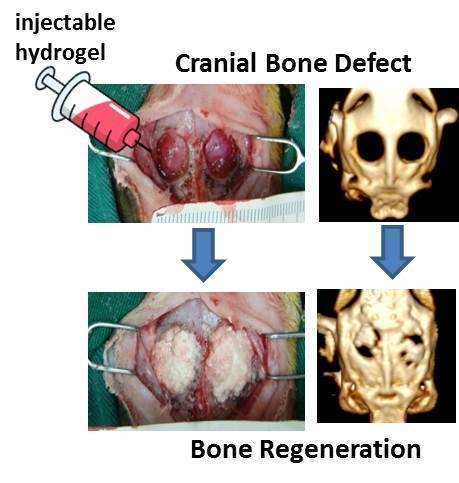
Bone Regeneration Using Adipose-Derived Stem Cells in Injectable Thermo-Gelling Hydrogel Scaffold Containing Platelet-Rich Plasma and Biphasic Calcium Phosphate
Abstract
:1. Introduction
2. Results and Discussion
2.1. Cell Proliferation
2.2. Live/Dead Staining
2.3. Alkaline Phosphatase (ALP) Activities
2.4. Von Kossa, Alizarin Red and ALP Stains
2.5. Calcium Content
2.6. Scanning Electron Microscopy/Energy Dispersive X-Ray (SEM/EDX) Analysis
2.7. Gene Expression by Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
2.8. In Vivo Studies
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Hyaluronic Acid-g-Chitosan-G-Poly(N-Isopropylacrylamide) (HA-CPN)
3.3. Preparation of Platelet-Rich Plasma (PRP)
3.4. Isolation of Rabbit Adipose-Derived Stem Cells (rASCs)
3.5. In Vitro Culture of rASCs in HA-CPN and HA-CPN/Platelet-Rich Plasma/Biphasic Calcium Phosphate (HA-CPN/PRP/BCP)
3.6. Cell Proliferation
3.7. Live/Dead Assay
3.8. ALP Activity
3.9. Alizarin Red, Von Kossa and ALP Stains
3.10. Calcium Content
3.11. Scanning Electron Microscopy/Energy Dispersive X-Ray (SEM/EDX) Analysis
3.12. Expression of Osteogenic Genes by qRT-PCR
3.13. In Vivo Experiment
3.14. Statistical Analysis
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
Abbreviations
| HA | Hyaluronic acid |
| PNIPAM | Poly(n-isopropylacrylamide) |
| HA-CPN | Hyaluronic acid-g-chitosan-g-PNIPAM |
| CPN | Chitosan-g-poly(n-isopropylacrylamide) |
| LCST | Lower critical solution temperature |
| PBS | Phosphate buffered saline |
| HAP | Hydroxyapatite |
| FBS | Fetal bovine serum |
| DMEM | Dulbecco’s modified Eagle medium |
| ASCs | Adipose-derived stem cells |
| rASCs | Rabbit adipose-derived stem cells |
| ECM | Extracellular matrix |
| H&E | Hematoxylin and eosin |
| PRP | Platelet-rich plasma |
| SEM | Scanning electron microscopy |
| BCP | Biphasic calcium phosphate |
| β-TCP | β-Tricalcium phosphate |
| BTE | Bone tissue engineering |
| AR | Alizarin red |
| ARS | Alizarin red S |
| 3D | Three-dimensional |
| IHC | Immunohistochemical |
| CT | Computed tomography |
| OD | Optical density |
| SEM/EDX | Scanning electron microscopy/energy dispersive X-ray |
| ALP | Alkaline phosphatase |
| NHS | n-Hydroxysuccinimide |
| EDC | 1-Ethyl-3-(3-dimethylaminopropyl) carbodiimide |
| DDI | Distilled de-ionized |
| MES | 2-Morpholinoethane sulfonic acid |
| OCT | Optimal cutting temperature |
| qRT-PCR | Quantitative real-time polymerase chain reaction |
References
- Sheikh, Z.; Sima, C.; Glogauer, M. Bone replacement materials and techniques used for achieving vertical alveolar bone augmentation. Materials 2015, 8, 2953. [Google Scholar] [CrossRef]
- Rogers, G.F.; Greene, A.K. Autogenous bone graft: Basic science and clinical implications. J. Craniofac. Surg. 2012, 23, 323–327. [Google Scholar] [CrossRef] [PubMed]
