Sex- and Age-Related Estrogen Signaling Alteration in Inflammatory Bowel Diseases: Modulatory Role of Estrogen Receptors
Abstract
:1. Introduction
2. Results
2.1. Patients Characteristics
2.2. 17β-Estradiol, CYP1A1 and SULT1E1 Levels in Serum of IBD Patients
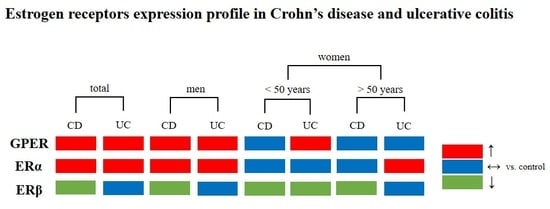
2.3. Estrogen Receptors Expression in IBD Patients
2.4. Estrogen Receptors Expression in Men with IBD
2.5. Estrogen Receptors Expression in Women with IBD under the Age of 50
2.6. Estrogen Receptors Expression in Women with IBD over the Age of 50
2.7. Estrogen Receptor ERα Spliced Variants Expression in IBD Patients
3. Discussion
4. Materials and Methods
4.1. Study Group and Colon Mucosa Sample Collection
4.2. Enzyme-Linked Immunosorbent Assay
4.3. RNA Isolation
4.4. Real-Time PCR
4.5. Western Blot Analysis
4.6. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
References
- Ananthakrishnan, A.N. Epidemiology and risk factors for IBD. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 205–217. [Google Scholar] [CrossRef] [PubMed]
- Nanini, H.F.; Bernardazzi, C.; Castro, F.; de Souza, H.S.P. Damage-associated molecular patterns in inflammatory bowel disease: From biomarkers to therapeutic targets. World J. Gastroenterol. 2018, 24, 4622–4634. [Google Scholar] [CrossRef] [PubMed]
- Wittkopf, N.; Neurath, M.F.; Becker, C. Immune-epithelial crosstalk at the intestinal surface. J. Gastroenterol. 2014, 49, 375–387. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, C.N.; Wajda, A.; Svenson, L.W.; MacKenzie, A.; Koehoorn, M.; Jackson, M.; Fedorak, R.; Israel, D.; Blanchard, J.F. The epidemiology of inflammatory bowel disease in Canada: A population-based study. Am. J. Gastroenterol. 2006, 101, 1559–1568. [Google Scholar] [CrossRef] [PubMed]
- Molinié, F.; Gower-Rousseau, C.; Yzet, T.; Merle, V.; Grandbastien, B.; Marti, R.; Lerebours, E.; Dupas, J.L.; Colombel, J.F.; Salomez, J.L.; et al. Opposite evolution in incidence of Crohn’s disease and ulcerative colitis in Northern France (1988–1999). Gut 2004, 53, 843–848. [Google Scholar] [CrossRef] [PubMed]
- Khalili, H.; Higuchi, L.M.; Ananthakrishnan, A.N.; Richter, J.M.; Feskanich, D.; Fuchs, C.S.; Chan, A.T. Oral contraceptives, reproductive factors and risk of inflammatory bowel disease. Gut 2013, 62, 1153–1159. [Google Scholar] [CrossRef]
- Khalili, H.; Granath, F.; Smedby, K.E.; Ekbom, A.; Neovius, M.; Chan, A.T.; Olen, O. Association between long-term oral contraceptive use and risk of Crohn’s disease complications in a nationwide study. Gastroenterology 2016, 150, 1561–1567. [Google Scholar] [CrossRef]
- Khalili, H.; Higuchi, L.M.; Ananthakrishnan, A.N.; Manson, J.E.; Feskanich, D.; Richter, J.M.; Fuchs, C.S.; Chan, A.T. Hormone therapy increases risk of ulcerative colitis but not Crohn’s disease. Gastroenterology 2012, 143, 1199–1206. [Google Scholar] [CrossRef]
- García Rodríguez, L.A.; González-Pérez, A.; Johansson, S.; Wallander, M.A. Risk factors for inflammatory bowel disease in the general population. Aliment. Pharm. 2005, 22, 309–315. [Google Scholar] [CrossRef]
