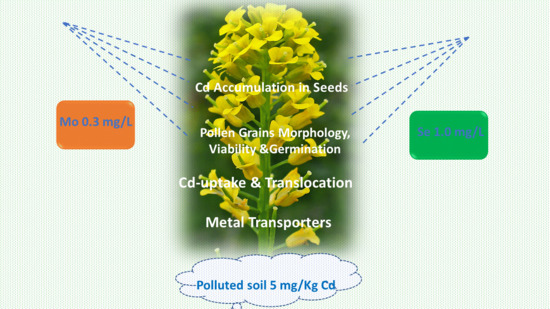
Can Selenium and Molybdenum Restrain Cadmium Toxicity to Pollen Grains in Brassica napus?
Abstract
:1. Introduction
2. Results
2.1. Plant Biomass
2.2. Cd Concentration and Accumulation
2.3. Se and Mo Contents in Oilseed Rape
2.4. Anther and Pollen Grains Morphology
2.4.1. Microscopic Studies
2.4.2. Scanning Electron Microscopy
2.5. Pollen Grains Fertility by I2/KI (Iodine) Staining
2.6. Pollen Germination and Pollen Tube Growth In Vitro
2.7. Relative Expression Analysis of Genes Related to Cd Uptake, Transport, and Detoxification
3. Discussion
4. Materials and Methods
4.1. Soil Properties, Selected Plant Species, and Experimental Setup
4.1.1. Soil Properties
4.1.2. Selected Plants
4.1.3. Pot Setup and Monitoring
4.2. Pollen Grain Studies
4.2.1. Development
4.2.2. Morphology by SEM
4.2.3. Fertility Using I2/KI Solution
4.2.4. In Vitro Germination
4.3. Analytical Tests
4.4. Total RNA Extraction and Quantitative RT-PCR
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gratão, P.L.; Polle, A.; Lea, P.J.; Azevedo, R.A. Making the life of heavy metal-stressed plants a little easier. Funct. Plant Biol. 2005, 32, 481–494. [Google Scholar] [CrossRef]
- Biao, Z.J.; Nan, H.W. Advances on physiological and ecological effects of cadmium on plants. Acta Ecol. Sin. 2000, 20, 514–523. [Google Scholar]
- Raskin, I.; Smith, R.D.; Salt, D.E. Phytoremediation of metals: Using plants to remove pollutants from the environment. Curr. Opin. Biotechnol. 1997, 8, 221–226. [Google Scholar] [CrossRef]
- Sanità di Toppi, L.; Gabbrielli, R. Response to cadmium in higher plants. Environ. Exp. Bot. 1999, 41, 105–130. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef] [PubMed]
- Selvam, A.; Wong, J.W.-C. Cadmium uptake potential of Brassica napus cocropped with Brassica parachinensis and Zea mays. J. Hazard. Mater. 2009, 167, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Ali, B.; Qian, P.; Jin, R.; Ali, S.; Khan, M.; Aziz, R.; Tian, T.; Zhou, W. Physiological and ultra-structural changes in Brassica napus seedlings induced by cadmium stress. Biol. Plant. 2014, 58, 131–138. [Google Scholar] [CrossRef]
- Zhang, W.; Rengel, Z.; Kuo, J.; Yan, G. Aluminium effects on pollen germination and tube growth of Chamelaucium uncinatum. A comparison with other Ca2+ antagonists. Ann. Bot. 1999, 84, 559–564. [Google Scholar] [CrossRef]
- Sawidis, T.; Reiss, H.-D. Effects of heavy metals on pollen tube growth and ultrastructure. Protoplasma 1995, 185, 113–122. [Google Scholar] [CrossRef]
- Strickland, R.C.; Chaney, W.R. Cadmium influence on respiratory gas exchange of Pinus resinosa pollen. Physiol. Plant. 1979, 47, 129–133. [Google Scholar] [CrossRef]
- Sabrine, H.; Afif, H.; Mohamed, B.; Hamadi, B.; Maria, H. Effects of cadmium and copper on pollen germination and fruit set in pea (Pisum sativum L.). Sci. Hortic. 2010, 125, 551–555. [Google Scholar] [CrossRef]
- Holub, Z.; Ostrolucka, G. The effect of cadmium (II) and lead (II) on pollen germination and pollen tube growth in Quercus cerris, Pinus nigra and Picea abies. Biologia 1983, 38, 393–400. [Google Scholar]
- Wang, X.; Gao, Y.; Feng, Y.; Li, X.; Wei, Q.; Sheng, X. Cadmium stress disrupts the endomembrane organelles and endocytosis during Picea wilsonii pollen germination and tube growth. PLoS ONE 2014, 9, e94721. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Dhingra, H. Sexual reproduction and cadmium partitioning in two mungbean genotypes raised in soils contaminated with cadmium. Indian J. Plant Physiol. 2005, 10, 151. [Google Scholar]
