Spatiotemporal Changes in Xylan-1/Xyloglucan and Xyloglucan Xyloglucosyl Transferase (XTH-Xet5) as a Step-In of Ultrastructural Cell Wall Remodelling in Potato–Potato Virus Y (PVYNTN) Hypersensitive and Susceptible Reaction
Abstract
:1. Introduction
2. Results
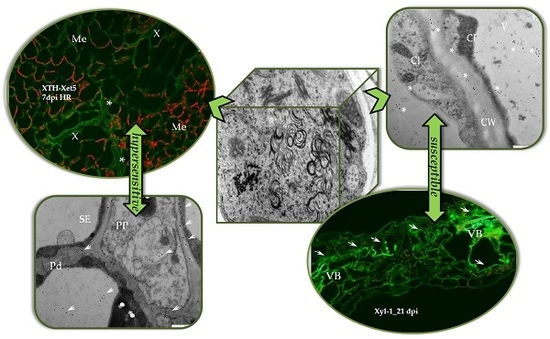
2.1. Localisation of Xylan-1/Xyloglucan during Compatible and Incompatible PVYNTN–Potato Interactions
2.2. Localisation of Xyloglucan Xyloglucosyl Transferase (XTH-Xet5) (E.C. 2.4.1.207) during Compatible and Incompatible PVYNTN–Potato Interactions
3. Discussion
4. Materials and Methods
4.1. Plant Material and Virus Inoculation
4.2. Immunofluorescence Localisation and the Assessment of the Quantitative Fluorescence Signal by Using the Corrected Total Cell Fluorescence Method (CTCF)
4.3. Quantitative Immunogold Localisation by Direct Estimation of the Relative Labelling Index (RLI)
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cai, X.K.; Spooner, D.M.; Jansky, S.H. A test of taxonomic and biogeographic predictivity: Resistance to Potato virus Y in wild relatives of the cultivated potato. Phytopathology 2011, 101, 1074–1080. [Google Scholar] [CrossRef] [PubMed]
- Rybicki, E.P. A top ten list for economically important plant viruses. Arch. Virol. 2015, 160, 17–20. [Google Scholar] [CrossRef] [PubMed]
- Dangl, J.L.; Dietrich, R.A.; Richberg, M.H. Death don’t have no mercy: Cell death programs in plant-microbe interactions. Plant Cell 1996, 8, 1793–1807. [Google Scholar] [CrossRef] [PubMed]
- Mur, L.A.; Kenton, P.; Lloyd, A.J.; Ougham, H.; Prats, E. The hypersensitive response; the centenary is upon us but how much do we know? J. Exp. Bot. 2008, 59, 501–520. [Google Scholar] [CrossRef] [PubMed]
- Alexander, M.M.; Cilia, M. A molecular tug-of-war: Global plant proteome changes during viral infection. Curr. Plant Biol. 2016, 5, 13–24. [Google Scholar] [CrossRef]
- Otulak, K.; Garbaczewsk, G. Localisation of hydrogen peroxide accumulation during Solanum tuberosum cv. Rywal hypersensitive response to Potato virus Y. Micron 2010, 41, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Nagy, P.D. Tombusvirus-host interactions: Co-opted evolutionarily conserved host factors take center court. Annu. Rev. Virol. 2016, 3, 491–515. [Google Scholar] [CrossRef] [PubMed]
- Otulak, K.; Garbaczewska, G. The participation of plant cell organelles in compatible and incompatible Potato virus Y-tobacco and -potato plant interaction. Acta Physiol. Plant. 2013, 36, 85–99. [Google Scholar] [CrossRef]
- Bacete, L.; Mélida, H.; Miedes, E.; Molina, A. Plant cell wall-mediated immunity: Cell wall changes trigger disease resistance responses. Plant J. 2018, 93, 614–636. [Google Scholar] [CrossRef] [PubMed]
- Pogorelko, G.; Lionetti, V.; Bellincampi, D.; Zabotina, O. Cell wall integrity: Targeted post-synthetic modifications to reveal its role in plant growth and defense against pathogens. Plant Signal. Behav. 2013, 8, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Otulak-Kozieł, K.; Kozieł, E.; Lockhart, B.E.L. Plant cell wall dynamics in compatible and incompatible potato response to infection caused by Potato virus Y (PVYNTN). Int. J. Mol. Sci. 2018, 19, 862. [Google Scholar] [CrossRef]
