The Anti-Inflammatory Effects of Vitamin D in Tumorigenesis
Abstract
:1. Introduction
2. Vitamin D and Epidemiology
3. Vitamin D and Metabolism
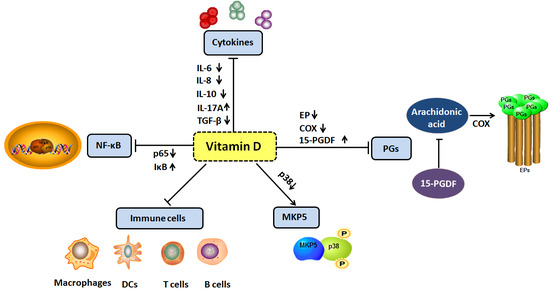
4. Vitamin D and Inflammation
4.1. Vitamin D and Cytokines
4.2. Vitamin D and Prostaglandins
4.3. Vitamin D and MAP Kinase Phosphatase 5
4.4. Vitamin D and Nuclear Factor Kappa B Signal Pathway
4.5. Vitamin D and Immune Cells
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bikle, D.D. Vitamin D metabolism, mechanism of action, and clinical applications. Chem. Biol. 2014, 21, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Jones, G. Metabolism and biomarkers of vitamin D. Scand. J. Clin. Lab. Investig. Suppl. 2012, 243, 7–13. [Google Scholar]
- Christakos, S.; Dhawan, P.; Liu, Y.; Peng, X.; Porta, A. New insights into the mechanisms of vitamin D action. J. Cell Biochem. 2003, 88, 695–705. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Zhuang, X.; Lin, L.; Yu, P.; Wang, Y.; Shi, Y.; Hu, G.; Sun, Y. New horizons in tumor microenvironment biology challenges and opportunities. BMC Med. 2015, 13, 45. [Google Scholar] [CrossRef] [PubMed]
- Ariztia, E.V.; Lee, C.J.; Gogoi, R.; Fishman, D.A. The tumor microenvironment: Key to early detection. Crit. Rev. Clin. Lab. Sci. 2006, 43, 393–425. [Google Scholar] [CrossRef] [PubMed]
- Grivennikov, S.I.; Greten, F.R.; Karin, M. Immunity, inflammation, and cancer. Cell 2010, 140, 883–899. [Google Scholar] [CrossRef] [PubMed]
- Gajewski, T.F.; Schreiber, H.; Fu, Y.X. Innate and adaptive immune cells in the tumor microenvironment. Nat. Immunol. 2013, 14, 1014–1022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zamarron, B.F.; Chen, W. Dual roles of immune cells and their factors in cancer development and progression. Int. J. Biol. Sci. 2011, 7, 651–658. [Google Scholar] [CrossRef] [PubMed]
- Tretli, S.; Schwartz, G.G.; Torjesen, P.A.; Robsahm, T.E. Serum levels of 25-hydroxyvitamin D and survival in Norwegian patients with cancer of breast, colon, lung, and lymphoma: A population-based study. Cancer Causes Control 2012, 23, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Tagliabue, E.; Raimondi, S.; Gandini, S. Vitamin D, Cancer Risk, and Mortality. Adv. Food Nutr. Res. 2015, 75, 1–52. [Google Scholar] [PubMed]
- Ordonez Mena, J.M.; Brenner, H. Vitamin D and cancer: An overview on epidemiological studies. Adv. Exp. Med. Biol. 2014, 810, 17–32. [Google Scholar] [PubMed]
- Pilz, S.; Tomaschitz, A.; Obermayer-Pietsch, B.; Dobnig, H.; Pieber, T.R. Epidemiology of vitamin D insufficiency and cancer mortality. Anticancer Res. 2009, 29, 3699–3704. [Google Scholar] [PubMed]
- Garland, C.F.; Garland, F.C. Do sunlight and vitamin D reduce the likelihood of colon cancer? Int. J. Epidemiol. 1980, 9, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Cho, M.; Peddi, P.F.; Ding, K.; Chen, L.; Thomas, D.; Wang, J.; Lockhart, A.C.; Tan, B.; Wang-Gillam, A. Vitamin D deficiency and prognostics among patients with pancreatic adenocarcinoma. J. Transl. Med. 2013, 11, 206. [Google Scholar] [CrossRef] [PubMed]
- Budhathoki, S.; Hidaka, A.; Yamaji, T.; Sawada, N.; Tanaka-Mizuno, S.; Kuchiba, A.; Charvat, H.; Goto, A.; Kojima, S.; Sudo, N.; et al. Plasma 25-hydroxyvitamin D concentration and subsequent risk of total and site specific cancers in Japanese population: Large case-cohort study within Japan Public Health Center-based Prospective Study cohort. BMJ 2018, 360, k671. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Chen, P.; Li, J.; Chu, R.; Xie, D.; Wang, H. Review: The impacts of circulating 25-hydroxyvitamin D levels on cancer patient outcomes: A systematic review and meta-analysis. J. Clin. Endocrinol. Metab. 2014, 99, 2327–2336. [Google Scholar] [CrossRef] [PubMed]
- Colston, K.W.; Hansen, C.M. Mechanisms implicated in the growth regulatory effects of vitamin D in breast cancer. Endocr. Relat. Cancer 2002, 9, 45–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gorham, E.D.; Garland, C.F.; Garland, F.C.; Grant, W.B.; Mohr, S.B.; Lipkin, M.; Newmark, H.L.; Giovannucci, E.; Wei, M.; Holick, M.F. Vitamin D and prevention of colorectal cancer. J. Steroid. Biochem. Mol. Biol. 2005, 97, 179–194. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Zhang, P.; Wang, F.; Yang, J.; Liu, Z.; Qin, H. Association between vitamin D and risk of colorectal cancer: A systematic review of prospective studies. J. Clin. Oncol. 2011, 29, 3775–3782. [Google Scholar] [CrossRef] [PubMed]