- Kane, S.V.; Reddy, D. Hormonal replacement therapy after menopause is protective of disease activity in women with inflammatory bowel disease. Am. J. Gastroenterol. 2008, 103, 1193–1196. [Google Scholar] [CrossRef]
- Betteridge, J.D.; Armbruster, S.P.; Maydonovitch, C.; Veerappan, G.R. Inflammatory bowel disease prevalence by age, gender, race, and geographic location in the U.S. military health care population. Inflamm. Bowel. Dis. 2013, 19, 1421–1427. [Google Scholar] [CrossRef] [PubMed]
- Ortizo, R.; Lee, S.Y.; Nguyen, E.T.; Jamal, M.M.; Bechtold, M.M.; Nguyen, D.L. Exposure to oral contraceptives increases the risk for development of inflammatory bowel disease: A meta-analysis of case-controlled and cohort studies. Eur. J. Gastroenterol. Hepatol. 2017, 29, 1064–1070. [Google Scholar] [CrossRef] [PubMed]
- Jacenik, D.; Cygankiewicz, A.I.; Krajewska, W.M. The G-protein-coupled estrogen receptor as a modulator of neoplastic transformation. Mol. Cell Endocrinol. 2016, 429, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.Y.; Yin, L. Estrogen receptor alpha-36 (ER-α36): A new player in human breast cancer. Mol. Cell Endocrinol. 2015, 3, 193–206. [Google Scholar] [CrossRef] [PubMed]
- Romano, S.N.; Gorelick, D.A. Crosstalk between nuclear and G-protein-coupled estrogen receptors. Gen. Comp. Endocrinol. 2018, 261, 190–197. [Google Scholar] [CrossRef]
- Straub, R.H. The complex role of estrogens in inflammation. Endocr. Rev. 2007, 28, 521–574. [Google Scholar] [CrossRef] [PubMed]
- Clegg, D.J. Minireview: The year in review of estrogen regulation of metabolism. Mol. Endocrinol. 2012, 26, 1957–1960. [Google Scholar] [CrossRef] [PubMed]
- Kovats, S. Estrogen receptors regulate innate immune cells and signaling pathways. Cell Immunol. 2015, 294, 63–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pierdominici, M.; Maselli, A.; Varano, B.; Barbati, C.; Cesaro, P.; Spada, C.; Zullo, A.; Lorenzetti, R.; Rosati, M.; Rainaldi, G.; et al. Linking estrogen receptor β expression with inflammatory bowel disease activity. Oncotarget. 2015, 6, 40443–40451. [Google Scholar] [CrossRef] [PubMed]
- Looijer-van Langen, M.; Hotte, N.; Dieleman, L.A.; Albert, E.; Mulder, C.; Madsen, K.L. Estrogen receptor-β signaling modulates epithelial barrier function. Am. J. Physiol. Gastrointest. Liver. Physiol. 2011, 300, G621–G626. [Google Scholar] [CrossRef]
- Goodman, W.A.; Havran, H.L.; Quereshy, H.A.; Kuang, S.; De Salvo, C.; Pizarro, T.T. Estrogen receptor α loss-of-function protects female mice from DSS-induced experimental colitis. Cell Mol. Gastroenterol. Hepatol. 2017, 5, 630.e1–633.e1. [Google Scholar] [CrossRef] [PubMed]
- Jacenik, D.; Zielińska, M.; Mokrowiecka, A.; Michlewska, S.; Małecka-Panas, E.; Kordek, R.; Fichna, J.; Krajewska, W.M. G-protein-coupled estrogen receptor mediates anti-inflammatory action in Crohn’s disease. Sci. Rep. 2019, 9, 6749. [Google Scholar] [CrossRef] [PubMed]
