Diversity and Function of Somatostatin-Expressing Interneurons in the Cerebral Cortex
Abstract
:1. Introduction
Brief Overview on the Diversity of GABAergic Interneurons in the Brain
2. Development of SOM+ Interneurons
3. Distribution and Morphological Variety of SOM+ Interneurons
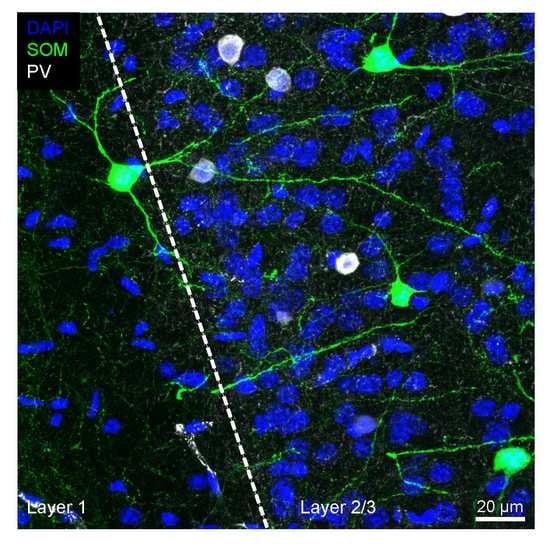
3.1. Distribution of SOM+ INs
3.2. Morphological Variety of SOM+ INs
4. Neurochemical Variety of SOM+ Interneurons
5. Electrophysiological Properties of SOM+ Interneurons
Firing Properties of SOM+ INs
6. Synaptic Connectivity of SOM+ INs
6.1. Synaptic Input onto SOM+ INs
6.2. Postsynaptic Targets of SOM+ INs
7. Role of SOM+ INs in Neuronal Circuits
7.1. Feedback Inhibition/Lateral Inhibition
7.2. Feed-Forward Inhibition
7.3. Disinhibition
8. Role of SOM+ Interneurons in Sensory Processing and in Learning and Memory
9. Role of SOM+ Interneurons in Mood Disorders
10. Outlook and Future Avenues in SOM+ IN Research
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| 5-HT | 5-hydroxytryptamine |
| ACh | Acetylcholine |
| AHP | Afterhyperpolarization |
| Ascl-1 | Achaete-scute homolog 1 |
| BDNF | Brain-derived neurotrophic factor |
| CCK | cholecystokinin |
| CGE | Caudal ganglionic eminence |
| CoupTF2 | Coup transcription factor 2 |
| D1 | Dopamine receptor 1 |
| D2 | Dopamine receptor 2 |
| DA | Dopamine |
| Dlx-1/-2 | Distal-less homeobox ½ |
| FS | Fast-spiking |
| GABA | Gamma aminobutyric acid |
| GFP | Green fluorescent protein |
| GIN | GFP-expressing inhibitory interneurons |
| L | Cortical layer |
| LTS | Low-threshold spike |
| MGE | Medial ganglionic eminence |
| NA | Noradrenaline |
| nNOS | Neuronal nitric oxide synthase |
| NO | Nitric oxide |
| NPY | Neuropeptide Y |
| POA | Preoptic area |
| PV | Parvalbumin |
| RSNP | Regular-spiking non-pyramidal |
| SOM | Somatostatin |
| V1 | Primary visual cortex |
| VIP | Vasoactive intestinal peptide |
References
- van Aerde, K.I.; Feldmeyer, D. Morphological and physiological characterization of pyramidal neuron subtypes in rat medial prefrontal cortex. Cereb Cortex 2015, 25, 788–805. [Google Scholar] [CrossRef] [PubMed]
- DeFelipe, J.; Lopez-Cruz, P.L.; Benavides-Piccione, R.; Bielza, C.; Larranaga, P.; Anderson, S.; Burkhalter, A.; Cauli, B.; Fairen, A.; Feldmeyer, D.; et al. New insights into the classification and nomenclature of cortical GABAergic interneurons. Nat. Rev. Neurosci. 2013, 14, 202–216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ascoli, G.A.; Alonso-Nanclares, L.; Anderson, S.A.; Barrionuevo, G.; Benavides-Piccione, R.; Burkhalter, A.; Buzsáki, G.; Cauli, B.; DeFelipe, J.; Feldmeyer, D.; et al. Petilla terminology: Nomenclature of features of GABAergic interneurons of the cerebral cortex. Nat. Rev. Neurosci. 2008, 9, 557. [Google Scholar] [PubMed]
- Tomioka, R.; Okamoto, K.; Furuta, T.; Fujiyama, F.; Iwasato, T.; Yanagawa, Y.; Obata, K.; Kaneko, T.; Tamamaki, N. Demonstration of long-range GABAergic connections distributed throughout the mouse neocortex. Eur. J. Neurosci. 2005, 21, 1587–1600. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lewis, D.A.; Hashimoto, T.; Volk, D.W. Cortical inhibitory neurons and schizophrenia. Nat. Rev. Neurosci. 2005, 6, 312–324. [Google Scholar] [CrossRef] [PubMed]
- Tripp, A.; Kota, R.S.; Lewis, D.A.; Sibille, E. Reduced somatostatin in subgenual anterior cingulate cortex in major depression. Neurobiol. Dis. 2011, 42, 116–124. [Google Scholar] [CrossRef] [Green Version]
- Seney, M.L.; Tripp, A.; McCune, S.; Lewis, D.A.; Sibille, E. Laminar and cellular analyses of reduced somatostatin gene expression in the subgenual anterior cingulate cortex in major depression. Neurobiol. Dis. 2015, 73, 213–219. [Google Scholar] [CrossRef]
- Lewis, D.A. Cortical circuit dysfunction and cognitive deficits in schizophrenia--implications for preemptive interventions. Eur. J. Neurosci. 2012, 35, 1871–1878. [Google Scholar] [CrossRef]
- Somogyi, P.; Klausberger, T. Defined types of cortical interneurone structure space and spike timing in the hippocampus. J. Physiol. 2005, 562, 9–26. [Google Scholar] [CrossRef]
- Feldmeyer, D.; Qi, G.; Emmenegger, V.; Staiger, J.F. Inhibitory interneurons and their circuit motifs in the many layers of the barrel cortex. Neuroscience 2018, 368, 132–151. [Google Scholar] [CrossRef]
- Markram, H.; Muller, E.; Ramaswamy, S.; Reimann, M.W.; Abdellah, M.; Sanchez, C.A.; Ailamaki, A.; Alonso-Nanclares, L.; Antille, N.; Arsever, S.; et al. Reconstruction and Simulation of Neocortical Microcircuitry. Cell 2015, 163, 456–492. [Google Scholar] [CrossRef] [PubMed]
- Riedemann, T.; Straub, T.; Sutor, B. Two types of somatostatin-expressing GABAergic interneurons in the superficial layers of the mouse cingulate cortex. PLoS ONE 2018, 13, e0200567. [Google Scholar] [CrossRef] [PubMed]
- Tasic, B.; Menon, V.; Nguyen, T.N.; Kim, T.K.; Jarsky, T.; Yao, Z.; Levi, B.; Gray, L.T.; Sorensen, S.A.; Dolbeare, T.; et al. Adult mouse cortical cell taxonomy revealed by single cell transcriptomics. Nat. Neurosci. 2016, 19, 335–346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tasic, B.; Yao, Z.; Graybuck, L.T.; Smith, K.A.; Nguyen, T.N.; Bertagnolli, D.; Goldy, J.; Garren, E.; Economo, M.N.; Viswanathan, S.; et al. Shared and distinct transcriptomic cell types across neocortical areas. Nature 2018, 563, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Zeisel, A.; Munoz-Manchado, A.B.; Codeluppi, S.; Lonnerberg, P.; La Manno, G.; Jureus, A.; Marques, S.; Munguba, H.; He, L.; Betsholtz, C.; et al. Brain structure. Cell types in the mouse cortex and hippocampus revealed by single-cell RNA-seq. Science 2015, 347, 1138–1142. [Google Scholar] [CrossRef] [PubMed]