- Skinner, H.G.; Michaud, D.S.; Giovannucci, E.; Willett, W.C.; Colditz, G.A.; Fuchs, C.S. Vitamin D intake and the risk for pancreatic cancer in two cohort studies. Cancer Epidemiol. Biomarkers Prev. 2006, 15, 1688–1695. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, A.V.; Trump, D.L.; Johnson, C.S.; Feldman, D. The role of vitamin D in cancer prevention and treatment. Endocrinol. Metab. Clin. N. Am. 2010, 39, 401–418. [Google Scholar] [CrossRef] [PubMed]
- Moukayed, M.; Grant, W.B. The roles of UVB and vitamin D in reducing risk of cancer incidence and mortality: A review of the epidemiology, clinical trials, and mechanisms. Rev. Endocr. Metab. Disord. 2017, 18, 167–182. [Google Scholar] [CrossRef] [PubMed]
- Giovannucci, E. The epidemiology of vitamin D and cancer incidence and mortality: A review (United States). Cancer Causes Control 2005, 16, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Grandi, N.; Raum, E.; Haug, U.; Arndt, V.; Brenner, H. Meta-analysis: Serum vitamin D and breast cancer risk. Eur. J. Cancer 2010, 46, 2196–2205. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Je, Y. Vitamin D intake, blood 25(OH)D levels, and breast cancer risk or mortality: A meta-analysis. Br. J. Cancer 2014, 110, 2772–2784. [Google Scholar] [CrossRef] [PubMed]
- Schuster, I. Cytochromes P450 are essential players in the vitamin D signaling system. Biochim. Biophys. Acta 2011, 1814, 186–199. [Google Scholar] [CrossRef] [PubMed]
- Segersten, U.; Correa, P.; Hewison, M.; Hellman, P.; Dralle, H.; Carling, T.; Akerstrom, G.; Westin, G. 25-hydroxyvitamin D3-1α-hydroxylase expression in normal and pathological parathyroid glands. J. Clin. Endocrinol. Metab. 2002, 87, 2967–2972. [Google Scholar] [CrossRef] [PubMed]
- Hsu, J.Y.; Feldman, D.; McNeal, J.E.; Peehl, D.M. Reduced 1α-hydroxylase activity in human prostate cancer cells correlates with decreased susceptibility to 25-hydroxyvitamin D3-induced growth inhibition. Cancer Res. 2001, 61, 2852–2856. [Google Scholar] [PubMed]
- Haussler, M.R.; Whitfield, G.K.; Haussler, C.A.; Hsieh, J.C.; Thompson, P.D.; Selznick, S.H.; Dominguez, C.E.; Jurutka, P.W. The nuclear vitamin D receptor: Biological and molecular regulatory properties revealed. J. Bone Miner. Res. 1998, 13, 325–349. [Google Scholar] [CrossRef] [PubMed]
- Hobaus, J.; Hummel, D.M.; Thiem, U.; Fetahu, I.S.; Aggarwal, A.; Mullauer, L.; Heller, G.; Egger, G.; Mesteri, I.; Baumgartner-Parzer, S.; et al. Increased copy-number and not DNA hypomethylation causes overexpression of the candidate proto-oncogene CYP24A1 in colorectal cancer. Int. J. Cancer 2013, 133, 1380–1388. [Google Scholar] [CrossRef] [PubMed]
- Albertson, D.G.; Ylstra, B.; Segraves, R.; Collins, C.; Dairkee, S.H.; Kowbel, D.; Kuo, W.L.; Gray, J.W.; Pinkel, D. Quantitative mapping of amplicon structure by array CGH identifies CYP24 as a candidate oncogene. Nat. Genet. 2000, 25, 144–146. [Google Scholar] [CrossRef] [PubMed]
- Borkowski, R.; Du, L.; Zhao, Z.; McMillan, E.; Kosti, A.; Yang, C.R.; Suraokar, M.; Wistuba, I.I.; Gazdar, A.F.; Minna, J.D.; et al. Genetic mutation of p53 and suppression of the miR-17 approximately 92 cluster are synthetic lethal in non-small cell lung cancer due to upregulation of vitamin D Signaling. Cancer Res. 2015, 75, 666–675. [Google Scholar] [CrossRef] [PubMed]
- Sharan, D.; Ajeesh, P.S.; Rameshkumar, R.; Mathankumar, M.; Paulina, R.J.; Manjula, M. Virtual reality based therapy for post operative rehabilitation of children with cerebral palsy. Work 2012, 41, 3612–3615. [Google Scholar] [PubMed]
- Deeb, K.K.; Trump, D.L.; Johnson, C.S. Vitamin D signalling pathways in cancer: Potential for anticancer therapeutics. Nat. Rev. Cancer 2007, 7, 684–700. [Google Scholar] [CrossRef] [PubMed]
- Mimori, K.; Tanaka, Y.; Yoshinaga, K.; Masuda, T.; Yamashita, K.; Okamoto, M.; Inoue, H.; Mori, M. Clinical significance of the overexpression of the candidate oncogene CYP24 in esophageal cancer. Ann. Oncol. 2004, 15, 236–241. [Google Scholar] [CrossRef] [PubMed]
- Lopes, N.; Sousa, B.; Martins, D.; Gomes, M.; Vieira, D.; Veronese, L.A.; Milanezi, F.; Paredes, J.; Costa, J.L.; Schmitt, F. Alterations in Vitamin D signalling and metabolic pathways in breast cancer progression: A study of VDR, CYP27B1 and CYP24A1 expression in benign and malignant breast lesions. BMC Cancer 2010, 10, 483. [Google Scholar] [CrossRef] [PubMed]
- De Souza, A.P.; Bonorino, C. Tumor immunosuppressive environment: Effects on tumor-specific and nontumor antigen immune responses. Expert. Rev. Anticancer Ther. 2009, 9, 1317–1332. [Google Scholar] [CrossRef] [PubMed]