- Kaur, N.; Sharma, S.; Kaur, S.; Nayyar, H. Selenium in agriculture: A nutrient or contaminant for crops? Arch. Agronom. Soil Sci. 2014, 60, 1593–1624. [Google Scholar] [CrossRef]
- Rayman, M.P. The importance of selenium to human health. Lancet 2000, 356, 233–241. [Google Scholar] [CrossRef] [Green Version]
- Hasanuzzaman, M.; Hossain, M.A.; Fujita, M. Selenium in higher plants: Physiological role, antioxidant metabolism and abiotic stress tolerance. J. Plant Sci. 2010, 5, 354–375. [Google Scholar]
- Feng, R.; Wei, C.; Tu, S. The roles of selenium in protecting plants against abiotic stresses. Environ. Exp. Bot. 2013, 87, 58–68. [Google Scholar] [CrossRef]
- Filek, M.; Zembala, M.; Hartikainen, H.; Miszalski, Z.; Kornaś, A.; Wietecka-Posłuszny, R.; Walas, P. Changes in wheat plastid membrane properties induced by cadmium and selenium in presence/absence of 2, 4-dichlorophenoxyacetic acid. Plant Cell Tissue Organ Cult. 2009, 96, 19–28. [Google Scholar] [CrossRef]
- Hristozkova, M.; Geneva, M.; Stancheva, I. Response of pea plants (Pisum sativum L.) to reduced supply with molybdenum and copper. Int. J. Agric. Biol. 2006, 8, 218–220. [Google Scholar]
- Vieira, R.F.; Cardoso, E.J.B.N.; Vieira, C.; Cassini, S.T.A. Foliar application of molybdenum in common beans. I. Nitrogenase and reductase activities in a soil of high fertility. J. Plant Nutr. 1998, 21, 169–180. [Google Scholar] [CrossRef]
- Agarwala, S.C.; Chatterjee, C.; Sharma, P.N.; Sharma, C.P.; Nautiyal, N. Pollen development in maize plants subjected to molybdenum deficiency. Can. J. Bot. 1979, 57, 1946–1950. [Google Scholar] [CrossRef]
- Marschner, H. Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Academic press: Cambridge, MA, USA, 2011. [Google Scholar]
- Gupta, U.C. Effect of methods of application and residual effect of molybdenum on the molybdenum concentration and yield of forages on podzol soils. Can. J. Soil Sci. 1979, 59, 183–189. [Google Scholar] [CrossRef]
- Kaiser, B.N.; Gridley, K.L.; Ngaire Brady, J.; Phillips, T.; Tyerman, S.D. The role of molybdenum in agricultural plant production. Ann. Bot. 2005, 96, 745–754. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Hu, C.; Zhao, X.; Tan, Q.; Sun, X.; Cao, A.; Cui, M.; Zhang, Y. Molybdenum improves antioxidant and osmotic-adjustment ability against salt stress in Chinese cabbage (Brassica Campestris L. Ssp. Pekinensis). Plant Soil 2012, 355, 375–383. [Google Scholar] [CrossRef]
- Yamane, Y.; Fukuchi, M.; Li, C.; Koizumi, T. Protective effect of sodium molybdate against the acute toxicity of cadmium chloride. Toxicology 1990, 60, 235–243. [Google Scholar] [CrossRef]
- Hadi, F.; Ali, N.; Fuller, M.P. Molybdenum (Mo) increases endogenous phenolics, proline and photosynthetic pigments and the phytoremediation potential of the industrially important plant Ricinus communis L. for removal of cadmium from contaminated soil. Environ. Sci. Pollut. Res. 2016, 23, 20408–20430. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, R.; Bashir, K.; Ishimaru, Y.; Nishizawa, N.K.; Nakanishi, H. The role of heavy-metal ATPases, HMAs, in zinc and cadmium transport in rice. Plant Signal. Behav. 2012, 7, 1605–1607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Connolly, E.L.; Fett, J.P.; Guerinot, M.L. Expression of the IRT1 metal transporter is controlled by metals at the levels of transcript and protein accumulation. Plant Cell 2002, 14, 1347–1357. [Google Scholar] [CrossRef] [PubMed]