- Tasic, B. Single cell transcriptomics in neuroscience: Cell classification and beyond. Curr. Opin. Neurobiol. 2018, 50, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Hjerling-Leffler, J.; Zagha, E.; Fishell, G.; Rudy, B. The largest group of superficial neocortical GABAergic interneurons expresses ionotropic serotonin receptors. J. Neurosci. 2010, 30, 16796–16808. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Roby, K.D.; Callaway, E.M. Immunochemical characterization of inhibitory mouse cortical neurons: Three chemically distinct classes of inhibitory cells. J. Comp. Neurol. 2010, 518, 389–404. [Google Scholar] [CrossRef]
- Rudy, B.; Fishell, G.; Lee, S.; Hjerling-Leffler, J. Three groups of interneurons account for nearly 100% of neocortical GABAergic neurons. Dev. Neurobiol. 2011, 71, 45–61. [Google Scholar] [CrossRef]
- Gonchar, Y.; Wang, Q.; Burkhalter, A. Multiple distinct subtypes of GABAergic neurons in mouse visual cortex identified by triple immunostaining. Front. Neuroanat. 2007, 1, 3. [Google Scholar] [CrossRef]
- Kubota, Y.; Shigematsu, N.; Karube, F.; Sekigawa, A.; Kato, S.; Yamaguchi, N.; Hirai, Y.; Morishima, M.; Kawaguchi, Y. Selective coexpression of multiple chemical markers defines discrete populations of neocortical GABAergic neurons. Cereb Cortex 2011, 21, 1803–1817. [Google Scholar] [CrossRef] [PubMed]
- Riedemann, T.; Schmitz, C.; Sutor, B. Immunocytochemical heterogeneity of somatostatin-expressing GABAergic interneurons in layers II and III of the mouse cingulate cortex: A combined immunofluorescence/design-based stereologic study. J. Comp. Neurol. 2016, 524, 2281–2299. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Wang, Y.; Markram, H. Organizing principles for a diversity of GABAergic interneurons and synapses in the neocortex. Science 2000, 287, 273–278. [Google Scholar] [CrossRef]
- Anderson, S.A.; Eisenstat, D.D.; Shi, L.; Rubenstein, J.L. Interneuron migration from basal forebrain to neocortex: Dependence on Dlx genes. Science 1997, 278, 474–476. [Google Scholar] [CrossRef] [PubMed]
- Tamamaki, N.; Fujimori, K.E.; Takauji, R. Origin and route of tangentially migrating neurons in the developing neocortical intermediate zone. J. Neurosci. 1997, 17, 8313–8323. [Google Scholar] [CrossRef] [PubMed]
- Lavdas, A.A.; Grigoriou, M.; Pachnis, V.; Parnavelas, J.G. The medial ganglionic eminence gives rise to a population of early neurons in the developing cerebral cortex. J. Neurosci. 1999, 19, 7881–7888. [Google Scholar] [CrossRef] [PubMed]
- Wichterle, H.; Garcia-Verdugo, J.M.; Herrera, D.G.; Alvarez-Buylla, A. Young neurons from medial ganglionic eminence disperse in adult and embryonic brain. Nat. Neurosci. 1999, 2, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Anderson, S.A.; Marin, O.; Horn, C.; Jennings, K.; Rubenstein, J.L. Distinct cortical migrations from the medial and lateral ganglionic eminences. Development 2001, 128, 353–363. [Google Scholar]
- Wonders, C.P.; Anderson, S.A. The origin and specification of cortical interneurons. Nat. Rev. Neurosci. 2006, 7, 687–696. [Google Scholar] [CrossRef] [PubMed]
- Gelman, D.; Griveau, A.; Dehorter, N.; Teissier, A.; Varela, C.; Pla, R.; Pierani, A.; Marin, O. A wide diversity of cortical GABAergic interneurons derives from the embryonic preoptic area. J. Neurosci. 2011, 31, 16570–16580. [Google Scholar] [CrossRef]
- Brandao, J.A.; Romcy-Pereira, R.N. Interplay of environmental signals and progenitor diversity on fate specification of cortical GABAergic neurons. Front. Cell. Neurosci. 2015, 9, 149. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.S.; Vogt, D.; Sandberg, M.; Rubenstein, J.L. Cortical interneuron development: A tale of time and space. Development 2017, 144, 3867–3878. [Google Scholar] [CrossRef] [PubMed]
- Batista-Brito, R.; Fishell, G. The developmental integration of cortical interneurons into a functional network. Curr. Top. Dev. Biol. 2009, 87, 81–118. [Google Scholar] [PubMed]
- Inan, M.; Welagen, J.; Anderson, S.A. Spatial and temporal bias in the mitotic origins of somatostatin- and parvalbumin-expressing interneuron subgroups and the chandelier subtype in the medial ganglionic eminence. Cereb Cortex 2012, 22, 820–827. [Google Scholar] [CrossRef] [PubMed]
- Basu, J.; Srinivas, K.V.; Cheung, S.K.; Taniguchi, H.; Huang, Z.J.; Siegelbaum, S.A. A cortico-hippocampal learning rule shapes inhibitory microcircuit activity to enhance hippocampal information flow. Neuron 2013, 79, 1208–1221. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, H.; Lu, J.; Huang, Z.J. The spatial and temporal origin of chandelier cells in mouse neocortex. Science 2013, 339, 70–74. [Google Scholar] [CrossRef]
- Nery, S.; Fishell, G.; Corbin, J.G. The caudal ganglionic eminence is a source of distinct cortical and subcortical cell populations. Nat. Neurosci. 2002, 5, 1279–1287. [Google Scholar] [CrossRef]