- Bethke, G.; Thao, A.; Xiong, G.; Li, B.; Soltis, N.E.; Hatsugai, N.; Hillmer, R.A.; Katagiri, F.; Kliebenstein, D.J.; Pauly, M.; et al. Pectin biosynthesis is critical for cell wall integrity and immunity in Arabidopsis thaliana. Plant Cell 2016, 28, 537–556. [Google Scholar] [CrossRef] [PubMed]
- Ellinger, D.; Voigt, C.A. Callose biosynthesis in Arabidopsis with a focus on pathogen response: What we have learned within the last decade. Ann. Bot. 2014, 114, 1349–1358. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Ma, L.; Zhao, J.; Li, Z.; Sun, F.; Lu, X. Comparative transcriptome analysis of two Rice varieties in response to Rice Stripe Virus and small Brown Planthoppers during early interaction. PLoS ONE 2013, 8, e82126. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, T.; Ogamino, T.; Hiraguri, A.; Nakazono-Nagaoka, E.; Uehara-Ichiki, T.; Nakajima, M.; Akutsu, K.; Omura, T.; Sasaya, T. Strong resistance against Rice grassy stunt virus is induced in transgenic rice plants expressing double-stranded RNA of the viral genes for nucleocapsid or movement proteins as targets for RNA interference. Phytopathology 2013, 103, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Zhang, Y.; Sun, H.; Liu, J.; Wang, Y.; Wang, X.; Li, D.; Yu, J.; Han, Ch. Transcriptome analysis of Beta macrocarpa and identification of differentially expressed transcripts in response to Beet necrotic yellow vein virus infection. PLoS ONE 2015, 10, e0132277. [Google Scholar] [CrossRef] [PubMed]
- Vorwerk, S.; Somerville, S.; Somerville, C. The role of plant cell wall polysaccharide composition in disease resistance. Trends Plant Sci. 2004, 9, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Rezzonico, F.; Rupp, O.; Fahrentrapp, J. Pathogen recognition in compatible plant-microbe interactions. Sci. Rep. 2017, 7, 6383. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Yao, S.; Liu, Y.; Yu, H.; Xu, P.; Sun, W.; Pu, Z.; Hou, H.; Bao, Y. Transcriptome analysis of tomato leaf spot pathogen Fusarium proliferatum: De novo assembly, expression profiling, and identification of candidate effectors. Int. J. Mol. Sci. 2018, 19, 31. [Google Scholar] [CrossRef] [PubMed]
- Sperotto, R.A.; Buffon, G.; Schwambach, J.; Ricachenevsky, F.K. Checkmite!? Is the resistance to Phytophagous mites on short and stocky wild Oryza species? Front. Plant Sci. 2018, 9, 321. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, T.; Satoh, K.; Kikuchi, S.; Omura, T. The repression of cell wall and plastid-related genes and the induction of defense-related genes in rice plants infected with Rice dwarf virus. Mol. Plant-Microbe Interact. 2007, 20, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Somerville, C.; Bauer, S.; Brininstool, G.; Facette, M.; Hamann, T.; Milne, J.; Osborne, E.; Paredez, A.; Persson, S.; Raab, T.; et al. Toward a systems approach to understanding plant cell walls. Science 2004, 306, 2206–2211. [Google Scholar] [CrossRef] [PubMed]
- Baumberger, N.; Ringli, C.; Keller, B. The chimeric leucine-rich repeat/extensin cell wall protein LRX1 is required for root hair morpho-genesis in Arabidopsis thaliana. Genes Dev. 2001, 15, 1128–1139. [Google Scholar] [CrossRef] [PubMed]