- Hussain, D.; Haydon, M.J.; Wang, Y.; Wong, E.; Sherson, S.M.; Young, J.; Camakaris, J.; Harper, J.F.; Cobbett, C.S. P-type ATPase heavy metal transporters with roles in essential zinc homeostasis in Arabidopsis. Plant Cell 2004, 16, 1327–1339. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.K.E.; Cobbett, C.S. HMA P-type ATPases are the major mechanism for root-to-shoot Cd translocation in Arabidopsis thaliana. New Phytol. 2009, 181, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Chao, D.-Y.; Silva, A.; Baxter, I.; Huang, Y.S.; Nordborg, M.; Danku, J.; Lahner, B.; Yakubova, E.; Salt, D.E. Genome-wide association studies identify heavy metal ATPase3 as the primary determinant of natural variation in leaf cadmium in Arabidopsis thaliana. PLoS. Genet. 2012, 8, e1002923. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mendoza-Cózatl, D.G.; Jobe, T.O.; Hauser, F.; Schroeder, J.I. Long-distance transport, vacuolar sequestration, tolerance, and transcriptional responses induced by cadmium and arsenic. Curr. Opin. Plant Biol. 2011, 14, 554–562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mendoza-Cózatl David, G.; Butko, E.; Springer, F.; Torpey Justin, W.; Komives Elizabeth, A.; Kehr, J.; Schroeder Julian, I. Identification of high levels of phytochelatins, glutathione and cadmium in the phloem sap of Brassica napus. A role for thiol-peptides in the long-distance transport of cadmium and the effect of cadmium on iron translocation. Plant J. 2008, 54, 249–259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heslop-Harrison, P. Genetics, genomics and breeding of oilseed brassicas. Ann. Bot. 2013, 112. [Google Scholar] [CrossRef] [Green Version]
- John, R.; Ahmad, P.; Gadgil, K.; Sharma, S. Heavy metal toxicity: Effect on plant growth, biochemical parameters and metal accumulation by Brassica juncea L. Int. J. Plant Prod. 2012, 3, 65–76. [Google Scholar]
- El-Ramady, H.; Abdalla, N.; Taha, H.S.; Alshaal, T.; El-Henawy, A.; Salah, E.-D.F.; Shams, M.S.; Youssef, S.M.; Shalaby, T.; Bayoumi, Y. Selenium and nano-selenium in plant nutrition. Environ. Chem. Lett. 2016, 14, 123–147. [Google Scholar] [CrossRef]
- Škarpa, P.; Kunzova, E.; Zukalova, H. Foliar fertilization with molybdenum in sunflower (Helianthus annuus L.). Plant Soil Environ. 2013, 59, 156–161. [Google Scholar] [CrossRef]
- Steiner, F.; Zoz, T. Foliar application of molybdenum improves nitrogen uptake and yield of sunflower. Afr. J. Agr. Res. 2015, 10, 1923–1928. [Google Scholar]
- Liu, H.; Hu, C.; Sun, X.; Tan, Q.; Nie, Z.; Su, J.; Liu, J.; Huang, H. Interactive effects of molybdenum and phosphorus fertilizers on grain yield and quality of Brassica napus. J. Food Agric. Environ. 2009, 7, 266–269. [Google Scholar]
- Zhang, M.; Hu, C.; Sun, X.; Zhao, X.; Tan, Q.; Zhang, Y.; Li, N. Molybdenum affects photosynthesis and ionic homeostasis of Chinese cabbage under salinity stress. Commun. Soil Sci. Plant Anal. 2014, 45, 2660–2672. [Google Scholar] [CrossRef]
- Wu, S.; Hu, C.; Tan, Q.; Xu, S.; Sun, X. Nitric oxide mediates molybdenum-induced antioxidant defense in wheat under drought stress. Front. Plant Sci. 2017, 8, 1085. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Hu, C.; Zhao, X.; Tan, Q.; Sun, X.; Li, N. Impact of molybdenum on Chinese cabbage response to selenium in solution culture. Soil Sci. Plant Nutr. 2012, 58, 595–603. [Google Scholar] [CrossRef] [Green Version]
- Hopper, J.L.; Parker, D.R. Plant availability of selenite and selenate as influenced by the competing ions phosphate and sulfate. Plant Soil 1999, 210, 199–207. [Google Scholar] [CrossRef]