- Wacker, M.; Holick, M.F. Vitamin D—effects on skeletal and extraskeletal health and the need for supplementation. Nutrients 2013, 5, 111–148. [Google Scholar] [CrossRef] [PubMed]
- Wobke, T.K.; Sorg, B.L.; Steinhilber, D. Vitamin D in inflammatory diseases. Front. Physiol. 2014, 5, 244. [Google Scholar] [PubMed]
- Bu, P.; Wang, L.; Chen, K.Y.; Srinivasan, T.; Murthy, P.K.; Tung, K.L.; Varanko, A.K.; Chen, H.J.; Ai, Y.; King, S.; et al. A miR-34a-Numb Feedforward Loop Triggered by Inflammation Regulates Asymmetric Stem Cell Division in Intestine and Colon Cancer. Cell Stem Cell 2016, 18, 189–202. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Liu, S.; Sams, G.H.; Curphey, D.P.; Santhanam, R.; Rush, L.J.; Schaefer, D.; Falkenberg, L.G.; Sullivan, L.; Jaroncyk, L.; et al. Aberrant overexpression of IL-15 initiates large granular lymphocyte leukemia through chromosomal instability and DNA hypermethylation. Cancer Cell 2012, 22, 645–655. [Google Scholar] [CrossRef] [PubMed]
- Tili, E.; Michaille, J.J.; Wernicke, D.; Alder, H.; Costinean, S.; Volinia, S.; Croce, C.M. Mutator activity induced by microRNA-155 (miR-155) links inflammation and cancer. Proc. Natl. Acad. Sci. USA 2011, 108, 4908–4913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El Rayes, T.; Catena, R.; Lee, S.; Stawowczyk, M.; Joshi, N.; Fischbach, C.; Powell, C.A.; Dannenberg, A.J.; Altorki, N.K.; Gao, D.; et al. Lung inflammation promotes metastasis through neutrophil protease-mediated degradation of Tsp-1. Proc. Natl. Acad. Sci. USA 2015, 112, 16000–16005. [Google Scholar] [CrossRef] [PubMed]
- Katoh, H.; Wang, D.; Daikoku, T.; Sun, H.; Dey, S.K.; Dubois, R.N. CXCR2-expressing myeloid-derived suppressor cells are essential to promote colitis-associated tumorigenesis. Cancer Cell 2013, 24, 631–644. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Xiao, J.; Zhou, X.; Xu, M.; Hu, C.; Xu, X.; Lu, Y.; Liu, C.; Xue, S.; Nie, L.; et al. STK4 regulates TLR pathways and protects against chronic inflammation-related hepatocellular carcinoma. J. Clin. Investig. 2015, 125, 4239–4254. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Ouyang, W.; Huang, C. Inflammation, a key event in cancer development. Mol. Cancer Res. 2006, 4, 221–233. [Google Scholar] [CrossRef] [PubMed]
- Balkwill, F.; Mantovani, A. Inflammation and cancer: Back to Virchow? Lancet 2001, 357, 539–545. [Google Scholar] [CrossRef]
- Cai, G.H.; Li, M.X.; Lu, L.; LokYi Chan, R.; Wang, J.H.; Cho, C.H. The current role and therapeutic targets of vitamin D in gastrointestinal inflammation and cancer. Curr. Pharm. Des. 2015, 21, 2917–2923. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Karin, M. Tumor-Elicited Inflammation and Colorectal Cancer. Adv. Cancer Res. 2015, 128, 173–196. [Google Scholar] [PubMed]
- Candido, J.; Hagemann, T. Cancer-related inflammation. J. Clin. Immunol. 2013, 33, S79–S84. [Google Scholar] [CrossRef] [PubMed]
- Raymond, E.; Dalgleish, A.; Damber, J.E.; Smith, M.; Pili, R. Mechanisms of action of tasquinimod on the tumour microenvironment. Cancer Chemother. Pharmacol. 2014, 73, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Zhang, Y.; Jia, T.; Sun, Y. Molecular mechanism underlying the tumor-promoting functions of carcinoma-associated fibroblasts. Tumour Biol. 2015, 36, 1385–1394. [Google Scholar] [CrossRef] [PubMed]
- Piazuelo, E.; Lanas, A. NSAIDS and gastrointestinal cancer. Prostaglandins Other Lipid Mediat. 2015, 120, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Groblewska, M.; Mroczko, B.; Wereszczynska-Siemiatkowska, U.; Kedra, B.; Lukaszewicz, M.; Baniukiewicz, A.; Szmitkowski, M. Serum interleukin 6 (IL-6) and C-reactive protein (CRP) levels in colorectal adenoma and cancer patients. Clin. Chem. Lab. Med. 2008, 46, 1423–1428. [Google Scholar] [CrossRef] [PubMed]
- Powell, I.J.; Dyson, G.; Land, S.; Ruterbusch, J.; Bock, C.H.; Lenk, S.; Herawi, M.; Everson, R.; Giroux, C.N.; Schwartz, A.G.; et al. Genes associated with prostate cancer are differentially expressed in African American and European American men. Cancer Epidemiol. Biomark. Prev. 2013, 22, 891–897. [Google Scholar] [CrossRef] [PubMed]
- Barrat, F.J.; Cua, D.J.; Boonstra, A.; Richards, D.F.; Crain, C.; Savelkoul, H.F.; de Waal-Malefyt, R.; Coffman, R.L.; Hawrylowicz, C.M.; O’Garra, A. In vitro generation of interleukin 10-producing regulatory CD4+ T cells is induced by immunosuppressive drugs and inhibited by T helper type 1 (Th1)- and Th2-inducing cytokines. J. Exp. Med. 2002, 195, 603–616. [Google Scholar] [CrossRef] [PubMed]