- Rose, F.R.; Oreffo, R.O. Bone tissue engineering: Hope vs hype. Biochem. Biophys. Res. Commun. 2002, 292, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Salgado, A.J.; Coutinho, O.P.; Reis, R.L. Bone tissue engineering: State of the art and future trends. Macromol. Biosci. 2004, 4, 743–765. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- LeGeros, R.Z. Calcium phosphate-based osteoinductive materials. Chem. Rev. 2008, 108, 4742–4753. [Google Scholar] [CrossRef] [PubMed]
- Rizzi, S.C.; Heath, D.J.; Coombes, A.G.; Bock, N.; Textor, M.; Downes, S. Biodegradable polymer/hydroxyapatite composites: Surface analysis and initial attachment of human osteoblasts. J. Biomed. Mater. Res. 2001, 55, 475–486. [Google Scholar] [CrossRef]
- Fellah, B.H.; Gauthier, O.; Weiss, P.; Chappard, D.; Layrolle, P. Osteogenicity of biphasic calcium phosphate ceramics and bone autograft in a goat model. Biomaterials 2008, 29, 1177–1188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daculsi, G.; LeGeros, R.; Nery, E.; Lynch, K.; Kerebel, B. Transformation of biphasic calcium phosphate ceramics in vivo: Ultrastructural and physicochemical characterization. J. Biomed. Mater. Res. A 1989, 23, 883–894. [Google Scholar] [CrossRef] [PubMed]
- Daculsi, G.; LeGeros, R.; Mitre, D. Crystal dissolution of biological and ceramic apatites. Calcif. Tissue Int. 1989, 45, 95–103. [Google Scholar] [CrossRef] [PubMed]
- LeGeros, R. Calcium phosphate materials in restorative dentistry: A review. Adv. Dent. Res. 1988, 2, 164–180. [Google Scholar] [CrossRef] [PubMed]
- Marra, K.G.; Szem, J.W.; Kumta, P.N.; DiMilla, P.A.; Weiss, L.E. In vitro analysis of biodegradable polymer blend/hydroxyapatite composites for bone tissue engineering. J. Biomed. Mater. Res. 1999, 47, 324–335. [Google Scholar] [CrossRef]
- Chen, J.-P.; Chang, Y.-S. Preparation and characterization of composite nanofibers of polycaprolactone and nanohydroxyapatite for osteogenic differentiation of mesenchymal stem cells. Colloids Surf. B Biointerfaces 2011, 86, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Baroth, S.; Bourges, X.; Goyenvalle, E.; Aguado, E.; Daculsi, G. Injectable biphasic calcium phosphate bioceramic: The hydros concept. Biomed. Mater. Eng. 2009, 19, 71–76. [Google Scholar] [PubMed]
- Slaughter, B.V.; Khurshid, S.S.; Fisher, O.Z.; Khademhosseini, A.; Peppas, N.A. Hydrogels in regenerative medicine. Adv. Mater. 2009, 21, 3307–3329. [Google Scholar] [CrossRef] [PubMed]
- Akalp, U.; Bryant, S.J.; Vernerey, F.J. Tuning tissue growth with scaffold degradation in enzyme-sensitive hydrogels: A mathematical model. Soft Matter 2016, 12, 7505–7520. [Google Scholar] [CrossRef] [PubMed]
- Tozzi, G.; De Mori, A.; Oliveira, A.; Roldo, M. Composite hydrogels for bone regeneration. Materials 2016, 9, 267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kretlow, J.D.; Klouda, L.; Mikos, A.G. Injectable matrices and scaffolds for drug delivery in tissue engineering. Adv. Drug Deliv. Rev. 2007, 59, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Huayang, S.A.; Lu, J.; Zibiao, L. Biodegradable polyester thermogelling system as emerging materials for therapeutic applications. Macromol. Mater. Eng. 2018, 303, 1700656. [Google Scholar]
- Liow, S.S.; Dou, Q.; Kai, D.; Karim, A.A.; Zhang, K.; Xu, F.; Loh, X.J. Thermogels: In situ gelling biomaterial. ACS Biomater. Sci. Eng. 2016, 2, 295–316. [Google Scholar] [CrossRef]