- Ramos, S.J.; Rutzke, M.A.; Hayes, R.J.; Faquin, V.; Guilherme, L.R.G.; Li, L. Selenium accumulation in lettuce germplasm. Planta 2011, 233, 649–660. [Google Scholar] [CrossRef] [PubMed]
- Schiavon, M.; Pittarello, M.; Pilon-Smits, E.A.H.; Wirtz, M.; Hell, R.; Malagoli, M. Selenate and molybdate alter sulfate transport and assimilation in Brassica juncea L. Czern.: Implications for phytoremediation. Environ. Exp. Bot. 2012, 75, 41–51. [Google Scholar] [CrossRef]
- DeTar, R.A.; Alford, É.R.; Pilon-Smits, E.A.H. Molybdenum accumulation, tolerance and molybdenum–selenium–sulfur interactions in astragalus selenium hyperaccumulator and nonaccumulator species. J. Plant Physiol. 2015, 183, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Filek, M.; Keskinen, R.; Hartikainen, H.; Szarejko, I.; Janiak, A.; Miszalski, Z.; Golda, A. The protective role of selenium in rape seedlings subjected to cadmium stress. J. Plant Physiol. 2008, 165, 833–844. [Google Scholar] [CrossRef] [PubMed]
- Saidi, I.; Chtourou, Y.; Djebali, W. Selenium alleviates cadmium toxicity by preventing oxidative stress in sunflower (Helianthus annuus) seedlings. J. Plant Physiol. 2014, 171, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Mroczek-Zdyrska, M.; Strubińska, J.; Hanaka, A. Selenium improves physiological parameters and alleviates oxidative stress in shoots of lead-exposed Vicia faba L. minor plants grown under phosphorus-deficient conditions. J. Plant Growth Regul. 2017, 36, 186–199. [Google Scholar] [CrossRef]
- Wu, Z.; Yin, X.; Bañuelos, G.S.; Lin, Z.-Q.; Liu, Y.; Li, M.; Yuan, L. Indications of selenium protection against cadmium and lead toxicity in oilseed rape (Brassica napus L.). Front. Plant Sci. 2016, 7, 1875. [Google Scholar] [CrossRef] [PubMed]
- Qing, X.; Zhao, X.; Hu, C.; Wang, P.; Zhang, Y.; Zhang, X.; Wang, P.; Shi, H.; Jia, F.; Qu, C. Selenium alleviates chromium toxicity by preventing oxidative stress in cabbage (Brassica campestris L. ssp. Pekinensis) leaves. Ecotoxicol. Environ. Saf. 2015, 114, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Gajewska, E.; Drobik, D.; Wielanek, M.; Sekulska-Nalewajko, J.; Gocławski, J.; Mazur, J.; Skłodowska, M. Alleviation of nickel toxicity in wheat (Triticum aestivum L.) seedlings by selenium supplementation. Biol. Lett. 2013, 50, 65–78. [Google Scholar] [CrossRef]
- Wang, X.; Tam, N.F.-Y.; Fu, S.; Ametkhan, A.; Ouyang, Y.; Ye, Z. Selenium addition alters mercury uptake, bioavailability in the rhizosphere and root anatomy of rice (Oryza sativa). Ann. Bot. 2014, 114, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Chao, C.Y.; Ma, D.J. The Effects of Selenium on Toxicity of Copper on Rape. Adv. Mater. Res. 2013, 610–613, 288–291. [Google Scholar] [CrossRef]
- Han, D.; Xiong, S.; Tu, S.; Liu, J.; Chen, C. Interactive effects of selenium and arsenic on growth, antioxidant system, arsenic and selenium species of Nicotiana tabacum L. Environ. Exp. Bot. 2015, 117, 12–19. [Google Scholar] [CrossRef]
- Ding, Y.; Wang, R.; Guo, J.; Wu, F.; Xu, Y.; Feng, R. The effect of selenium on the subcellular distribution of antimony to regulate the toxicity of antimony in paddy rice. Environ. Sci. Pollut. Res. 2015, 22, 5111–5123. [Google Scholar] [CrossRef] [PubMed]
- Landberg, T.; Greger, M. Influence of selenium on uptake and toxicity of copper and cadmium in pea (Pisum sativum) and wheat (Triticum aestivum). Physiol. Plant. 1994, 90, 637–644. [Google Scholar] [CrossRef]
- Bluemlein, K.; Klimm, E.; Raab, A.; Feldmann, J. Selenite enhances arsenate toxicity in Thunbergia alata. Environ. Chem. 2009, 6, 486–494. [Google Scholar] [CrossRef]