- Dauletbaev, N.; Herscovitch, K.; Das, M.; Chen, H.; Bernier, J.; Matouk, E.; Berube, J.; Rousseau, S.; Lands, L.C. Down-regulation of IL-8 by high-dose vitamin D is specific to hyperinflammatory macrophages and involves mechanisms beyond up-regulation of DUSP1. Br. J. Pharmacol. 2015, 172, 4757–4771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, D.P.; Li, J.; Tewari, A.K. Inflammation and prostate cancer: The role of interleukin 6 (IL-6). BJU Int. 2014, 113, 986–992. [Google Scholar] [CrossRef] [PubMed]
- Lippitz, B.E. Cytokine patterns in patients with cancer: A systematic review. Lancet Oncol. 2013, 14, e218–228. [Google Scholar] [CrossRef]
- Dalwadi, H.; Krysan, K.; Heuze-Vourc’h, N.; Dohadwala, M.; Elashoff, D.; Sharma, S.; Cacalano, N.; Lichtenstein, A.; Dubinett, S. Cyclooxygenase-2-dependent activation of signal transducer and activator of transcription 3 by interleukin-6 in non-small cell lung cancer. Clin. Cancer Res. 2005, 11, 7674–7682. [Google Scholar] [CrossRef] [PubMed]
- Cathcart, J.M.; Banach, A.; Liu, A.; Chen, J.; Goligorsky, M.; Cao, J. Interleukin-6 increases matrix metalloproteinase-14 (MMP-14) levels via down-regulation of p53 to drive cancer progression. Oncotarget 2016, 7, 61107–61120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nonn, L.; Peng, L.; Feldman, D.; Peehl, D.M. Inhibition of p38 by vitamin D reduces interleukin-6 production in normal prostate cells via mitogen-activated protein kinase phosphatase 5: Implications for prostate cancer prevention by vitamin D. Cancer Res. 2006, 66, 4516–4524. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.; Kong, H.; Li, Y. Prognostic value of interleukin-8 and MMP-9 in nasopharyngeal carcinoma. Eur. Arch. Otorhinolaryngol. 2014, 271, 503–509. [Google Scholar] [CrossRef] [PubMed]
- Haraguchi, M.; Komuta, K.; Akashi, A.; Matsuzaki, S.; Furui, J.; Kanematsu, T. Elevated IL-8 levels in the drainage vein of resectable Dukes’ C colorectal cancer indicate high risk for developing hepatic metastasis. Oncol. Rep. 2002, 9, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.K.; Simoes, B.M.; Howell, S.J.; Farnie, G.; Clarke, R.B. Recent advances reveal IL-8 signaling as a potential key to targeting breast cancer stem cells. Breast Cancer Res. 2013, 15, 210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bao, B.Y.; Yao, J.; Lee, Y.F. 1α,25-dihydroxyvitamin D3 suppresses interleukin-8-mediated prostate cancer cell angiogenesis. Carcinogenesis 2006, 27, 1883–1893. [Google Scholar] [CrossRef] [PubMed]
- Hidaka, M.; Wakabayashi, I.; Takeda, Y.; Fukuzawa, K. Vitamin D3 derivatives increase soluble CD14 release through ERK1/2 activation and decrease IL-8 production in intestinal epithelial cells. Eur. J. Pharmacol. 2013, 721, 305–312. [Google Scholar] [CrossRef] [PubMed]
- John, M.; Au, B.T.; Jose, P.J.; Lim, S.; Saunders, M.; Barnes, P.J.; Mitchell, J.A.; Belvisi, M.G.; Chung, K.F. Expression and release of interleukin-8 by human airway smooth muscle cells: Inhibition by Th-2 cytokines and corticosteroids. Am. J. Respir. Cell Mol. Biol. 1998, 18, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Schandene, L.; Alonso-Vega, C.; Willems, F.; Gerard, C.; Delvaux, A.; Velu, T.; Devos, R.; de Boer, M.; Goldman, M. B7/CD28-dependent IL-5 production by human resting T cells is inhibited by IL-10. J. Immunol. 1994, 152, 4368–4374. [Google Scholar] [PubMed]
- de Vries, J.E. Immunosuppressive and anti-inflammatory properties of interleukin 10. Ann. Med. 1995, 27, 537–541. [Google Scholar] [CrossRef] [PubMed]
- Dennis, K.L.; Blatner, N.R.; Gounari, F.; Khazaie, K. Current status of interleukin-10 and regulatory T-cells in cancer. Curr. Opin. Oncol. 2013, 25, 637–645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coquerelle, C.; Oldenhove, G.; Acolty, V.; Denoeud, J.; Vansanten, G.; Verdebout, J.M.; Mellor, A.; Bluestone, J.A.; Moser, M. Anti-CTLA-4 treatment induces IL-10-producing ICOS+ regulatory T cells displaying IDO-dependent anti-inflammatory properties in a mouse model of colitis. Gut 2009, 58, 1363–1373. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Liakou, C.I.; Kamat, A.; Pettaway, C.; Ward, J.F.; Tang, D.N.; Sun, J.; Jungbluth, A.A.; Troncoso, P.; Logothetis, C.; et al. Anti-CTLA-4 therapy results in higher CD4+ICOShi T cell frequency and IFN-γ levels in both nonmalignant and malignant prostate tissues. Proc. Natl. Acad. Sci. USA 2009, 106, 2729–2734. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Stolina, M.; Lin, Y.; Gardner, B.; Miller, P.W.; Kronenberg, M.; Dubinett, S.M. T cell-derived IL-10 promotes lung cancer growth by suppressing both T cell and APC function. J. Immunol. 1999, 163, 5020–5028. [Google Scholar] [PubMed]