- Vihola, H.; Laukkanen, A.; Valtola, L.; Tenhu, H.; Hirvonen, J. Cytotoxicity of thermosensitive polymers poly(n-isopropylacrylamide), poly(n-vinylcaprolactam) and amphiphilically modified poly(n-vinylcaprolactam). Biomaterials 2005, 26, 3055–3064. [Google Scholar] [CrossRef] [PubMed]
- Cooperstein, M.A.; Canavan, H.E. Assessment of cytotoxicity of (n-isopropyl acrylamide) and poly(n-isopropyl acrylamide)-coated surfaces. Biointerphases 2013, 8, 19. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.H.; Kim, S.H.; Park, K.D.; Jung, M.C.; Yang, W.I.; Han, S.W.; Noh, J.Y.; Lee, J.W. Chondrogenic differentiation of human mesenchymal stem cells using a thermosensitive poly(n-isopropylacrylamide) and water-soluble chitosan copolymer. Biomaterials 2004, 25, 5743–5751. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-P.; Cheng, T.-H. Preparation and evaluation of thermo-reversible copolymer hydrogels containing chitosan and hyaluronic acid as injectable cell carriers. Polymer 2009, 50, 107–116. [Google Scholar] [CrossRef]
- Chen, J.P.; Cheng, T.H. Thermo-responsive chitosan-graft-poly(n-isopropylacrylamide) injectable hydrogel for cultivation of chondrocytes and meniscus cells. Macromol. Biosci. 2006, 6, 1026–1039. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.H.; Kuo, C.Y.; Chen, S.H.; Mao, S.H.; Chang, C.Y.; Shalumon, K.T.; Chen, J.P. Thermosensitive injectable hydrogel for simultaneous intraperitoneal delivery of doxorubicin and prevention of peritoneal adhesion. Int. J. Mol. Sci. 2018, 19, 1373. [Google Scholar] [CrossRef] [PubMed]
- Levengood, S.L.; Zhang, M. Chitosan-based scaffolds for bone tissue engineering. J. Mater. Chem. B 2014, 2, 3161–3184. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Li, H.; Cheng, W.; Yang, Y.; Zhu, M.; Zhou, C. Novel injectable calcium phosphate/chitosan composites for bone substitute materials. Acta Biomater. 2006, 2, 557–565. [Google Scholar] [CrossRef] [PubMed]
- Sendemir-Urkmez, A.; Jamison, R.D. The addition of biphasic calcium phosphate to porous chitosan scaffolds enhances bone tissue development in vitro. J. Biomed. Mater. Res. Part A 2007, 81, 624–633. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.; Gong, Y.; Lao, L.; Mao, Z.; Gao, C. Gelatin/chitosan/hyaluronan ternary complex scaffold containing basic fibroblast growth factor for cartilage tissue engineering. J. Mater. Sci. Mater. Med. 2007, 18, 1961–1968. [Google Scholar] [CrossRef] [PubMed]
- Solchaga, L.A.; Dennis, J.E.; Goldberg, V.M.; Caplan, A.I. Hyaluronic acid-based polymers as cell carriers for tissue-engineered repair of bone and cartilage. J. Orthop. Res. 1999, 17, 205–213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chung, C.; Burdick, J.A. Influence of three-dimensional hyaluronic acid microenvironments on mesenchymal stem cell chondrogenesis. Tissue Eng. Part A 2009, 15, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.T.; Chen, C.T.; Chen, J.P. Osteogenic differentiation and ectopic bone formation of canine bone marrow-derived mesenchymal stem cells in injectable thermo-responsive polymer hydrogel. Tissue Eng. Part C Methods 2011, 17, 1139–1149. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-P.; Tsai, M.-J.; Liao, H.-T. Incorporation of biphasic calcium phosphate microparticles in injectable thermoresponsive hydrogel modulates bone cell proliferation and differentiation. Colloids Surf. B Biointerfaces 2013, 110, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Gimble, J.; Guilak, F. Adipose-derived adult stem cells: Isolation, characterization, and differentiation potential. Cytotherapy 2003, 5, 362–369. [Google Scholar] [CrossRef] [PubMed]
- Plachokova, A.S.; van den Dolder, J.; Stoelinga, P.J.; Jansen, J.A. Early effect of platelet-rich plasma on bone healing in combination with an osteoconductive material in rat cranial defects. Clin. Oral Implant. Res. 2007, 18, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Xu, H.; Sun, J.; Gong, X.; Zhao, H. Dual delivery of bmp-2 and bfgf from a new nano-composite scaffold, loaded with vascular stents for large-size mandibular defect regeneration. Int. J. Mol. Sci. 2013, 14, 12714. [Google Scholar] [CrossRef] [PubMed]
- Lacci, K.M.; Dardik, A. Platelet-rich plasma: Support for its use in wound healing. Yale J. Biol. Med. 2010, 83, 1–9. [Google Scholar] [PubMed]
- Marx, R.E. Platelet-rich plasma: Evidence to support its use. J. Oral Maxillofac. Surg. 2004, 62, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.-T.; Marra, K.G.; Rubin, J.P. Application of platelet-rich plasma and platelet-rich fibrin in fat grafting: Basic science and literature review. Tissue Eng. Part B Rev. 2014, 20, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Chang, W.; Tian, B.; Zeng, W.; Zhou, D. Bone regeneration combining platelet rich plasma with engineered bone tissue. J. Biomater. Tissue Eng. 2017, 7, 841–847. [Google Scholar] [CrossRef]
- Liao, H.-T.; Chen, J.-P.; Lee, M.-Y. Bone tissue engineering with adipose-derived stem cells in bioactive composites of laser-sintered porous polycaprolactone scaffolds and platelet-rich plasma. Materials 2013, 6, 4911–4929. [Google Scholar] [CrossRef] [PubMed]
- Griffin, X.L.; Smith, C.M.; Costa, M.L. The clinical use of platelet-rich plasma in the promotion of bone healing: A systematic review. Injury 2009, 40, 158–162. [Google Scholar] [CrossRef] [PubMed]
- Son, S.R.; Sarkar, S.K.; Nguyen-Thuy, B.L.; Padalhin, A.R.; Kim, B.R.; Jung, H.I.; Lee, B.T. Platelet-rich plasma encapsulation in hyaluronic acid/gelatin-bcp hydrogel for growth factor delivery in bcp sponge scaffold for bone regeneration. J. Biomater. Appl. 2015, 29, 988–1002. [Google Scholar] [CrossRef] [PubMed]
- Juan, S.; Yubao, L.; Yi, Z.; Qin, Z.; Lin, C.; Li, Z.; Mei, G.; Shibo, G. Characterization and cytocompatibility of biphasic calcium phosphate/polyamide 6 scaffolds for bone regeneration. J. Biomed. Mater. Res. B Appl. Biomater. 2010, 95B, 330–338. [Google Scholar]
- Liao, H.T.; James, I.B.; Marra, K.G.; Rubin, J.P. The effects of platelet-rich plasma on cell proliferation and adipogenic potential of adipose-derived stem cells. Tissue Eng. Part A 2015, 21, 2714–2722. [Google Scholar] [CrossRef] [PubMed]