- Cartes, P.; Jara, A.A.; Pinilla, L.; Rosas, A.; Mora, M.L. Selenium improves the antioxidant ability against aluminium–induced oxidative stress in ryegrass roots. Ann. Appl. Biol. 2010, 156, 297–307. [Google Scholar] [CrossRef]
- Fargasova, A.; Pastierová, J.; Svetková, K. Effect of Se-metal pair combinations (Cd, Zn, Cu, Pb) on photosynthetic pigments production and metal accumulation in Sinapis alba L. Seedlings. Plant Soil Environ. 2006, 52, 8–15. [Google Scholar] [CrossRef]
- Zhang, M.; Hu, C.; Zhao, X.; Tan, Q.; Sun, X. Co-application of molybdenum and selenium fertilizers increase uptake, recovery and harvest index of molybdenum and selenium in pepper crop. J. Plant Nutr. 2016, 39, 244–251. [Google Scholar] [CrossRef]
- Yousefi, N.; Chehregani, A.; Malayeri, B.; Lorestani, B.; Cheraghi, M. Investigating the effect of heavy metals on developmental stages of anther and pollen in Chenopodium botrys L. (Chenopodiaceae). Biol. Trace Elem. Res. 2011, 140, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Albooghobaish, N.; Zarinkamar, F. Effect of Lead Toxicity on Pollen grains in Matricaria chamomilla. In Proceedings of the International Conference on Bioscience, Biochemistry and Bioinformatics, Singapore, 26–28 February 2011; pp. 292–295. [Google Scholar]
- Torabi, F.; Majd, A.; Enteshari, S.; Irian, S.; Nabiuni, M. Effects of salinity on the development of hydroponically grown borage (Borago officinalis L.) male gametophyte. Not. Bot. Horti Agrobot. Cluj Napoca 2013, 41, 8. [Google Scholar] [CrossRef]
- Jiang, J.; Zhang, Z.; Cao, J. Pollen wall development: The associated enzymes and metabolic pathways. Plant Biol. 2013, 15, 249–263. [Google Scholar] [CrossRef] [PubMed]
- Møller, I.M. A more general mechanism of cytoplasmic male fertility? Trends Plant Sci. 2001, 6, 560. [Google Scholar] [CrossRef]
- Tedeschini, E.; Proietti, P.; Timorato, V.; D’Amato, R.; Nasini, L.; Dei Buono, D.; Businelli, D.; Frenguelli, G. Selenium as stressor and antioxidant affects pollen performance in Olea europaea. Flora-Morphol. Distrib. Funct. Ecol. Plants 2015, 215, 16–22. [Google Scholar] [CrossRef]
- Agarwala, S.; Sharma, C.; Farooq, S.; Chatterjee, C. Effect of molybdenum deficiency on the growth and metabolism of corn plants raised in sand culture. Can. J. Bot. 1978, 56, 1905–1908. [Google Scholar] [CrossRef]
- Levent, T.A.; Bürün, B.; Yokaş, İ.; Coban, E. The effects of heavy metals on pollen germination and pollen tube length in the tobacco plant. Turk. J. Biol. 2002, 26, 109–113. [Google Scholar]
- Xiong, Z.T.; Peng, Y.H. Response of pollen germination and tube growth to cadmium with special reference to low concentration exposure. Ecotoxicol. Environ. Saf. 2001, 48, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Sawidis, T. Effect of cadmium on pollen germination and tube growth in Lilium longiflorum and Nicotiana tabacum. Protoplasma 2008, 233, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Searcy, K.; Mulcahy, D. The parallel expression of metal tolerance in pollen and sporophytes of Silene dioica (L.) Clairv., S. alba (mill.) krause and Mimulus guttatus DC. Theor. Appl. Genet. 1985, 69, 597–602. [Google Scholar] [CrossRef] [PubMed]
- De Flora, S.; Bennicelli, C.; Bagnasco, M. Genotoxicity of mercury compounds. A review. Mut. Res. Rev. Genet. Toxicol. 1994, 317, 57–79. [Google Scholar] [CrossRef]
- Eshghi, S.; da Silva, J.A.T.; Ranjbar, R. Molybdenum and boron affect pollen germination of strawberry and fertile and infertile flowers of pomegranate. Fruit Veg. Cereal Sci. Biotechnol. 2010, 4, 148–150. [Google Scholar]