- Fogarty, M.; Grist, M.; Gelman, D.; Marin, O.; Pachnis, V.; Kessaris, N. Spatial genetic patterning of the embryonic neuroepithelium generates GABAergic interneuron diversity in the adult cortex. J. Neurosci. 2007, 27, 10935–10946. [Google Scholar] [CrossRef]
- Miyoshi, G.; Hjerling-Leffler, J.; Karayannis, T.; Sousa, V.H.; Butt, S.J.; Battiste, J.; Johnson, J.E.; Machold, R.P.; Fishell, G. Genetic fate mapping reveals that the caudal ganglionic eminence produces a large and diverse population of superficial cortical interneurons. J. Neurosci. 2010, 30, 1582–1594. [Google Scholar] [CrossRef]
- Anderson, S.; Mione, M.; Yun, K.; Rubenstein, J.L. Differential origins of neocortical projection and local circuit neurons: Role of Dlx genes in neocortical interneuronogenesis. Cereb Cortex 1999, 9, 646–654. [Google Scholar] [CrossRef]
- Casarosa, S.; Fode, C.; Guillemot, F. Mash1 regulates neurogenesis in the ventral telencephalon. Development 1999, 126, 525–534. [Google Scholar] [PubMed]
- Horton, S.; Meredith, A.; Richardson, J.A.; Johnson, J.E. Correct coordination of neuronal differentiation events in ventral forebrain requires the bHLH factor MASH1. Mol. Cell. Neurosci. 1999, 14, 355–369. [Google Scholar] [CrossRef] [PubMed]
- Vogt, D.; Hunt, R.F.; Mandal, S.; Sandberg, M.; Silberberg, S.N.; Nagasawa, T.; Yang, Z.; Baraban, S.C.; Rubenstein, J.L. Lhx6 directly regulates Arx and CXCR7 to determine cortical interneuron fate and laminar position. Neuron 2014, 82, 350–364. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.S.; Vogt, D.; Lindtner, S.; Sandberg, M.; Silberberg, S.N.; Rubenstein, J.L.R. Coup-TF1 and Coup-TF2 control subtype and laminar identity of MGE-derived neocortical interneurons. Development 2017, 144, 2837–2851. [Google Scholar] [CrossRef] [PubMed]
- Munguba, H.; Nikouei, K.; Hochgerner, H.; Oberst, P.; Kouznetsova, A.; Ryge, J.; Batista-Brito, R.; Munoz-Manchado, A.B.; Close, J.; Linnarsson, S.; et al. Transcriptional maintenance of cortical somatostatin interneuron subtype identity during migration. BioRxiv 2019, 593285. [Google Scholar] [CrossRef]
- Ciceri, G.; Dehorter, N.; Sols, I.; Huang, Z.J.; Maravall, M.; Marin, O. Lineage-specific laminar organization of cortical GABAergic interneurons. Nat. Neurosci. 2013, 16, 1199–1210. [Google Scholar] [CrossRef] [PubMed]
- Mi, D.; Li, Z.; Lim, L.; Li, M.; Moissidis, M.; Yang, Y.; Gao, T.; Hu, T.X.; Pratt, T.; Price, D.J.; et al. Early emergence of cortical interneuron diversity in the mouse embryo. Science 2018, 360, 81–85. [Google Scholar] [CrossRef] [Green Version]
- Gonchar, Y.; Burkhalter, A. Three distinct families of GABAergic neurons in rat visual cortex. Cereb Cortex 1997, 7, 347–358. [Google Scholar] [CrossRef] [Green Version]
- Nassar, M.; Simonnet, J.; Lofredi, R.; Cohen, I.; Savary, E.; Yanagawa, Y.; Miles, R.; Fricker, D. Diversity and overlap of parvalbumin and somatostatin expressing interneurons in mouse presubiculum. Front. Neural Circuits 2015, 9, 20. [Google Scholar] [CrossRef] [Green Version]
- Wouterlood, F.G.; Pothuizen, H. Sparse colocalization of somatostatin- and GABA-immunoreactivity in the entorhinal cortex of the rat. Hippocampus 2000, 10, 77–86. [Google Scholar] [CrossRef]
- Kowianski, P.; Morys, J.M.; Wojcik, S.; Dziewiatkowski, J.; Luczynska, A.; Spodnik, E.; Timmermans, J.P.; Morys, J. Neuropeptide-containing neurons in the endopiriform region of the rat: Morphology and colocalization with calcium-binding proteins and nitric oxide synthase. Brain Res. 2004, 996, 97–110. [Google Scholar] [CrossRef] [PubMed]
- Lepousez, G.; Csaba, Z.; Bernard, V.; Loudes, C.; Videau, C.; Lacombe, J.; Epelbaum, J.; Viollet, C. Somatostatin interneurons delineate the inner part of the external plexiform layer in the mouse main olfactory bulb. J. Comp. Neurol. 2010, 518, 1976–1994. [Google Scholar] [CrossRef] [PubMed]
- McDonald, A.J.; Zaric, V. GABAergic somatostatin-immunoreactive neurons in the amygdala project to the entorhinal cortex. Neuroscience 2015, 290, 227–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jinno, S.; Kosaka, T. Colocalization of parvalbumin and somatostatin-like immunoreactivity in the mouse hippocampus: Quantitative analysis with optical dissector. J. Comp. Neurol. 2000, 428, 377–388. [Google Scholar] [CrossRef]
- Liguz-Lecznar, M.; Urban-Ciecko, J.; Kossut, M. Somatostatin and Somatostatin-Containing Neurons in Shaping Neuronal Activity and Plasticity. Front. Neural Circuits 2016, 10, 48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halabisky, B.; Shen, F.; Huguenard, J.R.; Prince, D.A. Electrophysiological classification of somatostatin-positive interneurons in mouse sensorimotor cortex. J. Neurophysiol. 2006, 96, 834–845. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Hu, H.; Berrebi, A.S.; Mathers, P.H.; Agmon, A. Distinct subtypes of somatostatin-containing neocortical interneurons revealed in transgenic mice. J. Neurosci. 2006, 26, 5069–5082. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Roby, K.D.; Callaway, E.M. Mouse cortical inhibitory neuron type that coexpresses somatostatin and calretinin. J. Comp. Neurol. 2006, 499, 144–160. [Google Scholar] [CrossRef]
- Tremblay, R.; Lee, S.; Rudy, B. GABAergic Interneurons in the Neocortex: From Cellular Properties to Circuits. Neuron 2016, 91, 260–292. [Google Scholar] [CrossRef] [Green Version]