- Urry, Z.; Xystrakis, E.; Richards, D.F.; McDonald, J.; Sattar, Z.; Cousins, D.J.; Corrigan, C.J.; Hickman, E.; Brown, Z.; Hawrylowicz, C.M. Ligation of TLR9 induced on human IL-10-secreting Tregs by 1α,25-dihydroxyvitamin D3 abrogates regulatory function. J. Clin. Investig. 2009, 119, 387–398. [Google Scholar] [CrossRef] [PubMed]
- Sannino, G.; Armbruster, N.; Bodenhofer, M.; Haerle, U.; Behrens, D.; Buchholz, M.; Rothbauer, U.; Sipos, B.; Schmees, C. Role of BCL9L in transforming growth factor-β (TGF-β)-induced epithelial-to-mesenchymal-transition (EMT) and metastasis of pancreatic cancer. Oncotarget 2016, 7, 73725–73738. [Google Scholar] [CrossRef] [PubMed]
- Mori, S.; Kodaira, M.; Ito, A.; Okazaki, M.; Kawaguchi, N.; Hamada, Y.; Takada, Y.; Matsuura, N. Enhanced Expression of Integrin αvβ3 Induced by TGF-β Is Required for the Enhancing Effect of Fibroblast Growth Factor 1 (FGF1) in TGF-β-Induced Epithelial-Mesenchymal Transition (EMT) in Mammary Epithelial Cells. PLoS ONE 2015, 10, e0137486. [Google Scholar] [CrossRef] [PubMed]
- Artaza, J.N.; Norris, K.C. Vitamin D reduces the expression of collagen and key profibrotic factors by inducing an antifibrotic phenotype in mesenchymal multipotent cells. J. Endocrinol. 2009, 200, 207–221. [Google Scholar] [CrossRef] [PubMed]
- Telliez, A.; Furman, C.; Pommery, N.; Henichart, J.P. Mechanisms leading to COX-2 expression and COX-2 induced tumorigenesis: Topical therapeutic strategies targeting COX-2 expression and activity. Anticancer Agents Med. Chem. 2006, 6, 187–208. [Google Scholar] [CrossRef] [PubMed]
- Bonventre, J.V.; Huang, Z.; Taheri, M.R.; O’Leary, E.; Li, E.; Moskowitz, M.A.; Sapirstein, A. Reduced fertility and postischaemic brain injury in mice deficient in cytosolic phospholipase A2. Nature 1997, 390, 622–625. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Qiu, J.; Li, Q.; Shi, Z. Prostaglandin E2 Signaling: Alternative Target for Glioblastoma? Trend. Cancer 2017, 3, 75–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Funk, C.D.; FitzGerald, G.A. COX-2 inhibitors and cardiovascular risk. J. Cardiovasc. Pharmacol. 2007, 50, 470–479. [Google Scholar] [CrossRef] [PubMed]
- FitzGerald, G.A.; Loll, P. COX in a crystal ball: Current status and future promise of prostaglandin research. J. Clin. Investig. 2001, 107, 1335–1337. [Google Scholar] [CrossRef] [PubMed]
- Ricciotti, E.; FitzGerald, G.A. Prostaglandins and inflammation. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 986–1000. [Google Scholar] [CrossRef] [PubMed]
- Harizi, H.; Corcuff, J.B.; Gualde, N. Arachidonic-acid-derived eicosanoids: Roles in biology and immunopathology. Trends Mol. Med. 2008, 14, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Hughes-Fulford, M. Prostaglandin E2 and the protein kinase A pathway mediate arachidonic acid induction of c-fos in human prostate cancer cells. Br. J. Cancer 2000, 82, 2000–2006. [Google Scholar] [PubMed]
- Kawahara, K.; Hohjoh, H.; Inazumi, T.; Tsuchiya, S.; Sugimoto, Y. Prostaglandin E2-induced inflammation: Relevance of prostaglandin E receptors. Biochim. Biophys. Acta 2015, 1851, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Aoki, T.; Tsuruyama, T.; Narumiya, S. Definition of Prostaglandin E2-EP2 Signals in the Colon Tumor Microenvironment That Amplify Inflammation and Tumor Growth. Cancer Res. 2015, 75, 2822–2832. [Google Scholar] [CrossRef] [PubMed]
- Bazzani, L.; Donnini, S.; Finetti, F.; Christofori, G.; Ziche, M. PGE2/EP3/SRC signaling induces EGFR nuclear translocation and growth through EGFR ligands release in lung adenocarcinoma cells. Oncotarget 2017, 8, 31270–31287. [Google Scholar] [CrossRef] [PubMed]
- Moreno, J.; Krishnan, A.V.; Swami, S.; Nonn, L.; Peehl, D.M.; Feldman, D. Regulation of prostaglandin metabolism by calcitriol attenuates growth stimulation in prostate cancer cells. Cancer Res. 2005, 65, 7917–7925. [Google Scholar] [CrossRef] [PubMed]
- Rothwell, P.M.; Fowkes, F.G.; Belch, J.F.; Ogawa, H.; Warlow, C.P.; Meade, T.W. Effect of daily aspirin on long-term risk of death due to cancer: Analysis of individual patient data from randomised trials. Lancet 2011, 377, 31–41. [Google Scholar] [CrossRef]
- Bos, P.D.; Zhang, X.H.; Nadal, C.; Shu, W.; Gomis, R.R.; Nguyen, D.X.; Minn, A.J.; van de Vijver, M.J.; Gerald, W.L.; Foekens, J.A.; et al. Genes that mediate breast cancer metastasis to the brain. Nature 2009, 459, 1005–1009. [Google Scholar] [CrossRef] [PubMed]
