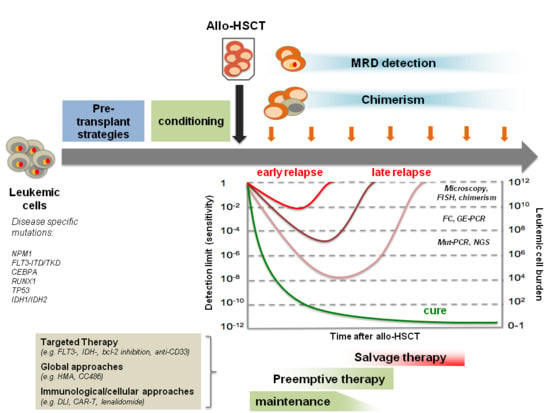
Relapse of Acute Myeloid Leukemia after Allogeneic Stem Cell Transplantation: Prevention, Detection, and Treatment
Abstract
:1. Introduction
2. Mutational Landscape and Pathophysiology of AML
3. Methods for Relapse Detection—Monitoring of Minimal Residual Disease (MRD)
4. Molecular MRD Assessment
5. Detection of Gene Mutations for MRD Assessment
6. Detection of Fusion Gene Transcripts for MRD Assessment
7. Quantification of Gene Expression for MRD Assessment
8. Chimerism Analyses for MRD Assessment
9. MRD Assessment by Multiparameter Flow Cytometry (MFC)
10. Prevention and Treatment of Relapse after Allo-SCT
11. FLT3 Inhibitors
12. Isocitrate Dehydrogenase (IDH) Inhibitors
13. Hypomethylating Agents and HDAC Inhibitors
14. Cellular Therapies
14.1. Donor Lymphocyte Infusions
14.2. Second Transplantation
14.3. New Cellular Therapies
15. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Döhner, H.; Estey, E.; Grimwade, D.; Amadori, S.; Appelbaum, F.R.; Büchner, T.; Dombret, H.; Ebert, B.L.; Fenaux, P.; Larson, R.A.; et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood 2017, 129, 424–447. [Google Scholar] [CrossRef] [PubMed]
- Gooley, T.A.; Chien, J.W.; Pergam, S.A.; Hingorani, S.; Sorror, M.L.; Boeckh, M. Reduced mortality after allogeneic hematopoietic cell transplantation. N. Engl. J. Med. 2010, 363, 2091–2101. [Google Scholar] [CrossRef] [PubMed]
- De Lima, M.; Porter, D.L.; Battiwalla, M.; Bishop, M.R.; Giralt, S.A.; Hardy, N.M. Proceedings from the National Cancer Institute’s Second International Workshop on the Biology, Prevention, and Treatment of Relapse after Hematopoietic Stem Cell Transplantation: Part III. Prevention and Treatment of Relapse after Allogeneic Transplantation. Biol. Blood Marrow Transplant. 2014, 20, 4–13. [Google Scholar] [PubMed] [Green Version]
- Bhagat, T.D.; Chen, S.; Bartenstein, M.; Barlowe, A.T.; Von Ahrens, D.; Choudhary, G.S. Epigenetically Aberrant Stroma In MDS Propagates Disease Via Wnt/β-Catenin Activation. Cancer Res. 2017, 77, 4846–4857. [Google Scholar] [CrossRef] [PubMed]
- Christopeit, M.; Kuss, O.; Finke, J.; Bacher, U.; Beelen, D.W.; Bornhäuser, M. Second Allograft for Hematologic Relapse of Acute Leukemia After First Allogeneic Stem-Cell Transplantation From Related and Unrelated Donors: The Role of Donor Change. J. Clin. Oncol. 2013, 31, 3259–3271. [Google Scholar] [CrossRef] [PubMed]
- Schmid, C.; de Wreede, L.C.; van Biezen, A.; Finke, J.; Ehninger, G.; Ganser, A. Outcome after relapse of myelodysplastic syndrome and secondary acute myeloid leukemia following allogeneic stem cell transplantation: A retrospective registry analysis on 698 patients by the Chronic Malignancies Working Party of the European Society of Blood and Marrow Transplantation. Haematologica 2018, 103, 237–245. [Google Scholar] [PubMed]
- Schmid, C.; Labopin, M.; Nagler, A.; Bornhäuser, M.; Finke, J.; Fassas, A. Donor lymphocyte infusion in the treatment of first hematological relapse after allogeneic stem-cell transplantation in adults with acute myeloid leukemia: A retrospective risk factors analysis and comparison with other strategies by the EBMT Acute Leukemia Working Party. J. Clin. Oncol. 2007, 25, 4938–4945. [Google Scholar] [PubMed]
- Schmid, C.; Labopin, M.; Nagler, A.; Niederwieser, D.; Castagna, L.; Tabrizi, R. Treatment, risk factors, and outcome of adults with relapsed AML after reduced intensity conditioning for allogeneic stem cell transplantation. Blood 2012, 119, 1599–1606. [Google Scholar] [CrossRef]
- Bullinger, L.; Döhner, K.; Döhner, H. Genomics of Acute Myeloid Leukemia Diagnosis and Pathways. J. Clin. Oncol. 2017, 35, 934–946. [Google Scholar] [CrossRef]
- Koreth, J.; Schlenk, R.; Kopecky, K.J.; Honda, S.; Sierra, J.; Djulbegovic, B.J. Allogeneic stem cell transplantation for acute myeloid leukemia in first complete remission: Systematic review and meta-analysis of prospective clinical trials. JAMA 2009, 301, 2349–2361. [Google Scholar] [CrossRef]
- Schlenk, R.F.; Döhner, K.; Krauter, J.; Fröhling, S.; Corbacioglu, A.; Bullinger, L. Mutations and treatment outcome in cytogenetically normal acute myeloid leukemia. N. Engl. J. Med. 2008, 358, 1909–1918. [Google Scholar] [CrossRef] [PubMed]
- Grimwade, D.; Freeman, S.D. Defining minimal residual disease in acute myeloid leukemia: Which platforms are ready for “prime time”? Blood 2014, 124, 3345–3355. [Google Scholar] [CrossRef]
- Grimwade, D.; Ivey, A.; Huntly, B.J.P. Molecular landscape of acute myeloid leukemia in younger adults and its clinical relevance. Blood 2016, 127, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Kayser, S.; Schlenk, R.F.; Grimwade, D.; Yosuico, V.E.D.; Walter, R.B. Minimal residual disease–directed therapy in acute myeloid leukemia. Blood 2015, 125, 2331–2335. [Google Scholar] [CrossRef] [PubMed]
- Schuurhuis, G.J.; Heuser, M.; Freeman, S.; Béné, M.-C.; Buccisano, F.; Cloos, J. Minimal/measurable residual disease in AML: A consensus document from the European LeukemiaNet MRD Working Party. Blood 2018, 131, 1275–1291. [Google Scholar] [CrossRef]
- DiNardo, C.D.; Stein, E.M.; de Botton, S.; Roboz, G.J.; Altman, J.K.; Mims, A.S. Durable Remissions with Ivosidenib in IDH1-Mutated Relapsed or Refractory AML. N. Engl. J. Med. 2018, 378, 2386–2398. [Google Scholar] [CrossRef]
- Stein, E.M.; DiNardo, C.D.; Pollyea, D.A.; Fathi, A.T.; Roboz, G.J.; Altman, J.K. Enasidenib in mutant IDH2 relapsed or refractory acute myeloid leukemia. Blood 2017, 130, 722–731. [Google Scholar] [CrossRef] [Green Version]