- de Lima, A.D.; Morrison, J.H. Ultrastructural analysis of somatostatin-immunoreactive neurons and synapses in the temporal and occipital cortex of the macaque monkey. J. Comp. Neurol. 1989, 283, 212–227. [Google Scholar] [CrossRef]
- Tamamaki, N.; Tomioka, R. Long-Range GABAergic Connections Distributed throughout the Neocortex and their Possible Function. Front. Neurosci. 2010, 4, 202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McGarry, L.M.; Packer, A.M.; Fino, E.; Nikolenko, V.; Sippy, T.; Yuste, R. Quantitative classification of somatostatin-positive neocortical interneurons identifies three interneuron subtypes. Front. Neural Circuits 2010, 4, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Packer, A.M.; Yuste, R. Dense, unspecific connectivity of neocortical parvalbumin-positive interneurons: A canonical microcircuit for inhibition? J. Neurosci. 2011, 31, 13260–13271. [Google Scholar] [CrossRef] [PubMed]
- Czeiger, D.; White, E.L. Comparison of the distribution of parvalbumin-immunoreactive and other synapses onto the somata of callosal projection neurons in mouse visual and somatosensory cortex. J. Comp. Neurol. 1997, 379, 198–210. [Google Scholar] [CrossRef]
- Druga, R. Neocortical inhibitory system. Folia Biol. 2009, 55, 201–217. [Google Scholar]
- DeFelipe, J.; Hendry, S.H.; Hashikawa, T.; Molinari, M.; Jones, E.G. A microcolumnar structure of monkey cerebral cortex revealed by immunocytochemical studies of double bouquet cell axons. Neuroscience 1990, 37, 655–673. [Google Scholar] [CrossRef]
- DeFelipe, J.; Hendry, S.H.; Jones, E.G. Synapses of double bouquet cells in monkey cerebral cortex visualized by calbindin immunoreactivity. Brain Res. 1989, 503, 49–54. [Google Scholar] [CrossRef]
- Kawaguchi, Y.; Kondo, S. Parvalbumin, somatostatin and cholecystokinin as chemical markers for specific GABAergic interneuron types in the rat frontal cortex. J. Neurocytol. 2002, 31, 277–287. [Google Scholar] [CrossRef]
- Tomioka, R.; Sakimura, K.; Yanagawa, Y. Corticofugal GABAergic projection neurons in the mouse frontal cortex. Front. Neuroanat. 2015, 9, 133. [Google Scholar] [CrossRef] [PubMed]
- Heynen, A.J.; Bilkey, D.K. Induction of RSA-like oscillations in both the in-vitro and in-vivo hippocampus. Neuroreport 1991, 2, 401–404. [Google Scholar] [CrossRef]
- Toth, K.; Borhegyi, Z.; Freund, T.F. Postsynaptic targets of GABAergic hippocampal neurons in the medial septum-diagonal band of broca complex. J. Neurosci. 1993, 13, 3712–3724. [Google Scholar] [CrossRef]
- Gulyas, A.I.; Hajos, N.; Katona, I.; Freund, T.F. Interneurons are the local targets of hippocampal inhibitory cells which project to the medial septum. Eur. J. Neurosci. 2003, 17, 1861–1872. [Google Scholar] [CrossRef]
- Jinno, S.; Klausberger, T.; Marton, L.F.; Dalezios, Y.; Roberts, J.D.; Fuentealba, P.; Bushong, E.A.; Henze, D.; Buzsaki, G.; Somogyi, P. Neuronal diversity in GABAergic long-range projections from the hippocampus. J. Neurosci. 2007, 27, 8790–8804. [Google Scholar] [CrossRef]
- Okhotin, V.E.; Kalinichenko, S.G. Subcortical white matter interstitial cells: Their connections, neurochemical specialization, and role in the histogenesis of the cortex. Neurosci. Behav. Physiol. 2003, 33, 177–194. [Google Scholar] [CrossRef]
- Wang, Y.; Toledo-Rodriguez, M.; Gupta, A.; Wu, C.; Silberberg, G.; Luo, J.; Markram, H. Anatomical, physiological and molecular properties of Martinotti cells in the somatosensory cortex of the juvenile rat. J. Physiol. 2004, 561, 65–90. [Google Scholar] [CrossRef]
- Xu, H.; Jeong, H.Y.; Tremblay, R.; Rudy, B. Neocortical somatostatin-expressing GABAergic interneurons disinhibit the thalamorecipient layer 4. Neuron 2013, 77, 155–167. [Google Scholar] [CrossRef]
- Marin-Padilla, M. The pyramidal cell and its local-circuit interneurons: A hypothetical unit of the Mammalian cerebral cortex. J. Cogn. Neurosci. 1990, 2, 180–194. [Google Scholar] [CrossRef]
- Kawaguchi, Y.; Kubota, Y. Physiological and morphological identification of somatostatin- or vasoactive intestinal polypeptide-containing cells among GABAergic cell subtypes in rat frontal cortex. J. Neurosci. 1996, 16, 2701–2715. [Google Scholar] [CrossRef]
- Shlosberg, D.; Patrick, S.L.; Buskila, Y.; Amitai, Y. Inhibitory effect of mouse neocortex layer I on the underlying cellular network. Eur. J. Neurosci. 2003, 18, 2751–2759. [Google Scholar] [CrossRef]
- Oliva, A.A., Jr.; Jiang, M.; Lam, T.; Smith, K.L.; Swann, J.W. Novel hippocampal interneuronal subtypes identified using transgenic mice that express green fluorescent protein in GABAergic interneurons. J. Neurosci. 2000, 20, 3354–3368. [Google Scholar] [CrossRef]
- Kubota, Y.; Kawaguchi, Y. Three classes of GABAergic interneurons in neocortex and neostriatum. Jpn. J. Physiol. 1994, 44, S145–S148. [Google Scholar]
- Sohn, J.; Hioki, H.; Okamoto, S.; Kaneko, T. Preprodynorphin-expressing neurons constitute a large subgroup of somatostatin-expressing GABAergic interneurons in the mouse neocortex. J. Comp. Neurol. 2014, 522, 1506–1526. [Google Scholar] [CrossRef]
- Perrenoud, Q.; Geoffroy, H.; Gauthier, B.; Rancillac, A.; Alfonsi, F.; Kessaris, N.; Rossier, J.; Vitalis, T.; Gallopin, T. Characterization of Type I and Type II nNOS-Expressing Interneurons in the Barrel Cortex of Mouse. Front. Neural Circuits 2012, 6, 36. [Google Scholar] [CrossRef] [Green Version]