- Regulski, M.; Regulska, K.; Prukala, W.; Piotrowska, H.; Stanisz, B.; Murias, M. COX-2 inhibitors: A novel strategy in the management of breast cancer. Drug Discov. Today 2016, 21, 598–615. [Google Scholar] [CrossRef] [PubMed]
- Clemente, M.; Sanchez-Archidona, A.R.; Sardon, D.; Diez, L.; Martin-Ruiz, A.; Caceres, S.; Sassi, F.; Dolores Perez-Alenza, M.; Illera, J.C.; Dunner, S.; et al. Different role of COX-2 and angiogenesis in canine inflammatory and non-inflammatory mammary cancer. Vet. J. 2013, 197, 427–432. [Google Scholar] [CrossRef] [PubMed]
- Su, J.L.; Shih, J.Y.; Yen, M.L.; Jeng, Y.M.; Chang, C.C.; Hsieh, C.Y.; Wei, L.H.; Yang, P.C.; Kuo, M.L. Cyclooxygenase-2 induces EP1- and HER-2/Neu-dependent vascular endothelial growth factor-C up-regulation: A novel mechanism of lymphangiogenesis in lung adenocarcinoma. Cancer Res. 2004, 64, 554–564. [Google Scholar] [CrossRef] [PubMed]
- Sawaoka, H.; Kawano, S.; Tsuji, S.; Tsujii, M.; Murata, H.; Hori, M. Effects of NSAIDs on proliferation of gastric cancer cells in vitro: Possible implication of cyclooxygenase-2 in cancer development. J. Clin. Gastroenterol. 1998, 27, S47–S52. [Google Scholar] [CrossRef] [PubMed]
- Nzeako, U.C.; Guicciardi, M.E.; Yoon, J.H.; Bronk, S.F.; Gores, G.J. COX-2 inhibits Fas-mediated apoptosis in cholangiocarcinoma cells. Hepatology 2002, 35, 552–559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hung, W.C.; Chang, H.C.; Pan, M.R.; Lee, T.H.; Chuang, L.Y. Induction of p27(KIP1) as a mechanism underlying NS398-induced growth inhibition in human lung cancer cells. Mol. Pharmacol. 2000, 58, 1398–1403. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, A.V.; Feldman, D. Molecular pathways mediating the anti-inflammatory effects of calcitriol: Implications for prostate cancer chemoprevention and treatment. Endocr. Relat. Cancer 2010, 17, R19–38. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Jiang, R.; Yang, Y.; Ding, S.; Deng, H. 1,25-Dihydroxyvitamin D3 inhibits growth of the breast cancer cell line MCF-7 and downregulates cytochrome P4501B1 through the COX-2/PGE2 pathway. Oncol. Rep. 2012, 28, 2131–2137. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.H.; Kim, B.G.; Robinson, J.; Fink, S.; Yan, M.; Sporn, M.B.; Markowitz, S.D.; Letterio, J.J. Synthetic triterpenoid induces 15-PGDH expression and suppresses inflammation-driven colon carcinogenesis. J. Clin. Investig. 2014, 124, 2472–2482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hazra, S.; Batra, R.K.; Tai, H.H.; Sharma, S.; Cui, X.; Dubinett, S.M. Pioglitazone and rosiglitazone decrease prostaglandin E2 in non-small-cell lung cancer cells by up-regulating 15-hydroxyprostaglandin dehydrogenase. Mol. Pharmacol. 2007, 71, 1715–1720. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.; Rerko, R.M.; Platzer, P.; Dawson, D.; Willis, J.; Tong, M.; Lawrence, E.; Lutterbaugh, J.; Lu, S.; Willson, J.K.; et al. 15-Hydroxyprostaglandin dehydrogenase, a COX-2 oncogene antagonist, is a TGF-β-induced suppressor of human gastrointestinal cancers. Proc. Natl. Acad. Sci. USA 2004, 101, 17468–17473. [Google Scholar] [CrossRef] [PubMed]
- Wolf, I.; O’Kelly, J.; Rubinek, T.; Tong, M.; Nguyen, A.; Lin, B.T.; Tai, H.H.; Karlan, B.Y.; Koeffler, H.P. 15-hydroxyprostaglandin dehydrogenase is a tumor suppressor of human breast cancer. Cancer Res. 2006, 66, 7818–7823. [Google Scholar] [CrossRef] [PubMed]
- Quidville, V.; Segond, N.; Lausson, S.; Frenkian, M.; Cohen, R.; Jullienne, A. 15-Hydroxyprostaglandin-dehydrogenase is involved in anti-proliferative effect of non-steroidal anti-inflammatory drugs COX-1 inhibitors on a human medullary thyroid carcinoma cell line. Prostaglandins Other Lipid Mediat. 2006, 81, 14–30. [Google Scholar] [CrossRef] [PubMed]
- Swami, S.; Krishnan, A.V.; Moreno, J.; Bhattacharyya, R.B.; Peehl, D.M.; Feldman, D. Calcitriol and genistein actions to inhibit the prostaglandin pathway: Potential combination therapy to treat prostate cancer. J. Nutr. 2007, 137, 205S–210S. [Google Scholar] [CrossRef] [PubMed]
- Gee, J.R.; Montoya, R.G.; Khaled, H.M.; Sabichi, A.L.; Grossman, H.B. Cytokeratin 20, AN43, PGDH, and COX-2 expression in transitional and squamous cell carcinoma of the bladder. Urol. Oncol. 2003, 21, 266–270. [Google Scholar] [CrossRef]