- Gersch, R.P.; Glahn, J.; Tecce, M.G.; Wilson, A.J.; Percec, I. Platelet rich plasma augments adipose-derived stem cell growth and differentiation. Aesthet. Surg. J. 2017, 37, 723–729. [Google Scholar] [CrossRef] [PubMed]
- Lai, F.; Kakudo, N.; Morimoto, N.; Taketani, S.; Hara, T.; Ogawa, T.; Kusumoto, K. Platelet-rich plasma enhances the proliferation of human adipose stem cells through multiple signaling pathways. Stem Cell Res. Ther. 2018, 9, 107. [Google Scholar] [CrossRef] [PubMed]
- Paduano, F.; Marrelli, M.; Amantea, M.; Rengo, C.; Rengo, S.; Goldberg, M.; Spagnuolo, G.; Tatullo, M. Adipose tissue as a strategic source of mesenchymal stem cells in bone regeneration: A topical review on the most promising craniomaxillofacial applications. Int. J. Mol. Sci. 2017, 18, 2140. [Google Scholar] [CrossRef] [PubMed]
- Ravichandran, R.; Venugopal, J.R.; Sundarrajan, S.; Mukherjee, S.; Ramakrishna, S. Precipitation of nanohydroxyapatite on plla/pblg/collagen nanofibrous structures for the differentiation of adipose derived stem cells to osteogenic lineage. Biomaterials 2012, 33, 846–855. [Google Scholar] [CrossRef] [PubMed]
- Anderson, H.C.; Sipe, J.B.; Hessle, L.; Dhanyamraju, R.; Atti, E.; Camacho, N.P.; Millan, J.L.; Dhamyamraju, R. Impaired calcification around matrix vesicles of growth plate and bone in alkaline phosphatase-deficient mice. Am. J. Pathol. 2004, 164, 841–847. [Google Scholar] [CrossRef]
- Park, K.W.; Yun, Y.P.; Kim, S.E.; Song, H.R. The effect of alendronate loaded biphasic calcium phosphate scaffolds on bone regeneration in a rat tibial defect model. Int. J. Mol. Sci. 2015, 16, 26738–26753. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.-T.; Shalumon, K.T.; Chang, K.-H.; Sheu, C.; Chen, J.-P. Investigation of synergistic effects of inductive and conductive factors in gelatin-based cryogels for bone tissue engineering. J. Mater. Chem. B 2016, 4, 1827–1841. [Google Scholar] [CrossRef]
- Shalumon, K.; Kuo, C.-Y.; Wong, C.-B.; Chien, Y.-M.; Chen, H.-A.; Chen, J.-P. Gelatin/nanohyroxyapatite cryogel embedded poly(lactic-co-glycolic acid)/nanohydroxyapatite microsphere hybrid scaffolds for simultaneous bone regeneration and load-bearing. Polymers 2018, 10, 620. [Google Scholar] [CrossRef]
- Ngiam, M.; Liao, S.; Patil, A.J.; Cheng, Z.; Chan, C.K.; Ramakrishna, S. The fabrication of nano-hydroxyapatite on PLGA and PLGA/collagen nanofibrous composite scaffolds and their effects in osteoblastic behavior for bone tissue engineering. Bone 2009, 45, 4–16. [Google Scholar] [CrossRef] [PubMed]
- McBeath, R.; Pirone, D.M.; Nelson, C.M.; Bhadriraju, K.; Chen, C.S. Cell shape, cytoskeletal tension, and rhoa regulate stem cell lineage commitment. Dev. Cell 2004, 6, 483–495. [Google Scholar] [CrossRef]