- Parra, P.; Gulyas, A.I.; Miles, R. How many subtypes of inhibitory cells in the hippocampus? Neuron 1998, 20, 983–993. [Google Scholar] [CrossRef]
- Battaglia, D.; Karagiannis, A.; Gallopin, T.; Gutch, H.W.; Cauli, B. Beyond the frontiers of neuronal types. Front. Neural Circuits 2013, 7, 13. [Google Scholar] [CrossRef] [Green Version]
- Harris, K.D.; Hochgerner, H.; Skene, N.G.; Magno, L.; Katona, L.; Bengtsson Gonzales, C.; Somogyi, P.; Kessaris, N.; Linnarsson, S.; Hjerling-Leffler, J. Classes and continua of hippocampal CA1 inhibitory neurons revealed by single-cell transcriptomics. PLoS Biol. 2018, 16, e2006387. [Google Scholar] [CrossRef]
- Rees, C.L.; White, C.M.; Ascoli, G.A. Neurochemical Markers in the Mammalian Brain: Structure, Roles in Synaptic Communication, and Pharmacological Relevance. Curr. Med. Chem. 2017, 24, 3077–3103. [Google Scholar] [CrossRef] [Green Version]
- Kawaguchi, Y. Physiological subgroups of nonpyramidal cells with specific morphological characteristics in layer II/III of rat frontal cortex. J. Neurosci. 1995, 15, 2638–2655. [Google Scholar] [CrossRef]
- Kawaguchi, Y. Groupings of nonpyramidal and pyramidal cells with specific physiological and morphological characteristics in rat frontal cortex. J. Neurophysiol. 1993, 69, 416–431. [Google Scholar] [CrossRef]
- Bacci, A.; Rudolph, U.; Huguenard, J.R.; Prince, D.A. Major differences in inhibitory synaptic transmission onto two neocortical interneuron subclasses. J. Neurosci. 2003, 23, 9664–9674. [Google Scholar] [CrossRef]
- Karagiannis, A.; Gallopin, T.; David, C.; Battaglia, D.; Geoffroy, H.; Rossier, J.; Hillman, E.M.; Staiger, J.F.; Cauli, B. Classification of NPY-expressing neocortical interneurons. J. Neurosci. 2009, 29, 3642–3659. [Google Scholar] [CrossRef]
- Riedemann, S.; Sutor, B.; Bergami, M.; Riedemann, T. Gad1-promotor-driven GFP expression in non-GABAergic neurons of the nucleus endopiriformis in a transgenic mouse line. J. Comp. Neurol. 2019. [Google Scholar] [CrossRef]
- Kawaguchi, Y.; Kubota, Y. Correlation of physiological subgroupings of nonpyramidal cells with parvalbumin- and calbindinD28k-immunoreactive neurons in layer V of rat frontal cortex. J. Neurophysiol. 1993, 70, 387–396. [Google Scholar] [CrossRef]
- Schreiber, S.; Samengo, I.; Herz, A.V. Two distinct mechanisms shape the reliability of neural responses. J. Neurophysiol. 2009, 101, 2239–2251. [Google Scholar] [CrossRef]
- Tiesinga, P.; Fellous, J.M.; Sejnowski, T.J. Regulation of spike timing in visual cortical circuits. Nat. Rev. Neurosci. 2008, 9, 97–107. [Google Scholar] [CrossRef] [Green Version]
- Gibson, J.R.; Beierlein, M.; Connors, B.W. Two networks of electrically coupled inhibitory neurons in neocortex. Nature 1999, 402, 75–79. [Google Scholar] [CrossRef]
- Beierlein, M.; Gibson, J.R.; Connors, B.W. Two dynamically distinct inhibitory networks in layer 4 of the neocortex. J. Neurophysiol. 2003, 90, 2987–3000. [Google Scholar] [CrossRef]
- Naka, A.; Veit, J.; Shababo, B.; Chance, R.K.; Risso, D.; Stafford, D.; Snyder, B.; Egladyous, A.; Chu, D.; Sridharan, S.; et al. Complementary networks of cortical somatostatin interneurons enforce layer specific control. Elife 2019, 8, e43696. [Google Scholar] [CrossRef]
- Yu, J.; Hu, H.; Agmon, A.; Svoboda, K. Principles Governing the Dynamics of GABAergic Interneurons in the Barrel Cortex. BioRxiv 2019. [Google Scholar] [CrossRef]
- Kwan, A.C.; Dan, Y. Dissection of cortical microcircuits by single-neuron stimulation in vivo. Curr. Biol. 2012, 22, 1459–1467. [Google Scholar] [CrossRef]
- Cruikshank, S.J.; Urabe, H.; Nurmikko, A.V.; Connors, B.W. Pathway-specific feedforward circuits between thalamus and neocortex revealed by selective optical stimulation of axons. Neuron 2010, 65, 230–245. [Google Scholar] [CrossRef]
- Silberberg, G.; Markram, H. Disynaptic inhibition between neocortical pyramidal cells mediated by Martinotti cells. Neuron 2007, 53, 735–746. [Google Scholar] [CrossRef]
- Obermayer, J.; Heistek, T.S.; Kerkhofs, A.; Goriounova, N.A.; Kroon, T.; Baayen, J.C.; Idema, S.; Testa-Silva, G.; Couey, J.J.; Mansvelder, H.D. Lateral inhibition by Martinotti interneurons is facilitated by cholinergic inputs in human and mouse neocortex. Nat. Commun. 2018, 9, 4101. [Google Scholar] [CrossRef] [Green Version]
- Hu, H.; Agmon, A. Differential Excitation of Distally versus Proximally Targeting Cortical Interneurons by Unitary Thalamocortical Bursts. J. Neurosci. 2016, 36, 6906–6916. [Google Scholar] [CrossRef]
- Wall, N.R.; De La Parra, M.; Sorokin, J.M.; Taniguchi, H.; Huang, Z.J.; Callaway, E.M. Brain-Wide Maps of Synaptic Input to Cortical Interneurons. J. Neurosci. 2016, 36, 4000–4009. [Google Scholar] [CrossRef] [Green Version]
- Kapfer, C.; Glickfeld, L.L.; Atallah, B.V.; Scanziani, M. Supralinear increase of recurrent inhibition during sparse activity in the somatosensory cortex. Nat. Neurosci. 2007, 10, 743–753. [Google Scholar] [CrossRef]
- Fanselow, E.E.; Richardson, K.A.; Connors, B.W. Selective, state-dependent activation of somatostatin-expressing inhibitory interneurons in mouse neocortex. J. Neurophysiol. 2008, 100, 2640–2652. [Google Scholar] [CrossRef]
