossu, M.L.; Delitala, A.P.; Serra, P.A.; Ventura, C.; Maioli, M. Melatonin and vitamin D interfere with the adipogenic fate of adipose-derived stem cells. Int. J. Mol. Sci. 2017, 18, 981. [Google Scholar] [CrossRef] [PubMed]
- Shulman, A.I.; Larson, C.; Mangelsdorf, D.J.; Ranganathan, R. Structural determinants of allosteric ligand activation in RXR heterodimers. Cell 2004, 116, 417–429. [Google Scholar] [CrossRef]
- Hsieh, J.-C.; Sisk, J.M.; Jurutka, P.W.; Haussler, C.A.; Slater, S.A.; Haussler, M.R.; Thompson, C.C. Physical and functional interaction between the vitamin D receptor and hairless corepressor, two proteins required for hair cycling. J. Biol. Chem. 2003, 278, 38665–38674. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.; Yamada, S.; Makishima, M. Dynamic and ligand-selective interactions of vitamin D receptor with retinoid X receptor and cofactors in living cells. Mol. Pharmacol. 2011, 80, 1147–1155. [Google Scholar] [CrossRef] [PubMed]
- Chuma, M.; Endo-Umeda, K.; Shimba, S.; Yamada, S.; Makishima, M. Hairless modulates ligand-dependent activation of the vitamin D receptor-retinoid x receptor heterodimer. Biol. Pharm. Bull. 2012, 35, 582–587. [Google Scholar] [CrossRef] [PubMed]
- Heyman, R.A.; Mangelsdorf, D.J.; Dyck, J.A.; Stein, R.B.; Eichele, G.; Evans, R.M.; Thaller, C. 9-cis retinoic acid is a high affinity ligand for the retinoid X receptor. Cell 1992, 68, 397–406. [Google Scholar] [CrossRef]
- Kane, M.A.; Folias, A.E.; Pingitore, A.; Perri, M.; Obrochta, K.M.; Krois, C.R.; Cione, E.; Ryu, J.Y.; Napoli, J.L. Identification of 9-cis-retinoic acid as a pancreas-specific autacoid that attenuates glucose-stimulated insulin secretion. Proc. Nat. Acad. Sci. USA 2010, 107, 21884–21889. [Google Scholar] [CrossRef] [PubMed]
- De Urquiza, A.M.; Liu, S.; Sjoberg, M.; Zetterstrom, R.H.; Griffiths, W.; Sjovall, J.; Perlmann, T. Docosahexaenoic acid, a ligand for the retinoid x receptor in mouse brain. Science 2000, 290, 2140–2144. [Google Scholar] [CrossRef] [PubMed]
- Niu, H.; Fujiwara, H.; di Martino, O.; Hadwiger, G.; Frederick, T.E.; Menéndez-Gutiérrez, M.P.; Ricote, M.; Bowman, G.R.; Welch, J.S. Endogenous retinoid X receptor ligands in mouse hematopoietic cells. Sci. Signal. 2017, 10. [Google Scholar] [CrossRef] [PubMed]
- Weber, K.; Erben, R.G. Differences in triglyceride and cholesterol metabolism and resistance to obesity in male and female vitamin D receptor knockout mice. J. Anim. Physiol. Anim. Nutr. 2013, 97, 675–683. [Google Scholar] [CrossRef] [PubMed]
- Ryan, J.W.; Starczak, Y.; Tsangari, H.; Sawyer, R.K.; Davey, R.A.; Atkins, G.J.; Morris, H.A.; Anderson, P.H. Sex-related differences in the skeletal phenotype of aged vitamin D receptor global knockout mice. J. Steroid Biochem. Mol. Biol. 2016, 164, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Jena, P.K.; Sheng, L.; Liu, H.X.; Kalanetra, K.M.; Mirsoian, A.; Murphy, W.J.; French, S.W.; Krishnan, V.V.; Mills, D.A.; Wan, Y.Y. Western diet-induced dysbiosis in farnesoid X receptor knockout mice causes persistent hepatic inflammation after antibiotic treatment. Am. J. Pathol. 2017, 187, 1800–1813. [Google Scholar] [CrossRef] [PubMed]
- Ishizawa, M.; Ogura, M.; Kato, S.; Makishima, M. Impairment of bilirubin clearance and intestinal interleukin-6 expression in bile duct-ligated vitamin D receptor null mice. PLoS ONE 2012, 7, e51664. [Google Scholar] [CrossRef] [PubMed]




© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ishizawa, M.; Akagi, D.; Makishima, M. Lithocholic Acid Is a Vitamin D Receptor Ligand That Acts Preferentially in the Ileum. Int. J. Mol. Sci. 2018, 19, 1975. https://doi.org/10.3390/ijms19071975
Ishizawa M, Akagi D, Makishima M. Lithocholic Acid Is a Vitamin D Receptor Ligand That Acts Preferentially in the Ileum. International Journal of Molecular Sciences. 2018; 19(7):1975. https://doi.org/10.3390/ijms19071975
Chicago/Turabian StyleIshizawa, Michiyasu, Daisuke Akagi, and Makoto Makishima. 2018. "Lithocholic Acid Is a Vitamin D Receptor Ligand That Acts Preferentially in the Ileum" International Journal of Molecular Sciences 19, no. 7: 1975. https://doi.org/10.3390/ijms19071975
APA StyleIshizawa, M., Akagi, D., & Makishima, M. (2018). Lithocholic Acid Is a Vitamin D Receptor Ligand That Acts Preferentially in the Ileum. International Journal of Molecular Sciences, 19(7), 1975. https://doi.org/10.3390/ijms19071975