- Krishnan, A.V.; Shinghal, R.; Raghavachari, N.; Brooks, J.D.; Peehl, D.M.; Feldman, D. Analysis of vitamin D-regulated gene expression in LNCaP human prostate cancer cells using cDNA microarrays. Prostate 2004, 59, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Park, J.I.; Lee, M.G.; Cho, K.; Park, B.J.; Chae, K.S.; Byun, D.S.; Ryu, B.K.; Park, Y.K.; Chi, S.G. Transforming growth factor-β1 activates interleukin-6 expression in prostate cancer cells through the synergistic collaboration of the Smad2, p38-NF-κB, JNK, and Ras signaling pathways. Oncogene 2003, 22, 4314–4332. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.K.; Choi, E.J. Pathological roles of MAPK signaling pathways in human diseases. Biochim. Biophys. Acta 2010, 1802, 396–405. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Shen, S.; Verma, I.M. NF-κB, an active player in human cancers. Cancer Immunol. Res. 2014, 2, 823–830. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Leung, D.Y.; Richers, B.N.; Liu, Y.; Remigio, L.K.; Riches, D.W.; Goleva, E. Vitamin D inhibits monocyte/macrophage proinflammatory cytokine production by targeting MAPK phosphatase-1. J. Immunol. 2012, 188, 2127–2135. [Google Scholar] [CrossRef] [PubMed]
- Cohen-Lahav, M.; Shany, S.; Tobvin, D.; Chaimovitz, C.; Douvdevani, A. Vitamin D decreases NF-κB activity by increasing IκBα levels. Nephrol. Dial. Transplant. 2006, 21, 889–897. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Mustafi, R.; Cerda, S.; Chumsangsri, A.; Xia, Y.R.; Li, Y.C.; Bissonnette, M. Lithocholic acid down-regulation of NF-κB activity through vitamin D receptor in colonic cancer cells. J. Steroid. Biochem. Mol. Biol. 2008, 111, 37–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tse, A.K.; Zhu, G.Y.; Wan, C.K.; Shen, X.L.; Yu, Z.L.; Fong, W.F. 1α,25-Dihydroxyvitamin D3 inhibits transcriptional potential of nuclear factor κB in breast cancer cells. Mol Immunol 2010, 47, 1728–1738. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. Vitamin D: A millenium perspective. J. Cell Biochem. 2003, 88, 296–307. [Google Scholar] [CrossRef] [PubMed]
- Munger, K.L.; Levin, L.I.; Hollis, B.W.; Howard, N.S.; Ascherio, A. Serum 25-hydroxyvitamin D levels and risk of multiple sclerosis. JAMA 2006, 296, 2832–2838. [Google Scholar] [CrossRef] [PubMed]
- Littorin, B.; Blom, P.; Scholin, A.; Arnqvist, H.J.; Blohme, G.; Bolinder, J.; Ekbom-Schnell, A.; Eriksson, J.W.; Gudbjornsdottir, S.; Nystrom, L.; et al. Lower levels of plasma 25-hydroxyvitamin D among young adults at diagnosis of autoimmune type 1 diabetes compared with control subjects: Results from the nationwide Diabetes Incidence Study in Sweden (DISS). Diabetologia 2006, 49, 2847–2852. [Google Scholar] [CrossRef] [PubMed]
- Merlino, L.A.; Curtis, J.; Mikuls, T.R.; Cerhan, J.R.; Criswell, L.A.; Saag, K.G. Vitamin D intake is inversely associated with rheumatoid arthritis: Results from the Iowa Women’s Health Study. Arthritis. Rheum. 2004, 50, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Sims, G.P.; Chen, X.X.; Gu, Y.Y.; Lipsky, P.E. Modulatory effects of 1,25-dihydroxyvitamin D3 on human B cell differentiation. J. Immunol. 2007, 179, 1634–1647. [Google Scholar] [CrossRef] [PubMed]
- Bittenbring, J.T.; Neumann, F.; Altmann, B.; Achenbach, M.; Reichrath, J.; Ziepert, M.; Geisel, J.; Regitz, E.; Held, G.; Pfreundschuh, M. Vitamin D deficiency impairs rituximab-mediated cellular cytotoxicity and outcome of patients with diffuse large B-cell lymphoma treated with but not without rituximab. J. Clin. Oncol. 2014, 32, 3242–3248. [Google Scholar] [CrossRef] [PubMed]
- Jeffery, L.E.; Burke, F.; Mura, M.; Zheng, Y.; Qureshi, O.S.; Hewison, M.; Walker, L.S.; Lammas, D.A.; Raza, K.; Sansom, D.M. 1,25-Dihydroxyvitamin D3 and IL-2 combine to inhibit T cell production of inflammatory cytokines and promote development of regulatory T cells expressing CTLA-4 and FoxP3. J. Immunol. 2009, 183, 5458–5467. [Google Scholar] [CrossRef] [PubMed]
- Edfeldt, K.; Liu, P.T.; Chun, R.; Fabri, M.; Schenk, M.; Wheelwright, M.; Keegan, C.; Krutzik, S.R.; Adams, J.S.; Hewison, M.; et al. T-cell cytokines differentially control human monocyte antimicrobial responses by regulating vitamin D. metabolism. Proc. Natl. Acad. Sci. USA 2010, 107, 22593–22598. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, V.; Kasapoglu, P.; Zamani, A.; Basiri, Z.; Tahamoli-Roudsari, A.; Alahgholi-Hajibehzad, M. Vitamin D3 inhibits the proliferation of T helper cells, downregulate CD4+ T cell cytokines and upregulate inhibitory markers. Hum. Immunol. 2018, 79, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Mayne, C.G.; Spanier, J.A.; Relland, L.M.; Williams, C.B.; Hayes, C.E. 1,25-Dihydroxyvitamin D3 acts directly on the T lymphocyte vitamin D receptor to inhibit experimental autoimmune encephalomyelitis. Eur. J. Immunol. 2011, 41, 822–832. [Google Scholar] [CrossRef] [PubMed]