- Stone, R.M.; Mandrekar, S.J.; Sanford, B.L.; Laumann, K.; Geyer, S.; Bloomfield, C.D. Midostaurin plus Chemotherapy for Acute Myeloid Leukemia with a FLT3 Mutation. N. Engl. J. Med. 2017, 377, 454–464. [Google Scholar] [CrossRef] [Green Version]
- DiNardo, C.D.; Pratz, K.; Pullarkat, V.; Jonas, B.A.; Arellano, M.; Becker, P.S.; Frankfurt, O.; Konopleva, M.; Wei, A.H.; Kantarjian, H.M.; et al. Venetoclax combined with decitabine or azacitidine in treatment-naive, elderly patients with acute myeloid leukemia. Blood 2019, 33, 7–17. [Google Scholar] [CrossRef]
- DiNardo, C.D.; Rausch, C.R.; Benton, C.; Kadia, T.; Jain, N.; Pemmaraju, N. Clinical experience with the BCL2-inhibitor venetoclax in combination therapy for relapsed and refractory acute myeloid leukemia and related myeloid malignancies. Am. J. Hematol. 2018, 93, 401–407. [Google Scholar] [CrossRef]
- Konopleva, M.; Pollyea, D.A.; Potluri, J.; Chyla, B.; Hogdal, L.; Busman, T.; McKeegan, E.; Salem, A.H.; Zhu, M.; Ricker, J.L.; et al. Efficacy and Biological Correlates of Response in a Phase II Study of Venetoclax Monotherapy in Patients with Acute Myelogenous Leukemia. Cancer Discov. 2016, 6, 1106–1117. [Google Scholar] [CrossRef] [Green Version]
- Hills, R.K.; Castaigne, S.; Appelbaum, F.R.; Delaunay, J.; Petersdorf, S.; Othus, M. Addition of gemtuzumab ozogamicin to induction chemotherapy in adult patients with acute myeloid leukaemia: A meta-analysis of individual patient data from randomised controlled trials. Lancet Oncol. 2014, 15, 986–996. [Google Scholar] [CrossRef]
- Lambert, J.; Pautas, C.; Terré, C.; Raffoux, E.; Turlure, P.; Caillot, D.; Legrand, O.; Thomas, X.; Gardin, C.; Gogat-Marchant, K.; et al. Gemtuzumab ozogamicin for de novo acute myeloid leukemia: Final efficacy and safety updates from the open-label, phase 3 ALFA-0701 trial. Haematologica 2019, 104, 113–119. [Google Scholar] [CrossRef]
- Lancet, J.E.; Uy, G.L.; Cortes, J.E.; Newell, L.F.; Lin, T.L.; Ritchie, E.K. CPX-351 (cytarabine and daunorubicin) Liposome for Injection Versus Conventional Cytarabine Plus Daunorubicin in Older Patients With Newly Diagnosed Secondary Acute Myeloid Leukemia. J. Clin. Oncol. 2018, 36, 2684–2692. [Google Scholar] [CrossRef] [PubMed]
- Dick, J.E.; Lapidot, T. Biology of normal and acute myeloid leukemia stem cells. Int. J. Hematol. 2005, 82, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Short, N.J.; Rytting, M.E.; Cortes, J.E. Acute myeloid leukaemia. Lancet 2018, 392, 593–606. [Google Scholar] [CrossRef]
- Ley, T.J.; Ding, L.; Walter, M.J.; McLellan, M.D.; Lamprecht, T.; Larson, D.E. DNMT3A mutations in acute myeloid leukemia. N. Engl. J. Med. 2010, 363, 2424–2433. [Google Scholar] [CrossRef]
- Cancer Genome Atlas Research Network. Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. N. Engl. J. Med. 2013, 368, 2059–2074. [Google Scholar]
- Mardis, E.R.; Ding, L.; Dooling, D.J.; Larson, D.E.; McLellan, M.D.; Chen, K. Recurring mutations found by sequencing an acute myeloid leukemia genome. N. Engl. J. Med. 2009, 361, 1058–1066. [Google Scholar] [CrossRef]
- Jaiswal, S.; Fontanillas, P.; Flannick, J.; Manning, A.; Grauman, P.V.; Mar, B.G. Age-Related Clonal Hematopoiesis Associated with Adverse Outcomes. N. Engl. J. Med. 2014, 371, 2488–2498. [Google Scholar] [CrossRef] [Green Version]
- Jaiswal, S.; Natarajan, P.; Silver, A.J.; Gibson, C.J.; Bick, A.G.; Shvartz, E. Clonal Hematopoiesis and Risk of Atherosclerotic Cardiovascular Disease. N. Engl. J. Med. 2017, 377, 111–121. [Google Scholar] [CrossRef]
- Papaemmanuil, E.; Gerstung, M.; Bullinger, L.; Gaidzik, V.I.; Paschka, P.; Roberts, N.D. Genomic Classification and Prognosis in Acute Myeloid Leukemia. N. Engl. J. Med. 2016, 374, 2209–2221. [Google Scholar] [CrossRef] [Green Version]
- Arber, D.A.; Orazi, A.; Hasserjian, R.; Thiele, J.; Borowitz, M.J.; Le Beau, M.M. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood 2016, 127, 2391–2405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bejanyan, N.; Weisdorf, D.J.; Logan, B.R.; Wang, H.-L.; Devine, S.M.; de Lima, M. Survival of patients with acute myeloid leukemia relapsing after allogeneic hematopoietic cell transplantation: A center for international blood and marrow transplant research study. Biol. Blood Marrow Transplant. 2015, 21, 454–459. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, T.; Rachlis, E.; Bug, G.; Stelljes, M.; Klein, S.; Steckel, N.K. Treatment of acute myeloid leukemia or myelodysplastic syndrome relapse after allogeneic stem cell transplantation with azacitidine and donor lymphocyte infusions--a retrospective multicenter analysis from the German Cooperative Transplant Study Group. Biol. Blood Marrow Transplant. 2015, 21, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Thol, F.; Gabdoulline, R.; Liebich, A.; Klement, P.; Schiller, J.; Kandziora, C. Measurable residual disease monitoring by NGS before allogeneic hematopoietic cell transplantation in AML. Blood 2018, 132, 1703–1713. [Google Scholar] [CrossRef] [PubMed]
- Tsirigotis, P.; Byrne, M.; Schmid, C.; Baron, F.; Ciceri, F.; Esteve, J. Relapse of AML after hematopoietic stem cell transplantation: Methods of monitoring and preventive strategies. A review from the ALWP of the EBMT. Bone Marrow Transplant. 2016, 51, 1431–1438. [Google Scholar] [CrossRef]
- Krönke, J.; Bullinger, L.; Teleanu, V.; Tschürtz, F.; Gaidzik, V.I.; Kühn, M.W.M. Clonal evolution in relapsed NPM1-mutated acute myeloid leukemia. Blood 2013, 122, 100–108. [Google Scholar] [CrossRef] [Green Version]