- Loddo, I.; Romano, C. Inflammatory bowel disease: Genetics, epigenetics, and pathogenesis. Front. Immunol. 2015, 6, 551. [Google Scholar] [CrossRef] [PubMed]
- Khor, B.; Gardet, A.; Xavier, R.J. Genetics and pathogenesis of inflammatory bowel disease. Nature 2011, 474, 307–317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ananthakrishnan, A.N. Environmental risk factors for inflammatory bowel disease. Gastroenterol. Hepatol. 2013, 9, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Bábíčková, J.; Tóthová, Ľ.; Lengyelová, E.; Bartoňová, A.; Hodosy, J.; Gardlík, R.; Celec, P. Sex differences in experimentally induced colitis in mice: A role for estrogens. Inflammation 2015, 38, 1996–2006. [Google Scholar] [CrossRef] [PubMed]
- Dumas, I.; Diorio, C. Estrogen pathway polymorphisms and mammographic density. Anticancer Res. 2011, 31, 4369–4386. [Google Scholar]
- Armstrong, C.M.; Allred, K.F.; Weeks, B.R.; Chapkin, R.S.; Allred, C.D. Estradiol has differential effects on acute colonic inflammation in the presence and absence of estrogen receptor β expression. Dig. Dis. Sci. 2017, 62, 1977–1984. [Google Scholar] [CrossRef]
- Principi, M.; Barone, M.; Pricci, M.; De Tullio, N.; Losurdo, G.; Ierardi, E.; Di Leo, A. Ulcerative colitis: From inflammation to cancer. Do estrogen receptors have a role? World J. Gastroenterol. 2014, 20, 11496–11504. [Google Scholar] [CrossRef]
- Girardi, B.; Principi, M.; Pricci, M.; Giorgio, F.; Iannone, A.; Losurdo, G.; Ierardi, E.; Di Leo, A.; Barone, M. Chemoprevention of inflammation-related colorectal cancer by silymarin-, acetyl-11-keto-beta-boswellic acid-, curcumin- and maltodextrin-enriched dietetic formulation in animal model. Carcinogenesis 2018, 39, 1274–1282. [Google Scholar] [CrossRef]
- Linares, P.M.; Algaba, A.; Urzainqui, A.; Guijarro-Rojas, M.; González-Tajuelo, R.; Garrido, J.; Chaparro, M.; Gisbert, J.P.; Bermejo, F.; Guerra, I.; et al. Ratio of circulating estrogen receptors beta and alpha (ERβ/ERα) indicates endoscopic activity in patients with Crohn’s Disease. Dig. Dis. Sci. 2017, 62, 2744–2754. [Google Scholar] [CrossRef] [PubMed]
- Vogel, C.; Marcotte, E.M. Insights into the regulation of protein abundance from proteomic and transcriptomic analyses. Nat. Rev. Genet. 2012, 13, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Cho, N.L.; Javid, S.H.; Carothers, A.M.; Redston, M.; Bertagnolli, M.M. Estrogen receptor α and β are inhibitory of Apc-dependent tumorigenesis in the proximal colon of Min/+ mice. Cancer Res. 2007, 67, 2366–2372. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.M.; Albanese, C.; Anderson, C.M.; Hilty, K.; Webb, P.; Uht, R.M.; Price, R.H., Jr.; Pestell, R.G.; Kushner, P.J. Opposing action of estrogen receptors α and β on cyclin D1 gene expression. J. Biol. Chem. 2002, 277, 24353–24360. [Google Scholar] [CrossRef] [PubMed]