- Zur Nieden, N.I.; Kempka, G.; Ahr, H.J. In vitro differentiation of embryonic stem cells into mineralized osteoblasts. Differentiation 2003, 71, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Heinemann, S.; Heinemann, C.; Bernhardt, R.; Reinstorf, A.; Nies, B.; Meyer, M.; Worch, H.; Hanke, T. Bioactive silica-collagen composite xerogels modified by calcium phosphate phases with adjustable mechanical properties for bone replacement. Acta Biomater. 2009, 5, 1979–1990. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, J.M.; Molinuevo, M.S.; Cortizo, M.S.; Cortizo, A.M. Development of an osteoconductive pcl–pdipf–hydroxyapatite composite scaffold for bone tissue engineering. J. Tissue Eng. Regen. Med. 2011, 5, e126–e135. [Google Scholar] [CrossRef] [PubMed]
- Robert, A.W.; Angulski, A.B.B.; Spangenberg, L.; Shigunov, P.; Pereira, I.T.; Bettes, P.S.L.; Naya, H.; Correa, A.; Dallagiovanna, B.; Stimamiglio, M.A. Gene expression analysis of human adipose tissue-derived stem cells during the initial steps of in vitro osteogenesis. Sci. Rep. 2018, 8, 4739. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wei, L.K.; Jian, X.F.; Huang, J.; Zou, H.; Zhang, S.Z.; Yuan, G.H. Isolation, culture and induced differentiation of rabbit mesenchymal stem cells into osteoblasts. Exp. Ther. Med. 2018, 15, 3715–3724. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Botchwey, E.A.; Levine, E.M.; Pollack, S.R.; Laurencin, C.T. Bioreactor-based bone tissue engineering: The influence of dynamic flow on osteoblast phenotypic expression and matrix mineralization. Proc. Natl. Acad. Sci. USA 2004, 101, 11203–11208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lai, G.J.; Shalumon, K.T.; Chen, J.P. Response of human mesenchymal stem cells to intrafibrillar nanohydroxyapatite content and extrafibrillar nanohydroxyapatite in biomimetic chitosan/silk fibroin/nanohydroxyapatite nanofibrous membrane scaffolds. Int. J. Nanomed. 2015, 10, 567–584. [Google Scholar]








© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liao, H.T.; Tsai, M.-J.; Brahmayya, M.; Chen, J.-P. Bone Regeneration Using Adipose-Derived Stem Cells in Injectable Thermo-Gelling Hydrogel Scaffold Containing Platelet-Rich Plasma and Biphasic Calcium Phosphate. Int. J. Mol. Sci. 2018, 19, 2537. https://doi.org/10.3390/ijms19092537
Liao HT, Tsai M-J, Brahmayya M, Chen J-P. Bone Regeneration Using Adipose-Derived Stem Cells in Injectable Thermo-Gelling Hydrogel Scaffold Containing Platelet-Rich Plasma and Biphasic Calcium Phosphate. International Journal of Molecular Sciences. 2018; 19(9):2537. https://doi.org/10.3390/ijms19092537
Chicago/Turabian StyleLiao, Han Tsung, Ming-Jin Tsai, Manuri Brahmayya, and Jyh-Ping Chen. 2018. "Bone Regeneration Using Adipose-Derived Stem Cells in Injectable Thermo-Gelling Hydrogel Scaffold Containing Platelet-Rich Plasma and Biphasic Calcium Phosphate" International Journal of Molecular Sciences 19, no. 9: 2537. https://doi.org/10.3390/ijms19092537
APA StyleLiao, H. T., Tsai, M. -J., Brahmayya, M., & Chen, J. -P. (2018). Bone Regeneration Using Adipose-Derived Stem Cells in Injectable Thermo-Gelling Hydrogel Scaffold Containing Platelet-Rich Plasma and Biphasic Calcium Phosphate. International Journal of Molecular Sciences, 19(9), 2537. https://doi.org/10.3390/ijms19092537