- Höllein, A.; Meggendorfer, M.; Dicker, F.; Jeromin, S.; Nadarajah, N.; Kern, W. NPM1 mutated AML can relapse with wild-type NPM1: Persistent clonal hematopoiesis can drive relapse. Blood Adv. 2018, 2, 3118–3125. [Google Scholar] [CrossRef]
- Gorello, P.; Cazzaniga, G.; Alberti, F.; Dell’Oro, M.G.; Gottardi, E.; Specchia, G. Quantitative assessment of minimal residual disease in acute myeloid leukemia carrying nucleophosmin (NPM1) gene mutations. Leukemia 2006, 20, 1103–1108. [Google Scholar] [CrossRef] [Green Version]
- Krönke, J.; Schlenk, R.F.; Jensen, K.-O.; Tschürtz, F.; Corbacioglu, A.; Gaidzik, V.I. Monitoring of minimal residual disease in NPM1-mutated acute myeloid leukemia: A study from the German-Austrian acute myeloid leukemia study group. J. Clin. Oncol. 2011, 29, 2709–2716. [Google Scholar] [CrossRef]
- Schnittger, S.; Kern, W.; Tschulik, C.; Weiss, T.; Dicker, F.; Falini, B. Minimal residual disease levels assessed by NPM1 mutation-specific RQ-PCR provide important prognostic information in AML. Blood 2009, 114, 2220–2231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ivey, A.; Hills, R.K.; Simpson, M.A.; Jovanovic, J.V.; Gilkes, A.; Grech, A. Assessment of Minimal Residual Disease in Standard-Risk AML. N. Engl. J. Med. 2016, 374, 422–433. [Google Scholar] [CrossRef] [Green Version]
- Forghieri, F.; Comoli, P.; Marasca, R.; Potenza, L.; Luppi, M. Minimal/Measurable Residual Disease Monitoring in NPM1-Mutated Acute Myeloid Leukemia: A Clinical Viewpoint and Perspectives. Int. J. Mol. Sci. 2018, 19, 3492. [Google Scholar] [CrossRef] [PubMed]
- Malmberg, E.B.R.; Ståhlman, S.; Rehammar, A.; Samuelsson, T.; Alm, S.J.; Kristiansson, E.; Abrahamsson, J.; Garelius, H.; Pettersson, L.; Ehinger, M.; et al. Patient-tailored analysis of minimal residual disease in acute myeloid leukemia using next-generation sequencing. Eur. J. Haematol. 2017, 98, 26–37. [Google Scholar] [CrossRef] [PubMed]
- Shayegi, N.; Kramer, M.; Bornhäuser, M.; Schaich, M.; Schetelig, J.; Platzbecker, U. The level of residual disease based on mutant NPM1 is an independent prognostic factor for relapse and survival in AML. Blood 2013, 122, 83–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Y.; Othus, M.; Walter, R.B.; Estey, E.H.; Wu, D.; Wood, B.L. Deep NPM1 Sequencing Following Allogeneic Hematopoietic Cell Transplantation Improves Risk Assessment in Adults with NPM1-Mutated AML. Biol. Blood Marrow Transplant. 2018, 24, 1615–1620. [Google Scholar] [CrossRef] [PubMed]
- Schlenk, R.F.; Kayser, S.; Bullinger, L.; Kobbe, G.; Casper, J.; Ringhoffer, M. Differential impact of allelic ratio and insertion site in FLT3-ITD–positive AML with respect to allogeneic transplantation. Blood 2014, 124, 3441–3449. [Google Scholar] [CrossRef] [PubMed]
- Cloos, J.; Goemans, B.F.; Hess, C.J.; van Oostveen, J.W.; Waisfisz, Q.; Corthals, S. Stability and prognostic influence of FLT3 mutations in paired initial and relapsed AML samples. Leukemia 2006, 20, 1217–1220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rebechi, M.T.; Pratz, K.W. Genomic instability is a principle pathologic feature of FLT3 ITD kinase activity in acute myeloid leukemia leading to clonal evolution and disease progression. Leuk. Lymphoma 2017, 58, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Shih, L.-Y.; Huang, C.-F.; Wu, J.-H.; Lin, T.-L.; Dunn, P.; Wang, P.-N. Internal tandem duplication of FLT3 in relapsed acute myeloid leukemia: A comparative analysis of bone marrow samples from 108 adult patients at diagnosis and relapse. Blood 2002, 100, 2387–2392. [Google Scholar] [CrossRef]
- Steensma, D.P.; Ebert, B.L. Clonal Hematopoiesis after Induction Chemotherapy for Acute Myeloid Leukemia. N. Engl. J. Med. 2018, 378, 1244–1245. [Google Scholar] [CrossRef]
- Jongen-Lavrencic, M.; Grob, T.; Hanekamp, D.; Kavelaars, F.G.; al Hinai, A.; Zeilemaker, A.; Erpelinck-Verschueren, C.A.J.; Gradowska, P.L.; Meijer, R.; Cloos, J.; et al. Molecular Minimal Residual Disease in Acute Myeloid Leukemia. N. Engl. J. Med. 2018, 378, 1189–1199. [Google Scholar] [CrossRef]
- Hirsch, P.; Tang, R.; Abermil, N.; Flandrin, P.; Moatti, H.; Favale, F. Precision and prognostic value of clone-specific minimal residual disease in acute myeloid leukemia. Haematologica 2017, 102, 1227–1237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rothenberg-Thurley, M.; Amler, S.; Goerlich, D.; Köhnke, T.; Konstandin, N.P.; Schneider, S. Persistence of pre-leukemic clones during first remission and risk of relapse in acute myeloid leukemia. Leukemia 2018, 32, 1598–1608. [Google Scholar] [CrossRef] [Green Version]
- Morita, K.; Kantarjian, H.M.; Wang, F.; Yan, Y.; Bueso-Ramos, C.; Sasaki, K. Clearance of Somatic Mutations at Remission and the Risk of Relapse in Acute Myeloid Leukemia. J. Clin. Oncol. 2018, 36, 1788–1797. [Google Scholar] [CrossRef] [PubMed]
- Klco, J.M.; Miller, C.A.; Griffith, M.; Petti, A.; Spencer, D.H.; Ketkar-Kulkarni, S. Association Between Mutation Clearance After Induction Therapy and Outcomes in Acute Myeloid Leukemia. JAMA 2015, 314, 811–822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frick, M.; Chan, W.; Arends, C.M.; Hablesreiter, R.; Halik, A.; Heuser, M. Role of Donor Clonal Hematopoiesis in Allogeneic Hematopoietic Stem-Cell Transplantation. J. Clin. Oncol. 2018. Available online: http://ascopubs.org/doi/10.1200/JCO.2018.79.2184 (accessed on 7 November 2018).