- Chen, N.; Sugihara, H.; Sur, M. An acetylcholine-activated microcircuit drives temporal dynamics of cortical activity. Nat. Neurosci. 2015, 18, 892–902. [Google Scholar] [CrossRef] [Green Version]
- Hilscher, M.M.; Leao, R.N.; Edwards, S.J.; Leao, K.E.; Kullander, K. Chrna2-Martinotti Cells Synchronize Layer 5 Type A Pyramidal Cells via Rebound Excitation. PLoS Biol. 2017, 15, e2001392. [Google Scholar] [CrossRef]
- Urban-Ciecko, J.; Jouhanneau, J.S.; Myal, S.E.; Poulet, J.F.A.; Barth, A.L. Precisely Timed Nicotinic Activation Drives SST Inhibition in Neocortical Circuits. Neuron 2018, 97, 611–625.e615. [Google Scholar] [CrossRef]
- Vitalis, T.; Verney, C. Sculpting Cerebral Cortex with Serotonin in Rodent and Primate. In Serotonin-A Chemical Messenger Between All Types of Living Cells; Shad, K.F., Ed.; IntechOpen: Rijeka, Republic of Croatia, 2017. [Google Scholar] [Green Version]
- Paspalas, C.D.; Papadopoulos, G.C. Serotoninergic afferents preferentially innervate distinct subclasses of peptidergic interneurons in the rat visual cortex. Brain Res. 2001, 891, 158–167. [Google Scholar] [CrossRef]
- Ferezou, I.; Cauli, B.; Hill, E.L.; Rossier, J.; Hamel, E.; Lambolez, B. 5-HT3 receptors mediate serotonergic fast synaptic excitation of neocortical vasoactive intestinal peptide/cholecystokinin interneurons. J. Neurosci. 2002, 22, 7389–7397. [Google Scholar] [CrossRef]
- Puig, M.V.; Gulledge, A.T. Serotonin and prefrontal cortex function: Neurons, networks, and circuits. Mol. Neurobiol. 2011, 44, 449–464. [Google Scholar] [CrossRef]
- Puig, M.V.; Watakabe, A.; Ushimaru, M.; Yamamori, T.; Kawaguchi, Y. Serotonin modulates fast-spiking interneuron and synchronous activity in the rat prefrontal cortex through 5-HT1A and 5-HT2A receptors. J. Neurosci. 2010, 30, 2211–2222. [Google Scholar] [CrossRef]
- Athilingam, J.C.; Ben-Shalom, R.; Keeshen, C.M.; Sohal, V.S.; Bender, K.J. Serotonin enhances excitability and gamma frequency temporal integration in mouse prefrontal fast-spiking interneurons. Elife 2017, 6, e31991. [Google Scholar] [CrossRef]
- Tseng, K.Y.; O’Donnell, P. D2 dopamine receptors recruit a GABA component for their attenuation of excitatory synaptic transmission in the adult rat prefrontal cortex. Synapse 2007, 61, 843–850. [Google Scholar] [CrossRef] [Green Version]
- Anastasiades, P.G.; Boada, C.; Carter, A.G. Cell-Type-Specific D1 Dopamine Receptor Modulation of Projection Neurons and Interneurons in the Prefrontal Cortex. Cereb Cortex 2018. [Google Scholar] [CrossRef]
- Koyanagi, Y.; Yamamoto, K.; Oi, Y.; Koshikawa, N.; Kobayashi, M. Presynaptic interneuron subtype- and age-dependent modulation of GABAergic synaptic transmission by beta-adrenoceptors in rat insular cortex. J. Neurophysiol. 2010, 103, 2876–2888. [Google Scholar] [CrossRef]
- Kawaguchi, Y.; Shindou, T. Noradrenergic excitation and inhibition of GABAergic cell types in rat frontal cortex. J. Neurosci. 1998, 18, 6963–6976. [Google Scholar] [CrossRef]
- Caputi, A.; Rozov, A.; Blatow, M.; Monyer, H. Two calretinin-positive GABAergic cell types in layer 2/3 of the mouse neocortex provide different forms of inhibition. Cereb Cortex 2009, 19, 1345–1359. [Google Scholar] [CrossRef]
- Pfeffer, C.K.; Xue, M.; He, M.; Huang, Z.J.; Scanziani, M. Inhibition of inhibition in visual cortex: The logic of connections between molecularly distinct interneurons. Nat. Neurosci. 2013, 16, 1068–1076. [Google Scholar] [CrossRef] [PubMed]
- Pi, H.J.; Hangya, B.; Kvitsiani, D.; Sanders, J.I.; Huang, Z.J.; Kepecs, A. Cortical interneurons that specialize in disinhibitory control. Nature 2013, 503, 521–524. [Google Scholar] [CrossRef] [PubMed]
- Walker, F.; Mock, M.; Feyerabend, M.; Guy, J.; Wagener, R.J.; Schubert, D.; Staiger, J.F.; Witte, M. Parvalbumin- and vasoactive intestinal polypeptide-expressing neocortical interneurons impose differential inhibition on Martinotti cells. Nat. Commun. 2016, 7, 13664. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Shen, S.; Cadwell, C.R.; Berens, P.; Sinz, F.; Ecker, A.S.; Patel, S.; Tolias, A.S. Principles of connectivity among morphologically defined cell types in adult neocortex. Science 2015, 350, aac9462. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Hu, H.; Agmon, A. Short-term plasticity of unitary inhibitory-to-inhibitory synapses depends on the presynaptic interneuron subtype. J. Neurosci. 2012, 32, 983–988. [Google Scholar] [CrossRef] [PubMed]
- Marlin, J.J.; Carter, A.G. GABA-A receptor inhibition of local calcium signaling in spines and dendrites. J. Neurosci. 2014, 34, 15898–15911. [Google Scholar] [CrossRef]
- Nigro, M.J.; Hashikawa-Yamasaki, Y.; Rudy, B. Diversity and Connectivity of Layer 5 Somatostatin-Expressing Interneurons in the Mouse Barrel Cortex. J. Neurosci. 2018, 38, 1622–1633. [Google Scholar] [CrossRef]
- Zhou, X.; Rickmann, M.; Hafner, G.; Staiger, J.F. Subcellular Targeting of VIP Boutons in Mouse Barrel Cortex is Layer-Dependent and not Restricted to Interneurons. Cereb Cortex 2017, 27, 5353–5368. [Google Scholar] [CrossRef] [Green Version]
- Kawaguchi, Y.; Kubota, Y. Neurochemical features and synaptic connections of large physiologically-identified GABAergic cells in the rat frontal cortex. Neuroscience 1998, 85, 677–701. [Google Scholar] [CrossRef]
- Kubota, Y.; Karube, F.; Nomura, M.; Kawaguchi, Y. The Diversity of Cortical Inhibitory Synapses. Front. Neural Circuits 2016, 10, 27. [Google Scholar] [CrossRef]