- Raohavan, V.; Baruah, H. Effect of time factor on the stimulation of pollen germination and pollen tube growth by certain auxins, vitamins, and trace elements. Physiol. Plant. 1959, 12, 441–451. [Google Scholar] [CrossRef]
- Bruyn, J.D. The in vitro germination of pollen of Setaria sphacelata. Physiol. Plant. 1966, 19, 365–376. [Google Scholar] [CrossRef]
- Korshunova, Y.O.; Eide, D.; Clark, W.G.; Guerinot, M.L.; Pakrasi, H.B. The IRT1 protein from Arabidopsis thaliana is a metal transporter with a broad substrate range. Plant Mol. Biol. 1999, 40, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Rogers, E.E.; Eide, D.J.; Guerinot, M.L. Altered selectivity in an Arabidopsis metal transporter. Proc. Natl. Acad. Sci. USA 2000, 97, 12356–12360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clemens, S.; Palmgren, M.G.; Krämer, U. A long way ahead: Understanding and engineering plant metal accumulation. Trends Plant Sci. 2002, 7, 309–315. [Google Scholar] [CrossRef]
- Verret, F.; Gravot, A.; Auroy, P.; Leonhardt, N.; David, P.; Nussaume, L.; Vavasseur, A.; Richaud, P. Overexpression of AtHMA4 enhances root-to-shoot translocation of zinc and cadmium and plant metal tolerance. FEBS Lett. 2004, 576, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Mills, R.F.; Francini, A.; Ferreira Da Rocha, P.S.C.; Baccarini, P.J.; Aylett, M.; Krijger, G.C.; Williams, L.E. The plant P1B-type ATPase AtHMA4 transports Zn and Cd and plays a role in detoxification of transition metals supplied at elevated levels. FEBS Lett. 2005, 579, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Zhao, X.; Sun, X.; Tan, Q.; Tang, Y.; Nie, Z.; Hu, C. Xylem transport and gene expression play decisive roles in cadmium accumulation in shoots of two oilseed rape cultivars (Brassica napus). Chemosphere 2015, 119, 1217–1223. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.K.E.; Jarvis, R.S.; Sherson, S.M.; Cobbett, C.S. Functional analysis of the heavy metal binding domains of the Zn/Cd-transporting ATPase, HMA2, in arabidopsis thaliana. New Phytol. 2009, 181, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Ueno, D.; Milner Matthew, J.; Yamaji, N.; Yokosho, K.; Koyama, E.; Clemencia Zambrano, M.; Kaskie, M.; Ebbs, S.; Kochian Leon, V.; Ma Jian, F. Elevated expression of TcHMA3 plays a key role in the extreme Cd tolerance in a Cd-hyperaccumulating ecotype of Thlaspi caerulescens. Plant J. 2011, 66, 852–862. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, M.; Shohag, M.J.I.; Tian, S.; Song, H.; Feng, Y.; Yang, X. Enhanced expression of SaHMA3 plays critical roles in Cd hyperaccumulation and hypertolerance in Cd hyperaccumulator Sedum alfredii Hance. Planta 2016, 243, 577–589. [Google Scholar] [CrossRef] [PubMed]
- Ueno, D.; Yamaji, N.; Kono, I.; Huang, C.F.; Ando, T.; Yano, M.; Ma, J.F. Gene limiting cadmium accumulation in rice. Proc. Natl. Acad. Sci. USA 2010, 107, 16500–16505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sasaki, A.; Yamaji, N.; Ma, J.F. Overexpression of OsHMA3 enhances Cd tolerance and expression of Zn transporter genes in rice. J. Exp. Bot. 2014, 65, 6013–6021. [Google Scholar] [CrossRef] [PubMed]
- Hidenori, M.; Saki, A.; Aya, H.; Kouichi, T.; Nobushige, N.; Tomohiko, K.; Kazunao, K.; Ikuko, K.; Kenji, S.; Hidekazu, T.; et al. OsHMA3, a P1B-type of ATPase affects root-to-shoot cadmium translocation in rice by mediating efflux into vacuoles. New Phytol. 2011, 189, 190–199. [Google Scholar]