- Döhner, H.; Weisdorf, D.J.; Bloomfield, C.D. Acute Myeloid Leukemia. N. Engl. J. Med. 2015, 373, 1136–1152. [Google Scholar] [CrossRef] [PubMed]
- Corbacioglu, A.; Scholl, C.; Schlenk, R.F.; Eiwen, K.; Du, J.; Bullinger, L. Prognostic Impact of Minimal Residual Disease inCBFB-MYH11–Positive Acute Myeloid Leukemia. J. Clin. Oncol. 2010, 28, 3724–3729. [Google Scholar] [CrossRef]
- Jourdan, E.; Boissel, N.; Chevret, S.; Delabesse, E.; Renneville, A.; Cornillet, P. Prospective evaluation of gene mutations and minimal residual disease in patients with core binding factor acute myeloid leukemia. Blood 2013, 121, 2213–2223. [Google Scholar] [CrossRef] [Green Version]
- Willekens, C.; Blanchet, O.; Renneville, A.; Cornillet-Lefebvre, P.; Pautas, C.; Guieze, R. Prospective long-term minimal residual disease monitoring using RQ-PCR in RUNX1-RUNX1T1-positive acute myeloid leukemia: Results of the French CBF-2006 trial. Haematologica 2016, 101, 328–335. [Google Scholar] [CrossRef]
- Yin, J.A.L.; O’Brien, M.A.; Hills, R.K.; Daly, S.B.; Wheatley, K.; Burnett, A.K. Minimal residual disease monitoring by quantitative RT-PCR in core binding factor AML allows risk stratification and predicts relapse: Results of the United Kingdom MRC AML-15 trial. Blood 2012, 120, 2826–2835. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wu, D.-P.; Liu, Q.-F.; Qin, Y.-Z.; Wang, J.-B.; Xu, L.-P. In adults with t(8;21)AM.L.; posttransplant RUNX1/RUNX1T1-based MRD monitoring, rather than c-KIT mutations, allows further risk stratification. Blood 2014, 124, 1880–1886. [Google Scholar] [CrossRef] [PubMed]
- Sockel, K.; Wermke, M.; Radke, J.; Kiani, A.; Schaich, M.; Bornhäuser, M. Minimal residual disease-directed preemptive treatment with azacitidine in patients with NPM1-mutant acute myeloid leukemia and molecular relapse. Haematologica 2011, 96, 1568–1570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cilloni, D.; Renneville, A.; Hermitte, F.; Hills, R.K.; Daly, S.; Jovanovic, J.V. Real-time quantitative polymerase chain reaction detection of minimal residual disease by standardized WT1 assay to enhance risk stratification in acute myeloid leukemia: A European LeukemiaNet study. J. Clin. Oncol. 2009, 27, 5195–5201. [Google Scholar] [CrossRef] [PubMed]
- Cilloni, D.; Saglio, G. WT1 as a universal marker for minimal residual disease detection and quantification in myeloid leukemias and in myelodysplastic syndrome. Acta Haematol. 2004, 112, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Candoni, A.; Tiribelli, M.; Toffoletti, E.; Cilloni, D.; Chiarvesio, A.; Michelutti, A. Quantitative assessment of WT1 gene expression after allogeneic stem cell transplantation is a useful tool for monitoring minimal residual disease in acute myeloid leukemia. Eur. J. Haematol. 2009, 82, 61–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lange, T.; Hubmann, M.; Burkhardt, R.; Franke, G.-N.; Cross, M.; Scholz, M. Monitoring of WT1 expression in PB and CD34(+) donor chimerism of BM predicts early relapse in AML and MDS patients after hematopoietic cell transplantation with reduced-intensity conditioning. Leukemia 2011, 25, 498–505. [Google Scholar] [CrossRef] [PubMed]
- Rautenberg, C.; Pechtel, S.; Hildebrandt, B.; Betz, B.; Dienst, A.; Nachtkamp, K. Wilms’ Tumor 1 Gene Expression Using a Standardized European LeukemiaNet-Certified Assay Compared to Other Methods for Detection of Minimal Residual Disease in Myelodysplastic Syndrome and Acute Myelogenous Leukemia after Allogeneic Blood Stem Cell Transplantation. Biol. Blood Marrow Transplant. 2018, 24, 2337–2343. [Google Scholar]
- Alizadeh, M.; Bernard, M.; Danic, B.; Dauriac, C.; Birebent, B.; Lapart, C. Quantitative assessment of hematopoietic chimerism after bone marrow transplantation by real-time quantitative polymerase chain reaction. Blood 2002, 99, 4618–4625. [Google Scholar] [CrossRef] [Green Version]
- Bornhauser, M.; Thiede, C. Chimerism analysis after allogeneic stem cell transplantation. Haematologica 2005, 90, 1301A. [Google Scholar]
- Thiede, C.; Bornhäuser, M.; Ehninger, G. Evaluation of STR informativity for chimerism testing--comparative analysis of 27 STR systems in 203 matched related donor recipient pairs. Leukemia 2004, 18, 248–254. [Google Scholar] [CrossRef]
- Kröger, N.; Bacher, U.; Bader, P.; Böttcher, S.; Borowitz, M.J.; Dreger, P. NCI first international workshop on the biology, prevention, and treatment of relapse after allogeneic hematopoietic stem cell transplantation: Report from the committee on disease-specific methods and strategies for monitoring relapse following allogeneic stem cell transplantation. part II: Chronic leukemias, myeloproliferative neoplasms, and lymphoid malignancies. Biol. Blood Marrow Transplant. 2010, 16, 1325–1346. [Google Scholar] [PubMed]
- Stahl, T.; Rothe, C.; Böhme, M.U.; Kohl, A.; Kröger, N.; Fehse, B.; Digital, P.C.R. Panel for Sensitive Hematopoietic Chimerism Quantification after Allogeneic Stem Cell Transplantation. Int. J. Mol. Sci. 2016, 17, 1515. [Google Scholar] [CrossRef] [PubMed]
- Stahl, T.; Böhme, M.U.; Kröger, N.; Fehse, B. Digital PCR to assess hematopoietic chimerism after allogeneic stem cell transplantation. Exp. Hematol. 2015, 43, 462–468. [Google Scholar] [CrossRef] [PubMed]