- Matthews, J.; Wihlén, B.; Tujague, M.; Wan, J.; Ström, A.; Gustafsson, J.A. Estrogen receptor (ER) beta modulates ERalpha-mediated transcriptional activation by altering the recruitment of c-Fos and c-Jun to estrogen-responsive promoters. Mol. Endocrinol. 2006, 20, 534–543. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, K.; Endoh, H.; Sasaki-Iwaoka, H.; Kanou, H.; Shimaya, E.; Hashimoto, S.; Kato, S.; Kawashima, H. A novel isoform of rat estrogen receptor beta with 18 amino acid insertion in the ligand binding domain as a putative dominant negative regular of estrogen action. Biochem. Biophys. Res. Commun. 1998, 246, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Kang, L.; Zhang, X.; Xie, Y.; Tu, Y.; Wang, D.; Liu, Z.; Wang, Z.Y. Involvement of estrogen receptor variant ER-α36, not GPR30, in nongenomic estrogen signaling. Mol. Endocrinol. 2010, 24, 709–721. [Google Scholar] [CrossRef]
- Prossnitz, E.R.; Hathaway, H.J. What have we learned about GPER function in physiology and disease from knockout mice? J. Steroid. Biochem. Mol. Biol. 2015, 153, 114–126. [Google Scholar] [CrossRef] [Green Version]
- Prossnitz, E.R.; Arterburn, J.B. International Union of Basic and Clinical Pharmacology. XCVII. G-protein-Coupled Estrogen Receptor and its pharmacologic modulators. Pharm. Rev. 2015, 67, 505–540. [Google Scholar] [CrossRef]
- Włodarczyk, M.; Sobolewska-Włodarczyk, A.; Cygankiewicz, A.I.; Jacenik, D.; Piechota-Polańczyk, A.; Stec-Michalska, K.; Krajewska, W.M.; Fichna, J.; Wiśniewska-Jarosińska, M. G-protein-Coupled Receptor 30 (GPR30) expression pattern in inflammatory bowel disease patients suggests its key role in the inflammatory process. A preliminary study. J. Gastrointestin. Liver Dis. 2017, 26, 29–35. [Google Scholar]
- Prossnitz, E.R.; Barton, M. Estrogen biology: New insights into GPER function and clinical opportunities. Mol. Cell Endocrinol. 2014, 389, 71–83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mendes-Oliveira, J.; Lopes Campos, F.; Videira, R.A.; Baltazar, G. GPER activation is effective in protecting against inflammation-induced nigral dopaminergic loss and motor function impairment. Brain Behav. Immun. 2017, 64, 296–307. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.Z.; Ding, Q.; Hu, J.; He, S.M.; Shi, F.; Ma, L.T. GPER expressed on microglia mediates the anti-inflammatory effect of estradiol in ischemic stroke. Brain Behav. 2016, 6, e00449. [Google Scholar] [CrossRef] [PubMed]








| Parameters (unit) | Control | CD | UC |
|---|---|---|---|
| White blood cell (G/L) | 8.6 ± 0.2 | 9.1 ± 1.1 | 8.4 ± 0.8 |
| Neutrophil (G/L) | 5.9 ± 0.2 | 6.9 ± 1.1 | 5.4 ± 0.6 |
| Lymphocyte (G/L) | 1.8 ± 0.3 | 1.4 ± 0.1 | 1.8 ± 0.2 |
| Monocyte (G/L) | 0.8 ± 0.6 | 1.0 ± 0.4 | 0.7 ± 0.1 |
| Eosinophil (G/L) | 0.18 ± 0.01 | 0.14 ± 0.02 | 0.14 ± 0.03 |
| Basophil (G/L) | 0.05 ± 0.01 | 0.03 ± 0.01 | 0.05 ± 0.02 |
| Red blood cell (T/L) | 4.4 ± 0.3 | 4.3 ± 0.1 | 4.3 ± 0.1 |
| Hemoglobin (g/dL) | 13.9 ± 0.4 | 12.7 ± 0.3 | 12.7 ± 0.3 |