- Sohn, J.; Okamoto, S.; Kataoka, N.; Kaneko, T.; Nakamura, K.; Hioki, H. Differential Inputs to the Perisomatic and Distal-Dendritic Compartments of VIP-Positive Neurons in Layer 2/3 of the Mouse Barrel Cortex. Front. Neuroanat. 2016, 10, 124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jackson, J.; Ayzenshtat, I.; Karnani, M.M.; Yuste, R. VIP+ interneurons control neocortical activity across brain states. J. Neurophysiol. 2016, 115, 3008–3017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adesnik, H.; Bruns, W.; Taniguchi, H.; Huang, Z.J.; Scanziani, M. A neural circuit for spatial summation in visual cortex. Nature 2012, 490, 226–231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roux, L.; Buzsaki, G. Tasks for inhibitory interneurons in intact brain circuits. Neuropharmacology 2015, 88, 10–23. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Xu, M.; Kamigaki, T.; Hoang Do, J.P.; Chang, W.C.; Jenvay, S.; Miyamichi, K.; Luo, L.; Dan, Y. Selective attention. Long-range and local circuits for top-down modulation of visual cortex processing. Science 2014, 345, 660–665. [Google Scholar] [CrossRef]
- Li, L.Y.; Xiong, X.R.; Ibrahim, L.A.; Yuan, W.; Tao, H.W.; Zhang, L.I. Differential Receptive Field Properties of Parvalbumin and Somatostatin Inhibitory Neurons in Mouse Auditory Cortex. Cereb Cortex 2015, 25, 1782–1791. [Google Scholar] [CrossRef] [PubMed]
- Tan, Z.; Hu, H.; Huang, Z.J.; Agmon, A. Robust but delayed thalamocortical activation of dendritic-targeting inhibitory interneurons. Proc. Natl. Acad. Sci. USA 2008, 105, 2187–2192. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.; Kruglikov, I.; Huang, Z.J.; Fishell, G.; Rudy, B. A disinhibitory circuit mediates motor integration in the somatosensory cortex. Nat. Neurosci. 2013, 16, 1662–1670. [Google Scholar] [CrossRef] [Green Version]
- Gentet, L.J.; Kremer, Y.; Taniguchi, H.; Huang, Z.J.; Staiger, J.F.; Petersen, C.C. Unique functional properties of somatostatin-expressing GABAergic neurons in mouse barrel cortex. Nat. Neurosci. 2012, 15, 607–612. [Google Scholar] [CrossRef]
- Munoz, W.; Tremblay, R.; Levenstein, D.; Rudy, B. Layer-specific modulation of neocortical dendritic inhibition during active wakefulness. Science 2017, 355, 954–959. [Google Scholar] [CrossRef] [Green Version]
- Munoz, W.; Tremblay, R.; Rudy, B. Channelrhodopsin-assisted patching: In vivo recording of genetically and morphologically identified neurons throughout the brain. Cell Rep. 2014, 9, 2304–2316. [Google Scholar] [CrossRef] [PubMed]
- Cottam, J.C.; Smith, S.L.; Hausser, M. Target-specific effects of somatostatin-expressing interneurons on neocortical visual processing. J. Neurosci. 2013, 33, 19567–19578. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Kaneko, M.; Tang, Y.; Alvarez-Buylla, A.; Stryker, M.P. A cortical disinhibitory circuit for enhancing adult plasticity. Elife 2015, 4, e05558. [Google Scholar] [CrossRef] [PubMed]
- Cone, J.J.; Scantlen, M.D.; Histed, M.H.; Maunsell, J.H.R. Different Inhibitory Interneuron Cell Classes Make Distinct Contributions to Visual Contrast Perception. Eneuro 2019, 6. [Google Scholar] [CrossRef] [PubMed]
- Ayaz, A.; Saleem, A.B.; Scholvinck, M.L.; Carandini, M. Locomotion controls spatial integration in mouse visual cortex. Curr. Biol. 2013, 23, 890–894. [Google Scholar] [CrossRef] [PubMed]
- Karnani, M.M.; Jackson, J.; Ayzenshtat, I.; Tucciarone, J.; Manoocheri, K.; Snider, W.G.; Yuste, R. Cooperative Subnetworks of Molecularly Similar Interneurons in Mouse Neocortex. Neuron 2016, 90, 86–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, J.W.; Kilb, W.; Kirischuk, S.; Unichenko, P.; Stuttgen, M.C.; Luhmann, H.J. Development of the whisker-to-barrel cortex system. Curr. Opin. Neurobiol. 2018, 53, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Dufour, A.; Rollenhagen, A.; Satzler, K.; Lubke, J.H.R. Development of Synaptic Boutons in Layer 4 of the Barrel Field of the Rat Somatosensory Cortex: A Quantitative Analysis. Cereb Cortex 2016, 26, 838–854. [Google Scholar] [CrossRef]
- Petersen, C.C. The functional organization of the barrel cortex. Neuron 2007, 56, 339–355. [Google Scholar] [CrossRef]
- Makino, H.; Komiyama, T. Learning enhances the relative impact of top-down processing in the visual cortex. Nat. Neurosci. 2015, 18, 1116–1122. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, Y.; Guan, S.; Hong, X.; Wang, Z.; Li, X. Neural substrate of initiation of cross-modal working memory retrieval. PLoS ONE 2014, 9, e103991. [Google Scholar] [CrossRef] [PubMed]
- Kato, H.K.; Gillet, S.N.; Isaacson, J.S. Flexible Sensory Representations in Auditory Cortex Driven by Behavioral Relevance. Neuron 2015, 88, 1027–1039. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Natan, R.G.; Briguglio, J.J.; Mwilambwe-Tshilobo, L.; Jones, S.I.; Aizenberg, M.; Goldberg, E.M.; Geffen, M.N. Complementary control of sensory adaptation by two types of cortical interneurons. Elife 2015, 4, e09868. [Google Scholar] [CrossRef] [PubMed]