- Bornhäuser, M.; Oelschlaegel, U.; Platzbecker, U.; Bug, G.; Lutterbeck, K.; Kiehl, M.G. Monitoring of donor chimerism in sorted CD34+ peripheral blood cells allows the sensitive detection of imminent relapse after allogeneic stem cell transplantation. Haematologica 2009, 94, 1613–1617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bastos-Oreiro, M.; Perez-Corral, A.; Martínez-Laperche, C.; Bento, L.; Pascual, C.; Kwon, M. Prognostic impact of minimal residual disease analysis by flow cytometry in patients with acute myeloid leukemia before and after allogeneic hemopoietic stem cell transplantation. Eur. J. Haematol. 2014, 93, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Kwon, M.; Martínez-Laperche, C.; Infante, M.; Carretero, F.; Balsalobre, P.; Serrano, D. Evaluation of minimal residual disease by real-time quantitative PCR of Wilms’ tumor 1 expression in patients with acute myelogenous leukemia after allogeneic stem cell transplantation: Correlation with flow cytometry and chimerism. Biol. Blood Marrow Transplant. 2012, 18, 1235–1242. [Google Scholar] [CrossRef] [PubMed]
- Rossi, G.; Carella, A.M.; Minervini, M.M.; Savino, L.; Fontana, A.; Pellegrini, F. Minimal residual disease after allogeneic stem cell transplant: A comparison among multiparametric flow cytometry, Wilms tumor 1 expression and chimerism status (Complete chimerism versus Low Level Mixed Chimerism) in acute leukemia. Leuk. Lymphoma 2013, 54, 2660–2666. [Google Scholar] [CrossRef]
- Zhao, X.; Yan, C.; Liu, D.; Xu, L.; Liu, Y.; Liu, K. Combined use of WT1 and flow cytometry monitoring can promote sensitivity of predicting relapse after allogeneic HSCT without affecting specificity. Ann. Hematol. 2013, 92, 1111–1119. [Google Scholar] [CrossRef]
- Lee, C.J.; Savani, B.N.; Mohty, M.; Gorin, N.C.; Labopin, M.; Ruggeri, A.; Schmid, C.; Baron, F.; Esteve, J.; Giebel, S.; et al. Post-remission strategies for the prevention of relapse following allogeneic hematopoietic cell transplantation for high-risk acute myeloid leukemia: expert review from the Acute Leukemia Working Party of the European Society for Blood and Marrow Transplantation. Bone Marrow Transplant. 2018. Available online: https://www.nature.com/articles/s41409-018-0286-2 (accessed on 13 August 2018).
- Orti, G.; Barba, P.; Fox, L.; Salamero, O.; Bosch, F.; Valcarcel, D. Donor lymphocyte infusions in AML and MDS: Enhancing the graft-versus-leukemia effect. Exp. Hematol. 2017, 48, 1–11. [Google Scholar] [CrossRef]
- Schroeder, T.; Czibere, A.; Platzbecker, U.; Bug, G.; Uharek, L.; Luft, T. Azacitidine and donor lymphocyte infusions as first salvage therapy for relapse of AML or MDS after allogeneic stem cell transplantation. Leukemia 2013, 27, 1229–1235. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, T.; Rautenberg, C.; Haas, R.; Kobbe, G. Hypomethylating agents after allogeneic blood stem cell transplantation. Stem Cell Investig. 2016, 3, 84. [Google Scholar] [CrossRef] [PubMed]
- Aldoss, I.; Yang, D.; Aribi, A.; Ali, H.; Sandhu, K.; Al Malki, M.M. Efficacy of the combination of venetoclax and hypomethylating agents in relapsed/refractory acute myeloid leukemia. Haematologica 2018, 103, e404–e407. [Google Scholar] [CrossRef] [PubMed]
- Small, D. FLT3 mutations: Biology and treatment. Hematol. Am. Soc. Hematol. Educ. Program 2006, 1, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.C.; Wang, Q.; Chin, C.-S.; Salerno, S.; Damon, L.E.; Levis, M.J. Validation of ITD mutations in FLT3 as a therapeutic target in human acute myeloid leukaemia. Nature 2012, 485, 260–263. [Google Scholar] [CrossRef] [Green Version]
- Zarrinkar, P.P.; Gunawardane, R.N.; Cramer, M.D.; Gardner, M.F.; Brigham, D.; Belli, B. AC220 is a uniquely potent and selective inhibitor of FLT3 for the treatment of acute myeloid leukemia (AML). Blood 2009, 114, 2984–2992. [Google Scholar] [CrossRef] [Green Version]
- Cortes, J.E.; Khaled, S.K.; Martinelli, G.; Perl, A.E.; Ganguly, S.; Russell, N.H. Efficacy and Safety of Single-Agent Quizartinib (Q), a Potent and Selective FLT3 Inhibitor (FLT3i), in Patients (pts) with FLT3-Internal Tandem Duplication (FLT3-ITD)-Mutated Relapsed/Refractory (R/R) Acute Myeloid Leukemia (AML) Enrolled in the Global, Phase 3, Randomized Controlled Quantum-R Trial. Blood 2018, 132 (Suppl. 1), 563. [Google Scholar]
- Galanis, A.; Ma, H.; Rajkhowa, T.; Ramachandran, A.; Small, D.; Cortes, J. Crenolanib is a potent inhibitor of FLT3 with activity against resistance-conferring point mutants. Blood 2014, 123, 94–100. [Google Scholar] [CrossRef]
- Collins, R.; Kantarjian, H.M.; Levis, M.J.; Perl, A.E.; Ramachandran, A.; Ravandi, F. Clinical activity of Crenolanib in patients with D835 mutant FLT3-positive relapsed/refractory acute myeloid leukemia (AML). J. Clin. Oncol. 2014, 32 (Suppl. 15), 7027. [Google Scholar] [CrossRef]
- Jetani, H.; Garcia-Cadenas, I.; Nerreter, T.; Thomas, S.; Rydzek, J.; Meijide, J.B. CAR T-cells targeting FLT3 have potent activity against FLT3-ITD+ AML and act synergistically with the FLT3-inhibitor crenolanib. Leukemia 2018, 32, 1168–1179. [Google Scholar] [CrossRef]