- Ventelon-Debout, M.; Delalande, F.; Brizard, J.P.; Diemer, H.; Van Dorsselaer, A.; Brugidou, C. Proteome analysis of cultivar- specific deregulations of Oryza sativa indica and O. sativa japonica cellular suspensions undergoing Rice yellow mottle virus infection. Proteomics 2004, 4, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Seifert, G.J.; Blaukopf, C. Irritable walls: The plant extracellular matrix and signaling. Plant Physiol. 2010, 153, 467–478. [Google Scholar] [CrossRef] [PubMed]
- Lotan, T.; Fluhr, R. Xylanase, a novel elicitor of pathogenesis-related proteins in tobacco, uses a non-ethylene pathway for induction. Plant Physiol. 1990, 93, 811–817. [Google Scholar] [CrossRef] [PubMed]
- Hillung, J.; García-García, F.; Dopazo, J.; Cuevas, J.M.; Elena, S.F. The transcriptomics of an experimentally evolved plant-virus interaction. Sci. Rep. 2016, 6, 24901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Lilley, C.J.; Imren, M.; Knox, J.P.; Urwin, P.E. The complex cell wall composition of syncytia induced by plant parasitic cyst nematodes reflects both function and host plant. Front. Plant Sci. 2017, 8, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Northcote, D.H.; Davey, R.; Lay, J. Use of antisera to localize callose, xylan and arabinogalactan in the cell-plate, primary and secondary walls of plant cells. Planta 1989, 178, 353–366. [Google Scholar] [CrossRef] [PubMed]
- Pauly, M.; Gille, S.; Liu, L.; Mansoori, N.; de Souza, A.; Schultink, A.; Xiong, G. Hemicellulose biosynthesis. Planta 2013, 238, 627–642. [Google Scholar] [CrossRef] [PubMed]
- Davies, L.J.; Lilley, C.J.; Knox, J.P.; Urwin, P.E. Syncytia formed by adult female Heterodera schachtii in Arabidopsis thaliana roots have a distinct cell wall molecular architecture. New Phytol. 2012, 196, 238–246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fry, S.C. Polysaccharide-modifying enzymes in the plant cell wall. Annu. Rev. 1995, 46, 497–520. [Google Scholar] [CrossRef]
- Sulová, Z.; Takacova, M.; Steele, N.M.; Fry, S.C.; Farkaš, V. Xyloglucan endotransglycosylase: Evidence for the existence of a relatively stable glycosyl-enzyme intermediate. Biochem. J. 1998, 330, 1475–1480. [Google Scholar] [CrossRef]
- Rose, J.K.C.; Braam, J.; Fry, S.C.; Nishitani, K. The XTH family of enzymes involved in xyloglucan endotransglucosylation and endohydrolysis: Current perspectives and a new unifying nomenclature. Plant Cell Physiol. 2002, 43, 1421–1435. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.K.; Kim, J.E.; Park, J.; Eom, T.J.; Kim, W.T. Constitutive expression of abiotic stress-inducible hot pepper CaXTH3, which encodes a xyloglucan endotransglucosylase/hydrolase homolog, improves drought and salt tolerance in transgenic Arabidopsis plants. FEBS Lett. 2006, 580, 3136–3144. [Google Scholar] [CrossRef] [PubMed]
- Fry, S.C.; Smith, R.C.; Renwick, K.F.; Martin, D.J.; Hodge, S.K.; Matthews, K.J. Xyloglucan endotransglycosylase, a new wall-loosening enzyme activity from plants. Biochem. J. 1992, 282, 821–828. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zabotina, O.A.; van de Ven, W.T.; Freshour, G.; Drakakaki, G.; Cavalier, D.; Mouille, G.; Hahn, M.G.; Keegstra, K.; Raikhel, N.V. Arabidopsis XXT5 gene encodes a putative alpha-1,6-xylosyltransferase that is involved in xyloglucan biosynthesis. Plant J. 2008, 56, 101–115. [Google Scholar] [CrossRef] [PubMed]