- Palmer, M.T.; Lee, Y.K.; Maynard, C.L.; Oliver, J.R.; Bikle, D.D.; Jetten, A.M.; Weaver, C.T. Lineage-specific effects of 1,25-dihydroxyvitamin D3 on the development of effector CD4 T cells. J. Biol. Chem. 2011, 286, 997–1004. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Luo, F.; Xing, J.C.; Zhang, F.; Xu, J.Z.; Zhang, Z.H. 1,25(OH)2D3 inhibited Th17 cells differentiation via regulating the NF-κB activity and expression of IL-17. Cell Prolif. 2018, e12461. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.T.; Stenger, S.; Li, H.; Wenzel, L.; Tan, B.H.; Krutzik, S.R.; Ochoa, M.T.; Schauber, J.; Wu, K.; Meinken, C.; et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science 2006, 311, 1770–1773. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liu, W.; Sun, T.; Huang, Y.; Wang, Y.; Deb, D.K.; Yoon, D.; Kong, J.; Thadhani, R.; Li, Y.C. 1,25-Dihydroxyvitamin D promotes negative feedback regulation of TLR signaling via targeting microRNA-155-SOCS1 in macrophages. J. Immunol. 2013, 190, 3687–3695. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Nino, M.D.; Bozic, M.; Cordoba-Lanus, E.; Valcheva, P.; Gracia, O.; Ibarz, M.; Fernandez, E.; Navarro-Gonzalez, J.F.; Ortiz, A.; Valdivielso, J.M. Beyond proteinuria: VDR activation reduces renal inflammation in experimental diabetic nephropathy. Am. J. Physiol. Renal. Physiol. 2012, 302, F647–F657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Helming, L.; Bose, J.; Ehrchen, J.; Schiebe, S.; Frahm, T.; Geffers, R.; Probst-Kepper, M.; Balling, R.; Lengeling, A. 1α,25-Dihydroxyvitamin D3 is a potent suppressor of interferon γ-mediated macrophage activation. Blood 2005, 106, 4351–4358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steinman, R.M.; Banchereau, J. Taking dendritic cells into medicine. Nature 2007, 449, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Berer, A.; Stockl, J.; Majdic, O.; Wagner, T.; Kollars, M.; Lechner, K.; Geissler, K.; Oehler, L. 1,25-Dihydroxyvitamin D3 inhibits dendritic cell differentiation and maturation in vitro. Exp. Hematol. 2000, 28, 575–583. [Google Scholar] [CrossRef]
- Canning, M.O.; Grotenhuis, K.; de Wit, H.; Ruwhof, C.; Drexhage, H.A. 1-α,25-Dihydroxyvitamin D3 (1,25(OH)2D3) hampers the maturation of fully active immature dendritic cells from monocytes. Eur. J. Endocrinol. 2001, 145, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Szeles, L.; Keresztes, G.; Torocsik, D.; Balajthy, Z.; Krenacs, L.; Poliska, S.; Steinmeyer, A.; Zuegel, U.; Pruenster, M.; Rot, A.; et al. 1,25-dihydroxyvitamin D3 is an autonomous regulator of the transcriptional changes leading to a tolerogenic dendritic cell phenotype. J. Immunol. 2009, 182, 2074–2083. [Google Scholar] [CrossRef] [PubMed]
- Takeda, M.; Yamashita, T.; Sasaki, N.; Nakajima, K.; Kita, T.; Shinohara, M.; Ishida, T.; Hirata, K. Oral administration of an active form of vitamin D3 (calcitriol) decreases atherosclerosis in mice by inducing regulatory T cells and immature dendritic cells with tolerogenic functions. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 2495–2503. [Google Scholar] [CrossRef] [PubMed]
- Sommer, A.; Fabri, M. Vitamin D regulates cytokine patterns secreted by dendritic cells to promote differentiation of IL-22-producing T cells. PLoS ONE 2015, 10, e0130395. [Google Scholar] [CrossRef] [PubMed]


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, W.; Zhang, L.; Xu, H.-J.; Li, Y.; Hu, C.-M.; Yang, J.-Y.; Sun, M.-Y. The Anti-Inflammatory Effects of Vitamin D in Tumorigenesis. Int. J. Mol. Sci. 2018, 19, 2736. https://doi.org/10.3390/ijms19092736
Liu W, Zhang L, Xu H-J, Li Y, Hu C-M, Yang J-Y, Sun M-Y. The Anti-Inflammatory Effects of Vitamin D in Tumorigenesis. International Journal of Molecular Sciences. 2018; 19(9):2736. https://doi.org/10.3390/ijms19092736
Chicago/Turabian StyleLiu, Wei, Lei Zhang, Hui-Jing Xu, Yan Li, Chuan-Min Hu, Jing-Yan Yang, and Mei-Yan Sun. 2018. "The Anti-Inflammatory Effects of Vitamin D in Tumorigenesis" International Journal of Molecular Sciences 19, no. 9: 2736. https://doi.org/10.3390/ijms19092736
APA StyleLiu, W., Zhang, L., Xu, H. -J., Li, Y., Hu, C. -M., Yang, J. -Y., & Sun, M. -Y. (2018). The Anti-Inflammatory Effects of Vitamin D in Tumorigenesis. International Journal of Molecular Sciences, 19(9), 2736. https://doi.org/10.3390/ijms19092736