- Garz, A.-K.; Wolf, S.; Grath, S.; Gaidzik, V.; Habringer, S.; Vick, B. Azacitidine combined with the selective FLT3 kinase inhibitor crenolanib disrupts stromal protection and inhibits expansion of residual leukemia-initiating cells in FLT3-ITD AML with concurrent epigenetic mutations. Oncotarget 2017, 8, 108738–108759. [Google Scholar] [CrossRef]
- Metzelder, S.K.; Schroeder, T.; Finck, A.; Scholl, S.; Fey, M.; Götze, K. High activity of sorafenib in FLT3-ITD-positive acute myeloid leukemia synergizes with allo-immune effects to induce sustained responses. Leukemia 2012, 26, 2353–2359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Safaian, N.N.; Czibere, A.; Bruns, I.; Fenk, R.; Reinecke, P.; Dienst, A. Sorafenib (Nexavar) induces molecular remission and regression of extramedullary disease in a patient with FLT3-ITD+ acute myeloid leukemia. Leuk. Res. 2009, 33, 348–350. [Google Scholar] [CrossRef]
- Schroeder, T.; Zohren, F.; Saure, C.; Bruns, I.; Czibere, A.; Safaian, N.N. Sorafenib treatment in 13 patients with acute myeloid leukemia and activating FLT3 mutations in combination with chemotherapy or as monotherapy. Acta Haematol. 2010, 124, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Mathew, N.R.; Baumgartner, F.; Braun, L.; O’Sullivan, D.; Thomas, S.; Waterhouse, M. Sorafenib promotes graft-versus-leukemia activity in mice and humans through IL-15 production in FLT3-ITD-mutant leukemia cells. Nat. Med. 2018, 24, 282–291. [Google Scholar] [CrossRef] [PubMed]
- Burchert, A.; Bug, G.; Finke, J.; Stelljes, M.; Rollig, C.; Wäsch, R. Sorafenib As Maintenance Therapy Post Allogeneic Stem Cell Transplantation for FLT3-ITD Positive AML: Results from the Randomized, Double-Blind, Placebo-Controlled Multicentre Sormain Trial. Blood 2018, 132 (Suppl. 1), 661. [Google Scholar]
- DiNardo, C.D.; Stein, E.M. SOHO State of the Art Update and Next Questions: IDH Therapeutic Targeting in AML. Clin. Lymphoma Myeloma Leuk. 2018, 18, 769–772. [Google Scholar] [CrossRef]
- Amatangelo, M.D.; Quek, L.; Shih, A.; Stein, E.M.; Roshal, M.; David, M.D. Enasidenib induces acute myeloid leukemia cell differentiation to promote clinical response. Blood 2017, 130, 732–741. [Google Scholar] [CrossRef] [Green Version]
- Fathi, A.T.; DiNardo, C.D.; Kline, I.; Kenvin, L.; Gupta, I.; Attar, E.C. Differentiation Syndrome Associated With Enasidenib, a Selective Inhibitor of Mutant Isocitrate Dehydrogenase 2: Analysis of a Phase 1/2 Study. JAMA Oncol. 2018, 4, 1106–1110. [Google Scholar] [CrossRef]
- Jacoby, M.A.; Duncavage, E.J.; Chang, G.S.; Miller, C.A.; Shao, J.; Elliott, K. Subclones dominate at MDS progression following allogeneic hematopoietic cell transplant. JCI Insight 2018, 3, 5. [Google Scholar] [CrossRef]
- Quek, L.; Ferguson, P.; Metzner, M.; Ahmed, I.; Kennedy, A.; Garnett, C. Mutational analysis of disease relapse in patients allografted for acute myeloid leukemia. Blood Adv. 2016, 1, 193–204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fenaux, P.; Mufti, G.J.; Hellstrom-Lindberg, E.; Santini, V.; Finelli, C.; Giagounidis, A. Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: A randomised, open-label, phase III study. Lancet Oncol. 2009, 10, 223–232. [Google Scholar] [CrossRef]
- Fenaux, P.; Mufti, G.J.; Hellström-Lindberg, E.; Santini, V.; Gattermann, N.; Germing, U. Azacitidine prolongs overall survival compared with conventional care regimens in elderly patients with low bone marrow blast count acute myeloid leukemia. J. Clin. Oncol. 2010, 28, 562–569. [Google Scholar] [CrossRef] [PubMed]
- Kantarjian, H.M.; Thomas, X.G.; Dmoszynska, A.; Wierzbowska, A.; Mazur, G.; Mayer, J. Multicenter, randomized, open-label, phase III trial of decitabine versus patient choice, with physician advice, of either supportive care or low-dose cytarabine for the treatment of older patients with newly diagnosed acute myeloid leukemia. J. Clin. Oncol. 2012, 30, 2670–2677. [Google Scholar] [CrossRef] [PubMed]
- Mohty, M.; Chevallier, P. Azacitidine after allo-SCT: The good without the bad? Blood 2012, 119, 3199–3200. [Google Scholar] [CrossRef] [PubMed]
- Atanackovic, D.; Luetkens, T.; Kloth, B.; Fuchs, G.; Cao, Y.; Hildebrandt, Y. Cancer-testis antigen expression and its epigenetic modulation in acute myeloid leukemia. Am. J. Hematol. 2011, 86, 918–922. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiappinelli, K.B.; Strissel, P.L.; Desrichard, A.; Li, H.; Henke, C.; Akman, B. Inhibiting DNA Methylation Causes an Interferon Response in Cancer via dsRNA Including Endogenous Retroviruses. Cell 2017, 169, 361. [Google Scholar] [CrossRef] [Green Version]
- Goodyear, O.C.; Dennis, M.; Jilani, N.Y.; Loke, J.; Siddique, S.; Ryan, G. Azacitidine augments expansion of regulatory T cells after allogeneic stem cell transplantation in patients with acute myeloid leukemia (AML). Blood 2012, 119, 3361–3369. [Google Scholar] [CrossRef] [Green Version]
- Santourlidis, S.; Trompeter, H.-I.; Weinhold, S.; Eisermann, B.; Meyer, K.L.; Wernet, P. Crucial role of DNA methylation in determination of clonally distributed killer cell Ig-like receptor expression patterns in NK cells. J. Immunol. 2002, 169, 4253–4261. [Google Scholar] [CrossRef]