- Faik, A.; Price, N.J.; Raikhel, N.V.; Keegstra, K. An Arabidopsis gene encoding an alpha-xylosyltransferase involved in xyloglucan biosynthesis. Proc. Natl. Acad. Sci. USA 2002, 99, 7797–7802. [Google Scholar] [CrossRef] [PubMed]
- Hrmova, M.; Farkaš, V.; Lahnstein, J.; Fincher, G.B. A barley xyloglucan xyloglucosyl transferase covalently links xyloglucan, cellulosic substrates, and (1,3;1,4)-β-d-glucans. J. Biol. Chem. 2007, 282, 12951–12962. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, M.; Satoh, S.; Iwai, H. Distribution of XTH, expansin, and secondary-wall-related CesA in floral and fruit abscission zones during fruit development in tomato (Solanum lycopersicum). Front. Plant Sci. 2015, 6, 323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodrigues, S.P.; Ventura, J.A.; Aguilar, C.; Nakayasu, E.S.; Almeida, I.C.; Fernandes, P.M.; Zingali, R.B. Proteomic analysis of papaya (Carica papaya L.) displaying typical sticky disease symptoms. Proteomics 2011, 11, 2592–2602. [Google Scholar] [CrossRef] [PubMed]
- DeBlasio, S.L.; Johnson, R.; Mahoney, J.; Karasev, A.; Gray, S.M.; MacCoss, M.J.; Cilia, M. Insights into the Polerovirus-plant interactome revealed by coimmunoprecipitation and mass spectrometry. Mol. Plant-Microbe Interact. 2015, 28, 467–481. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Guo, R.; Jie, F.; Nettleton, D.; Peng, J.; Carr, T.; Yeakley, J.M.; Fan, J.B.; Whitham, S.A. Spatial analysis of arabidopsis thaliana gene expression in response to Turnip mosaic virus infection. Mol. Plant Microbe Interact. 2007, 20, 358–370. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Li, F.; Liu, D.; Jiang, C.; Cui, L.; Shen, L.; Liu, G.; Yang, A. Dynamic expression analysis of early response genes induced by potato virus Y in PVY-resistant Nicotiana tabacum. Plant Cell Rep. 2016, 36, 297–311. [Google Scholar] [CrossRef] [PubMed]
- Antosiewicz, D.M.; Purugganan, M.M.; Polisensky, D.H.; Braam, J. Cellular localization of Arabidopsis xyloglucan endotransglycosylase-related proteins during development and after wind stimulation. Plant Physiol. 1997, 115, 1319–1328. [Google Scholar] [CrossRef] [PubMed]
- Saab, I.N.; Sachs, M.M. Complete cDNA and genomic se-quence encoding a flooding-responsive gene from maize (Zea mays L.) homologous to xyloglucan endotransglycosylase. Plant Physiol. 1995, 108, 439–440. [Google Scholar] [CrossRef] [PubMed]
- Zabotina, O.A.; Avci, U.; Cavalier, D.; Pattathil, S.; Chou, Y.H.; Eberhard, S.; Danhof, L.; Keegstra, K.; Hahn, M.G. Mutations in multiple XXT genes of Arabidopsis reveal the complexity of xyloglucan biosynthesis. Plant Physiol. 2012, 159, 1367–1384. [Google Scholar] [CrossRef] [PubMed]
- Worden, N.; Park, E.; Drakakaki, G. Trans-Golgi network: An intersection of trafficking cell wall components. J. Integr. Plant Biol. 2012, 54, 875–886. [Google Scholar] [CrossRef] [PubMed]
- The European Cultivated Potato Database. Available online: https://www.europotato.org/quick_search.php (accessed on 24 April 2018).