- Yavorska, I.; Wehr, M. Somatostatin-Expressing Inhibitory Interneurons in Cortical Circuits. Front. Neural Circuits 2016, 10, 76. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.X.; Kim, A.N.; Peters, A.J.; Komiyama, T. Subtype-specific plasticity of inhibitory circuits in motor cortex during motor learning. Nat. Neurosci. 2015, 18, 1109–1115. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Jeong, H.; Lee, J.; Ghim, J.W.; Her, E.S.; Lee, S.H.; Jung, M.W. Distinct Roles of Parvalbumin- and Somatostatin-Expressing Interneurons in Working Memory. Neuron 2016, 92, 902–915. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamigaki, T.; Dan, Y. Delay activity of specific prefrontal interneuron subtypes modulates memory-guided behavior. Nat. Neurosci. 2017, 20, 854–863. [Google Scholar] [CrossRef]
- Scheuss, V.; Bonhoeffer, T. Function of dendritic spines on hippocampal inhibitory neurons. Cereb Cortex 2014, 24, 3142–3153. [Google Scholar] [CrossRef]
- Keck, T.; Scheuss, V.; Jacobsen, R.I.; Wierenga, C.J.; Eysel, U.T.; Bonhoeffer, T.; Hubener, M. Loss of sensory input causes rapid structural changes of inhibitory neurons in adult mouse visual cortex. Neuron 2011, 71, 869–882. [Google Scholar] [CrossRef]
- Post, R.J.; Warden, M.R. Depression: The search for separable behaviors and circuits. Curr. Opin. Neurobiol. 2018, 49, 192–200. [Google Scholar] [CrossRef]
- Miller, E.K.; Cohen, J.D. An integrative theory of prefrontal cortex function. Annu. Rev. Neurosci. 2001, 24, 167–202. [Google Scholar] [CrossRef] [PubMed]
- Hamani, C.; Mayberg, H.; Stone, S.; Laxton, A.; Haber, S.; Lozano, A.M. The subcallosal cingulate gyrus in the context of major depression. Biol. Psychiatry 2011, 69, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Guilloux, J.P.; Douillard-Guilloux, G.; Kota, R.; Wang, X.; Gardier, A.M.; Martinowich, K.; Tseng, G.C.; Lewis, D.A.; Sibille, E. Molecular evidence for BDNF- and GABA-related dysfunctions in the amygdala of female subjects with major depression. Mol. Psychiatry 2012, 17, 1130–1142. [Google Scholar] [CrossRef] [PubMed]
- Tripp, A.; Oh, H.; Guilloux, J.P.; Martinowich, K.; Lewis, D.A.; Sibille, E. Brain-derived neurotrophic factor signaling and subgenual anterior cingulate cortex dysfunction in major depressive disorder. Am. J. Psychiatry 2012, 169, 1194–1202. [Google Scholar] [CrossRef] [PubMed]
- Oh, H.; Piantadosi, S.C.; Rocco, B.R.; Lewis, D.A.; Watkins, S.C.; Sibille, E. The Role of Dendritic Brain-Derived Neurotrophic Factor Transcripts on Altered Inhibitory Circuitry in Depression. Biol. Psychiatry 2019, 85, 517–526. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, T.; Jefferson, S.J.; Hooper, A.; Yee, P.P.; Maguire, J.; Luscher, B. Disinhibition of somatostatin-positive interneurons by deletion of postsynaptic GABAA receptors. Mol. Psychiatry 2017, 22, 787. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, T.; Jefferson, S.J.; Hooper, A.; Yee, P.H.; Maguire, J.; Luscher, B. Disinhibition of somatostatin-positive GABAergic interneurons results in an anxiolytic and antidepressant-like brain state. Mol. Psychiatry 2017, 22, 920–930. [Google Scholar] [CrossRef] [PubMed]
- Engin, E.; Stellbrink, J.; Treit, D.; Dickson, C.T. Anxiolytic and antidepressant effects of intracerebroventricularly administered somatostatin: Behavioral and neurophysiological evidence. Neuroscience 2008, 157, 666–676. [Google Scholar] [CrossRef]
- Inoue, M.; Nakajima, S.; Nakajima, Y. Somatostatin induces an inward rectification in rat locus coeruleus neurones through a pertussis toxin-sensitive mechanism. J. Physiol. 1988, 407, 177–198. [Google Scholar] [CrossRef]
- Galarraga, E.; Vilchis, C.; Tkatch, T.; Salgado, H.; Tecuapetla, F.; Perez-Rosello, T.; Perez-Garci, E.; Hernandez-Echeagaray, E.; Surmeier, D.J.; Bargas, J. Somatostatinergic modulation of firing pattern and calcium-activated potassium currents in medium spiny neostriatal neurons. Neuroscience 2007, 146, 537–554. [Google Scholar] [CrossRef]
- Lucas, S.J.; Armstrong, D.L. Protein phosphatase modulation of somatostatin receptor signaling in the mouse hippocampus. Neuropharmacology 2015, 99, 232–241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riedemann, T.; Sutor, B. Long-lasting actions of somatostatin on pyramidal cell excitability in the mouse cingulate cortex. Neurosci. Lett. 2019, 698, 217–223. [Google Scholar] [CrossRef] [PubMed]
- van den Pol, A.N. Neuropeptide transmission in brain circuits. Neuron 2012, 76, 98–115. [Google Scholar] [CrossRef] [PubMed]
- Sauer, J.F.; Struber, M.; Bartos, M. Impaired fast-spiking interneuron function in a genetic mouse model of depression. Elife 2015, 4, e04979. [Google Scholar] [CrossRef] [PubMed]



© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Riedemann, T. Diversity and Function of Somatostatin-Expressing Interneurons in the Cerebral Cortex. Int. J. Mol. Sci. 2019, 20, 2952. https://doi.org/10.3390/ijms20122952
Riedemann T. Diversity and Function of Somatostatin-Expressing Interneurons in the Cerebral Cortex. International Journal of Molecular Sciences. 2019; 20(12):2952. https://doi.org/10.3390/ijms20122952
Chicago/Turabian StyleRiedemann, Therese. 2019. "Diversity and Function of Somatostatin-Expressing Interneurons in the Cerebral Cortex" International Journal of Molecular Sciences 20, no. 12: 2952. https://doi.org/10.3390/ijms20122952
APA StyleRiedemann, T. (2019). Diversity and Function of Somatostatin-Expressing Interneurons in the Cerebral Cortex. International Journal of Molecular Sciences, 20(12), 2952. https://doi.org/10.3390/ijms20122952