- Schroeder, T.; Fröbel, J.; Cadeddu, R.-P.; Czibere, A.; Dienst, A.; Platzbecker, U. Salvage therapy with azacitidine increases regulatory T cells in peripheral blood of patients with AML or MDS and early relapse after allogeneic blood stem cell transplantation. Leukemia 2013, 27, 1910–1913. [Google Scholar] [CrossRef]
- De Lima, M.; Giralt, S.; Thall, P.F.; de Padua Silva, L.; Jones, R.B.; Komanduri, K. Maintenance therapy with low-dose azacitidine after allogeneic hematopoietic stem cell transplantation for recurrent acute myelogenous leukemia or myelodysplastic syndrome: A dose and schedule finding study. Cancer 2010, 116, 5420–5431. [Google Scholar] [CrossRef] [PubMed]
- El-Cheikh, J.; Massoud, R.; Fares, E.; Kreidieh, N.; Mahfouz, R.; Charafeddine, M. Low-dose 5-azacytidine as preventive therapy for relapse of AML and MDS following allogeneic HCT. Bone Marrow Transplant. 2017, 52, 918–921. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Kim, Y.-J.; Lee, J.; Jeon, S.; Hong, T.; Park, G. Model-based adaptive phase I trial design of post-transplant decitabine maintenance in myelodysplastic syndrome. J. Hematol. Oncol. 2015, 8, 118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oshikawa, G.; Kakihana, K.; Saito, M.; Aoki, J.; Najima, Y.; Kobayashi, T. Post-transplant maintenance therapy with azacitidine and gemtuzumab ozogamicin for high-risk acute myeloid leukaemia. Br. J. Haematol. 2015, 169, 756–759. [Google Scholar] [CrossRef] [PubMed]
- Pusic, I.; Choi, J.; Fiala, M.A.; Gao, F.; Holt, M.; Cashen, A.F. Maintenance Therapy with Decitabine after Allogeneic Stem Cell Transplantation for Acute Myelogenous Leukemia and Myelodysplastic Syndrome. Biol. Blood Marrow Transplant. 2015, 21, 1761–1769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Lima, M.; Oran, B.; Champlin, R.E.; Papadopoulos, E.B.; Giralt, S.A.; Scott, B.L. CC-486 Maintenance after Stem Cell Transplantation in Patients with Acute Myeloid Leukemia or Myelodysplastic Syndromes. Biol. Blood Marrow Transplant. 2018, 24, 2017–2024. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, T.; Rautenberg, C.; Haas, R.; Germing, U.; Kobbe, G. Hypomethylating agents for treatment and prevention of relapse after allogeneic blood stem cell transplantation. Int. J. Hematol. 2018, 107, 138–150. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, T.; Rautenberg, C.; Krüger, W.; Platzbecker, U.; Bug, G.; Steinmann, J. Treatment of relapsed AML and MDS after allogeneic stem cell transplantation with decitabine and DLI-a retrospective multicenter analysis on behalf of the German Cooperative Transplant Study Group. Ann. Hematol. 2018, 97, 335–342. [Google Scholar] [CrossRef]
- Platzbecker, U.; Wermke, M.; Radke, J.; Oelschlaegel, U.; Seltmann, F.; Kiani, A. Azacitidine for treatment of imminent relapse in MDS or AML patients after allogeneic HSCT: Results of the RELAZA trial. Leukemia 2012, 26, 381–389. [Google Scholar] [CrossRef]
- Platzbecker, U.; Middeke, J.M.; Sockel, K.; Herbst, R.; Wolf, D.; Baldus, C.D. Measurable residual disease-guided treatment with azacitidine to prevent haematological relapse in patients with myelodysplastic syndrome and acute myeloid leukaemia (RELAZA2): An open-label, multicentre, phase 2 trial. Lancet Oncol. 2018, 19, 1668–1679. [Google Scholar] [CrossRef]
- Rautenberg, C.; Nachtkamp, K.; Dienst, A.; Schmidt, P.V.; Heyn, C.; Kondakci, M. Sorafenib and azacitidine as salvage therapy for relapse of FLT3-ITD mutated AML after allo-SCT. Eur. J. Haematol. 2017, 98, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Ravandi, F.; Alattar, M.L.; Grunwald, M.R.; Rudek, M.A.; Rajkhowa, T.; Richie, M.A. Phase 2 study of azacytidine plus sorafenib in patients with acute myeloid leukemia and FLT-3 internal tandem duplication mutation. Blood 2013, 121, 4655–4662. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sid, S.; Rey, J.; Charbonnier, A.; D’Incan, E.; Mohty, B.; Blaise, D. Treatment of Post-transplant Relapse of FLT3-ITD Mutated AML Using 5-Azacytidine and Sorafenib Bitherapy. Clin. Lymphoma Myeloma Leuk. 2017, 17, 241–242. [Google Scholar] [CrossRef] [PubMed]
- Toprak, S.K. Donor lymphocyte infusion in myeloid disorders. Transfus. Apher. Sci. 2018, 57, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Kolb, H.J.; Mittermüller, J.; Clemm, C.; Holler, E.; Ledderose, G.; Brehm, G. Donor leukocyte transfusions for treatment of recurrent chronic myelogenous leukemia in marrow transplant patients. Blood 1990, 76, 2462–2465. [Google Scholar] [PubMed]
- Liga, M.; Triantafyllou, E.; Tiniakou, M.; Lambropoulou, P.; Karakantza, M.; Zoumbos, N.C. High alloreactivity of low-dose prophylactic donor lymphocyte infusion in patients with acute leukemia undergoing allogeneic hematopoietic cell transplantation with an alemtuzumab-containing conditioning regimen. Biol. Blood Marrow Transplant. 2013, 19, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Montero, A.; Savani, B.N.; Shenoy, A.; Read, E.J.; Carter, C.S.; Leitman, S.F. T-cell depleted peripheral blood stem cell allotransplantation with T-cell add-back for patients with hematological malignancies: Effect of chronic GVHD on outcome. Biol. Blood Marrow Transplant. 2006, 12, 1318–1325. [Google Scholar] [CrossRef]