- Otulak, K.; Kozieł, E.; Garbaczewska, G. Ultastructural impact of tobacco rattle virus on tobacco and pepper ovary and anther tissues. J. Phytopatol. 2016, 164, 226–241. [Google Scholar] [CrossRef]
- Tomczyńska, I.; Jupe, F.; Hein, I.; Marczewski, W.; Śliwka, J. Hypersensitive response to Potato virus Y in potato cultivar Sárpo Mira is conferred by the Ny-Smira gene located on the long arm of chromosome IX. Mol. Breed. 2014, 34, 471–480. [Google Scholar] [CrossRef] [Green Version]
- Chrzanowska, M.; Doroszewska, T. Comparison between PVY isolates obtained from potato and tobacco plants grown in Poland. Phytopathol. Pol. 1997, 13, 63–71. [Google Scholar]
- Grupa, A.; Otulak-Kozieł, K.; Syller, J. Serological, molecular and immunofluorescent evidence for interference competition between isolates of Potato virus Y. Plant Pathol. 2018. [Google Scholar] [CrossRef]
- Burgess, A.; Vigneron, S.; Brioudes, E.; Labbé, J.C.; Lorca, T.; Castro, A. Loss of human Greatwall results in G2 arrest and multiple mitotic defects due to deregulation of the cyclin B-Cdc2/PP2A balance. Proc. Natl. Acad. Sci. USA 2010, 107, 12564–12569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- The University of Sydney Official Website of the Bosh Institute. Available online: https://sydney.edu.au/medicine/bosch/facilities/advanced-microscopy/user-support/ImageJ_FL_Image_Analysis.pdf (accessed on 6 May 2018).
- Mayhew, T.M. Quantifying immunogold localization on electron microscopic thin sections: A compendium of new approaches for plant cell biologists. J. Exp. Bot. 2011, 62, 4101–4113. [Google Scholar] [CrossRef] [PubMed]
- Otulak, K.; Kozieł, E.; Lockhart, B.E.L.; Garbaczewska, G. Ultrastructural effects of PVYNTN infection of Capsicum annuum L. cv. Yolo Wonder generative organs; a first step in describing seed transmission. Phytopathol. Mediterr. 2017, 56, 379–391. [Google Scholar] [CrossRef]










| Sample | Parameters of Immunogold Labelling | ||||
|---|---|---|---|---|---|
| G0 | Ge | RLI | Χ2Value | Χ2as % | |
| (A) Immunogold localisation of xyl-1/xyloglucan: | |||||
| 1. Mock-inoculated cv. Irys potato plants | |||||
| cell wall | 27 | 3 | 9.00 * | 192.00 | 56.30 * |
| endoplasmic reticulum (ER) | 14 | 3 | 4.67 * | 40.33 | 11.83 * |
| Golgi apparatus | 12 | 2 | 6.00 * | 50.00 | 14.66 * |
| chloroplasts | 0 | 6 | 0.00 | 6.00 | 1.76 |
| mitochondrion | 1 | 7 | 0.14 | 5.14 | 1.51 |
| vacuole | 1 | 3 | 0.33 | 1.33 | 0.39 |
| cytoplasm | 0 | 4 | 0.00 | 4.00 | 1.47 |
| vesicles | 17 | 4 | 4.25 * | 42.25 | 12.39 * |