| Sodium (mmol/dL) | 139.6 ± 4.0 | 137.6 ± 0.5 | 137.0 ± 1.5 |
| Potassium (mmol/dL) | 4.3 ± 0.2 | 4.0 ± 0.1 | 4.0 ± 0.1 |
| C-reactive protein (CRP; mg/dL) | 1.1 ± 0.2 | 31.8 ± 9.1 ** | 35.4 ± 11.3 *** |
| Inflammation Biomarkers | Control | CD | UC |
|---|---|---|---|
| Interleukin-6 (IL-6) | 4.11 ± 1.25 | 30.98 ± 13.18 ** | 50.33 ± 21.14 * |
| Interleukin-10 (IL-10) | 1.57 ± 0.53 | 2.21 ± 0.78 | 6.00 ± 2.83 |
| C-C chemokine motif ligand 18 (CCL18) | 1.08 ± 0.35 | 4.94 ± 1.64 | 2.16 ± 0.36 * |
| Tumor necrosis factor-α (TNF-α) | 4.64 ± 1.01 | 5.62 ± 1.40 | 5.89 ± 1.76 |
| Nuclear factor kappa-light-chain-enhancer of activated B cells (NFκB) | 102.10 ± 11.21 | 138.10 ± 34.29 | 116.90 ± 29.76 |
| Subjects and Age of Subjects | Control | CD | UC | |
|---|---|---|---|---|
| Total | n | 31 | 31 | 42 |
| mean ± SD | 63.08 ± 15.79 | 48.40 ± 17.55 | 45.22 ± 12.63 | |
| range | 22–86 | 29–86 | 22–81 | |
| Male | n | 12 | 19 | 24 |
| mean ± SD | 63.27 ± 12.67 | 49.43 ± 48.90 | 43.24 ± 11.07 | |
| range | 32–83 | 29–86 | 29–65 | |
| Female under the age of 50 years | n | 9 | 7 | 12 |
| mean ± SD | 41.60 ± 9.52 | 36.14 ± 7.92 | 39.43 ± 6.26 | |
| range | 22–49 | 29–48 | 22–47 | |
| Female over the age of 50 years | n | 10 | 5 | 6 |
| mean ± SD | 73.60 ± 9.69 | 66.25 ± 3.30 | 65.00 ± 9.59 | |
| range | 57–86 | 64–71 | 52–81 |
| Gene | Primer | Sequence | Amplicon Length (bp) |
|---|---|---|---|
| ERα36 | Forward Reverse | CAAGTGGTTTCCTCGTGTCTAAAG TGTTGAGTGTTGGTTGCCAGG | 290 |
| ERα46 | Forward Reverse | GTGCTCCCCAAATTTCCTTTCA GCCTTCGCCATTGAAGTCAC | 440 |
| GAPDH | Forward Reverse | CTTCGCTCTCTGCTCCTCCTGTTCG ACCAGGCGCCCAATACGACCAAAT | 225 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jacenik, D.; Cygankiewicz, A.I.; Mokrowiecka, A.; Małecka-Panas, E.; Fichna, J.; Krajewska, W.M. Sex- and Age-Related Estrogen Signaling Alteration in Inflammatory Bowel Diseases: Modulatory Role of Estrogen Receptors. Int. J. Mol. Sci. 2019, 20, 3175. https://doi.org/10.3390/ijms20133175
Jacenik D, Cygankiewicz AI, Mokrowiecka A, Małecka-Panas E, Fichna J, Krajewska WM. Sex- and Age-Related Estrogen Signaling Alteration in Inflammatory Bowel Diseases: Modulatory Role of Estrogen Receptors. International Journal of Molecular Sciences. 2019; 20(13):3175. https://doi.org/10.3390/ijms20133175
Chicago/Turabian StyleJacenik, Damian, Adam I. Cygankiewicz, Anna Mokrowiecka, Ewa Małecka-Panas, Jakub Fichna, and Wanda M. Krajewska. 2019. "Sex- and Age-Related Estrogen Signaling Alteration in Inflammatory Bowel Diseases: Modulatory Role of Estrogen Receptors" International Journal of Molecular Sciences 20, no. 13: 3175. https://doi.org/10.3390/ijms20133175
APA StyleJacenik, D., Cygankiewicz, A. I., Mokrowiecka, A., Małecka-Panas, E., Fichna, J., & Krajewska, W. M. (2019). Sex- and Age-Related Estrogen Signaling Alteration in Inflammatory Bowel Diseases: Modulatory Role of Estrogen Receptors. International Journal of Molecular Sciences, 20(13), 3175. https://doi.org/10.3390/ijms20133175