- Howden, R.; Goldsbrough, P.B.; Andersen, C.R.; Cobbett, C.S. Cadmium-sensitive, cad1 mutants of Arabidopsis thaliana are phytochelatin deficient. Plant Physiol. 1995, 107, 1059. [Google Scholar] [CrossRef] [PubMed]
- Lillie, R.D. Histopathologic Technic and Practical Histochemistry, 3rd ed.; Blakiston Division: New York, NY, USA, 1965. [Google Scholar]
- Chang, F.; Zhang, Z.; Jin, Y.; Ma, H. Cell biological analyses of anther morphogenesis and pollen viability in arabidopsis and rice. In Flower Development: Methods and Protocols; Riechmann, J.L., Wellmer, F., Eds.; Springer: New York, NY, USA, 2014; pp. 203–216. [Google Scholar]
- Shivanna, K.R.; Sawhney, V.K. Polyethylene glycol improves the in vitro growth of brassica pollen tubes without loss in germination. J. Exp. Bot. 1995, 46, 1771–1774. [Google Scholar] [CrossRef]
- Searcy, K.; Mulcahy, D. Comparison of the response to aluminum toxicity in gametophyte and sporophyte of four tomato (Lycopersicon esculentum Mill.) cultivars. Theor. Appl. Genet. 1990, 80, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Sakan, S.; Đorđević, D.; Dević, G.; Relić, D.; Anđelković, I.; Ðuričić, J. A study of trace element contamination in river sediments in serbia using microwave-assisted aqua regia digestion and multivariate statistical analysis. Microchem. J. 2011, 99, 492–502. [Google Scholar] [CrossRef]
- Wan, Y.H. Determination of soil available molybdenum and plant molybdenum by polarographic catalytic wave analysis. Chin. J. Soil Sci. 1988, 19, 43–46. [Google Scholar]
- Li, P.; Dong, Q.; Ge, S.; He, X.; Verdier, J.; Li, D.; Zhao, J. Metabolic engineering of proanthocyanidin production by repressing the isoflavone pathways and redirecting anthocyanidin precursor flux in legume. Plant Biotechnol. J. 2016, 14, 1604–1618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative pcr and the 2−ΔΔCt method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Zhu, Y.; Song, J.; Xu, L.; Sun, C.; Zhang, X.; Xu, Y.; He, L.; Sun, W.; Xu, H.; et al. Transcriptional data mining of Salvia miltiorrhiza in response to methyl jasmonate to examine the mechanism of bioactive compound biosynthesis and regulation. Physiol. Plant. 2014, 152, 241–255. [Google Scholar] [CrossRef] [PubMed]












© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ismael, M.A.; Elyamine, A.M.; Zhao, Y.Y.; Moussa, M.G.; Rana, M.S.; Afzal, J.; Imran, M.; Zhao, X.H.; Hu, C.X. Can Selenium and Molybdenum Restrain Cadmium Toxicity to Pollen Grains in Brassica napus? Int. J. Mol. Sci. 2018, 19, 2163. https://doi.org/10.3390/ijms19082163
Ismael MA, Elyamine AM, Zhao YY, Moussa MG, Rana MS, Afzal J, Imran M, Zhao XH, Hu CX. Can Selenium and Molybdenum Restrain Cadmium Toxicity to Pollen Grains in Brassica napus? International Journal of Molecular Sciences. 2018; 19(8):2163. https://doi.org/10.3390/ijms19082163
Chicago/Turabian StyleIsmael, Marwa A., Ali Mohamed Elyamine, Yuan Yuan Zhao, Mohamed G. Moussa, Muhammad Shoaib Rana, Javaria Afzal, Muhammad Imran, Xiao Hu Zhao, and Cheng Xiao Hu. 2018. "Can Selenium and Molybdenum Restrain Cadmium Toxicity to Pollen Grains in Brassica napus?" International Journal of Molecular Sciences 19, no. 8: 2163. https://doi.org/10.3390/ijms19082163
APA StyleIsmael, M. A., Elyamine, A. M., Zhao, Y. Y., Moussa, M. G., Rana, M. S., Afzal, J., Imran, M., Zhao, X. H., & Hu, C. X. (2018). Can Selenium and Molybdenum Restrain Cadmium Toxicity to Pollen Grains in Brassica napus? International Journal of Molecular Sciences, 19(8), 2163. https://doi.org/10.3390/ijms19082163