| Column total | 341.06 | 100 | |||
| 2. PVYNTN inoculated cv. Irys potato plants | |||||
| cell wall | 77 | 15 | 5.13 * | 256.27 | 23.89 * |
| endoplasmic reticulum (ER) | 32 | 6 | 5.33 * | 112.67 | 10.50 * |
| Golgi apparatus | 30 | 4 | 7.50 * | 169.00 | 15.75 * |
| chloroplasts | 0 | 5 | 0.00 | 5.00 | 0.47 |
| mitochondrion | 0 | 4 | 0.00 | 4.00 | 0.37 |
| vacuole | 64 | 10 | 6.40 * | 291.60 | 27.18 * |
| cytoplasm | 1 | 4 | 0.25 | 2.25 | 0.21 |
| vesicles | 74 | 15 | 4.93 * | 232.07 | 21.63 * |
| Column total | 1072.85 | 100 | |||
| 3. Mock-inoculated cv. Sárpo Mira potato plants | |||||
| cell wall | 30 | 3 | 10.00 * | 243.00 | 35.69 * |
| endoplasmic reticulum (ER) | 11 | 2 | 5.50 | 40.50 | 5.95 |
| Golgi apparatus | 15 | 2 | 7.50 * | 84.50 | 12.41 * |
| chloroplasts | 2 | 1 | 2.00 | 1.00 | 0.15 |
| mitochondrion | 4 | 3 | 1.33 | 0.33 | 0.05 |
| vacuole | 21 | 2 | 10.50 * | 180.50 | 26.51 * |
| cytoplasm | 0 | 3 | 0.00 | 3.00 | 0.44 |
| vesicles | 18 | 2 | 9.00 * | 128.00 | 18.80 * |
| Column total | 680.83 | 100 | |||
| 4. PVYNTN inoculated cv. Sárpo Mira potato plants | |||||
| cell wall | 9 | 2 | 4.50 * | 24.50 | 20.04 * |
| endoplasmic reticulum (ER) | 7 | 4 | 1.75 | 2.25 | 1.84 |
| Golgi apparatus | 8 | 2 | 4.00 * | 18.00 | 14.72 * |
| chloroplasts | 0 | 3 | 0.00 | 3.00 | 2.45 |
| mitochondrion | 0 | 3 | 0.00 | 3.00 | 2.45 |
| vacuole | 7 | 2 | 3.50 * | 12.50 | 10.22 * |
| cytoplasm | 12 | 3 | 4.00 * | 27.00 | 22.09 * |
| vesicles | 10 | 2 | 5.00 * | 32.00 | 26.18 * |
| Column total | 122.25 | 100 | |||
| (B) Immunogold localisation of XTH-Xet5: | |||||
| 1. Mock-inoculated cv. Irys potato plants | |||||
| cell wall | 19 | 3 | 6.33 * | 85.33 | 25.45 * |
| endoplasmic reticulum (ER) | 22 | 5 | 4.40 * | 57.80 | 17.24 * |
| Golgi apparatus | 11 | 2 | 5.50 * | 40.50 | 12.08 * |
| chloroplasts | 0 | 4 | 0.00 | 4.00 | 1.19 |
| mitochondrion | 0 | 4 | 0.00 | 4.00 | 1.19 |
| vacuole | 19 | 3 | 6.33 * | 85.33 | 25.45 * |
| cytoplasm | 4 | 2 | 2.00 | 2.00 | 0.60 |
| vesicles | 16 | 3 | 5.33 * | 56.33 | 16.80 * |
| Column total | 335.30 | 100 | |||
| 2. PVYNTN inoculated cv. Irys potato plants | |||||
| cell wall | 13 | 4 | 3.25 * | 20.25 | 17.62 * |
| endoplasmic reticulum (ER) | 3 | 2 | 1.50 | 0.50 | 0.44 |
| Golgi apparatus | 9 | 2 | 4.50 * | 24.50 | 21.32 * |
| chloroplasts | 2 | 3 | 0.67 | 0.33 | 0.29 |
| mitochondrion | 0 | 3 | 0.00 | 3.00 | 2.61 |
| vacuole | 9 | 2 | 4.50 * | 24.50 | 21.32 * |
| cytoplasm | 5 | 3 | 1.67 | 1.33 | 1.16 |
| vesicles | 11 | 2 | 5.50 * | 40.50 | 35.24 * |
| Column total | 114.92 | 100 | |||