- Naparstek, E.; Or, R.; Nagler, A.; Cividalli, G.; Engelhard, D.; Aker, M. T-cell-depleted allogeneic bone marrow transplantation for acute leukaemia using Campath-1 antibodies and post-transplant administration of donor’s peripheral blood lymphocytes for prevention of relapse. Br. J. Haematol. 1995, 89, 506–515. [Google Scholar] [CrossRef]
- Schmid, C.; Schleuning, M.; Schwerdtfeger, R.; Hertenstein, B.; Mischak-Weissinger, E.; Bunjes, D. Long-term survival in refractory acute myeloid leukemia after sequential treatment with chemotherapy and reduced-intensity conditioning for allogeneic stem cell transplantation. Blood 2006, 108, 1092–1099. [Google Scholar] [CrossRef] [Green Version]
- Jedlickova, Z.; Schmid, C.; Koenecke, C.; Hertenstein, B.; Baurmann, H.; Schwerdtfeger, R. Long-term results of adjuvant donor lymphocyte transfusion in AML after allogeneic stem cell transplantation. Bone Marrow Transplant. 2016, 51, 663–667. [Google Scholar] [CrossRef]
- Schmid, C.; Labopin, M.; Schaap, N.; Veelken, H.; Schleuning, M.; Stadler, M. Prophylactic donor lymphocyte infusion after allogeneic stem cell transplantation in acute leukaemia—A matched pair analysis by the Acute Leukaemia Working Party of EBMT. Br. J. Haematol. 2018. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1111/bjh.15691 (accessed on 22 November 2018).
- Wang, Y.; Liu, D.-H.; Fan, Z.-P.; Sun, J.; Wu, X.-J.; Ma, X. Prevention of relapse using DLI can increase survival following HLA-identical transplantation in patients with advanced-stage acute leukemia: A multi-center study. Clin. Transplant. 2012, 26, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, T.; Fukuda, T.; Nakashima, M.; Henzan, T.; Kusakabe, S.; Kobayashi, N. Donor Lymphocyte Infusion for Relapsed Hematological Malignancies after Unrelated Allogeneic Bone Marrow Transplantation Facilitated by the Japan Marrow Donor Program. Biol. Blood Marrow Transplant. 2017, 23, 938–944. [Google Scholar] [CrossRef] [PubMed]
- Tischer, J.; Engel, N.; Fritsch, S.; Prevalsek, D.; Hubmann, M.; Schulz, C. Second haematopoietic SCT using HLA-haploidentical donors in patients with relapse of acute leukaemia after a first allogeneic transplantation. Bone Marrow Transplant. 2014, 49, 895–901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crucitti, L.; Crocchiolo, R.; Toffalori, C.; Mazzi, B.; Greco, R.; Signori, A. Incidence, risk factors and clinical outcome of leukemia relapses with loss of the mismatched HLA after partially incompatible hematopoietic stem cell transplantation. Leukemia 2015, 29, 1143–1152. [Google Scholar] [CrossRef] [PubMed]
- Toffalori, C.; Cavattoni, I.; Deola, S.; Mastaglio, S.; Giglio, F.; Mazzi, B. Genomic loss of patient-specific HLA in acute myeloid leukemia relapse after well-matched unrelated donor HSCT. Blood 2012, 119, 4813–4815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vago, L.; Perna, S.K.; Zanussi, M.; Mazzi, B.; Barlassina, C.; Stanghellini, M.T.L. Loss of mismatched HLA in leukemia after stem-cell transplantation. N. Engl. J. Med. 2009, 361, 478–488. [Google Scholar] [CrossRef]
- Fan, M.; Li, M.; Gao, L.; Geng, S.; Wang, J.; Wang, Y. Chimeric antigen receptors for adoptive T cell therapy in acute myeloid leukemia. J. Hematol. Oncol. 2017, 10, 151. [Google Scholar] [CrossRef]


| Multiparametric Flow Cytometry | Molecular Genetics (Fusion Transcripts, Point Mutations, Gene Overexpression) | Chimerism | |
|---|---|---|---|
| Methods/Approaches | LAIP/DfN | qPCR // digital droplet PCR (ddPCR) // NGS | qPCR // Indel-PCR // STR-based // XY-FISH |
| Sensitivity | 10−3–10−4 | 10−3–10−6 | 10−2–10−3 10−4 –10−5 |
| Advantages | broad applicability | high sensitivity and specificity | applicable in all patients after allo-SCT |
| Disadvantages/Perspectives | need for standardization | mostly restricted to a small part of patients need for standardization MRD monitoring based on mutations not yet established | low sensitivity and specificity not directly detecting leukemic cells CD34+ sorted chimerism offers increased sensitivity |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rautenberg, C.; Germing, U.; Haas, R.; Kobbe, G.; Schroeder, T. Relapse of Acute Myeloid Leukemia after Allogeneic Stem Cell Transplantation: Prevention, Detection, and Treatment. Int. J. Mol. Sci. 2019, 20, 228. https://doi.org/10.3390/ijms20010228
Rautenberg C, Germing U, Haas R, Kobbe G, Schroeder T. Relapse of Acute Myeloid Leukemia after Allogeneic Stem Cell Transplantation: Prevention, Detection, and Treatment. International Journal of Molecular Sciences. 2019; 20(1):228. https://doi.org/10.3390/ijms20010228
Chicago/Turabian StyleRautenberg, Christina, Ulrich Germing, Rainer Haas, Guido Kobbe, and Thomas Schroeder. 2019. "Relapse of Acute Myeloid Leukemia after Allogeneic Stem Cell Transplantation: Prevention, Detection, and Treatment" International Journal of Molecular Sciences 20, no. 1: 228. https://doi.org/10.3390/ijms20010228
APA StyleRautenberg, C., Germing, U., Haas, R., Kobbe, G., & Schroeder, T. (2019). Relapse of Acute Myeloid Leukemia after Allogeneic Stem Cell Transplantation: Prevention, Detection, and Treatment. International Journal of Molecular Sciences, 20(1), 228. https://doi.org/10.3390/ijms20010228