| 3. Mock-inoculated cv. Sárpo Mira potato plants | |||||
| cell wall | 10 | 2 | 5.00 * | 32.00 | 53.93 * |
| endoplasmic reticulum (ER) | 3 | 2 | 1.50 | 0.50 | 0.84 |
| Golgi apparatus | 5 | 3 | 1.67 | 1.33 | 2.25 |
| chloroplasts | 0 | 3 | 0.00 | 3.00 | 5.06 |
| mitochondrion | 0 | 3 | 0.00 | 3.00 | 5.06 |
| vacuole | 7 | 2 | 3.50 * | 12.50 | 21.07 * |
| cytoplasm | 0 | 3 | 0.00 | 3.00 | 5.06 |
| vesicles | 3 | 1 | 3.00 | 4.00 | 6.74 |
| Column total | 59.33 | 100 | |||
| 4. PVYNTN inoculated cv. Sárpo Mira potato plants | |||||
| cell wall | 28 | 4 | 7.00 * | 144.00 | 23.80 * |
| endoplasmic reticulum (ER) | 6 | 2 | 3.00 | 8.00 | 1.32 |
| Golgi apparatus | 22 | 4 | 5.50 * | 81.00 | 13.38 * |
| chloroplasts | 1 | 6 | 0.17 | 4.17 | 0.69 |
| mitochondrion | 0 | 6 | 0.00 | 6.00 | 0.99 |
| vacuole | 26 | 4 | 6.50 * | 121.00 | 19.99 * |
| cytoplasm | 30 | 4 | 7.50 * | 169.00 | 27.93 * |
| vesicles | 14 | 2 | 7.00 * | 72.00 | 11.90 * |
| Column total | 605.17 | 100 | |||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Otulak-Kozieł, K.; Kozieł, E.; Bujarski, J.J. Spatiotemporal Changes in Xylan-1/Xyloglucan and Xyloglucan Xyloglucosyl Transferase (XTH-Xet5) as a Step-In of Ultrastructural Cell Wall Remodelling in Potato–Potato Virus Y (PVYNTN) Hypersensitive and Susceptible Reaction. Int. J. Mol. Sci. 2018, 19, 2287. https://doi.org/10.3390/ijms19082287
Otulak-Kozieł K, Kozieł E, Bujarski JJ. Spatiotemporal Changes in Xylan-1/Xyloglucan and Xyloglucan Xyloglucosyl Transferase (XTH-Xet5) as a Step-In of Ultrastructural Cell Wall Remodelling in Potato–Potato Virus Y (PVYNTN) Hypersensitive and Susceptible Reaction. International Journal of Molecular Sciences. 2018; 19(8):2287. https://doi.org/10.3390/ijms19082287
Chicago/Turabian StyleOtulak-Kozieł, Katarzyna, Edmund Kozieł, and Józef J. Bujarski. 2018. "Spatiotemporal Changes in Xylan-1/Xyloglucan and Xyloglucan Xyloglucosyl Transferase (XTH-Xet5) as a Step-In of Ultrastructural Cell Wall Remodelling in Potato–Potato Virus Y (PVYNTN) Hypersensitive and Susceptible Reaction" International Journal of Molecular Sciences 19, no. 8: 2287. https://doi.org/10.3390/ijms19082287
APA StyleOtulak-Kozieł, K., Kozieł, E., & Bujarski, J. J. (2018). Spatiotemporal Changes in Xylan-1/Xyloglucan and Xyloglucan Xyloglucosyl Transferase (XTH-Xet5) as a Step-In of Ultrastructural Cell Wall Remodelling in Potato–Potato Virus Y (PVYNTN) Hypersensitive and Susceptible Reaction. International Journal of Molecular Sciences, 19(8), 2287. https://doi.org/10.3390/ijms19082287