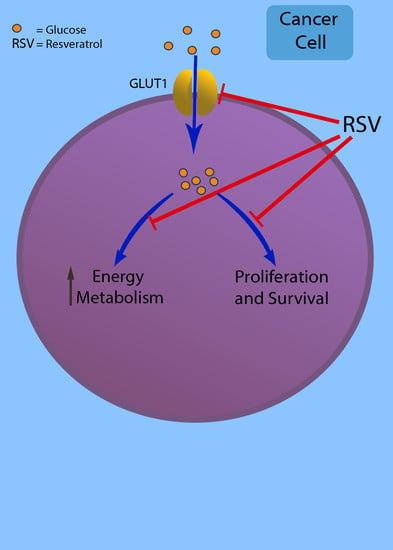
Glut 1 in Cancer Cells and the Inhibitory Action of Resveratrol as A Potential Therapeutic Strategy
Abstract
:1. Introduction
2. Glucose Uptake and Cancer Metabolism
3. Glucose Uptake and Glucose Transporters
3.1. Glucose Transporters
3.2. GLUT1: Kinetic Properties and Mechanism of Transport
3.3. GLUT1 Overexpression in Cancer Cells
3.4. How Is GLUT1 Regulated?
4. Inhibition of Glucose Uptake by Resveratrol
5. Inhibition of GLUT1 by Other Small Molecules
6. Conclusion and Final Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| RSV | Resveratrol |
| GLUT1 | Glucose transporter 1 |
| HK | Hexokinase |
| OXPHOS | Oxidative phosphorylation |
| UCP2 | Uncoupling protein 2 |
| PET | Positron emission tomography |
| FDG | 2-(18F)-fluoro-2-deoxy-D-glucose |
| SGLT | Sodium-glucose linked transporters |
| SWEET | Sugars will eventually be exported transporters |
| HIF-1alpha | Hypoxia-inducible factor |
| PDK1 | Pyruvate dehydrogenase kinase 1 |
| PFK1 | Phosphofructokinase |
| CML | Chronic myelogenous leukemia |
| PKM2 | Pyruvate kinase 2 |
| VDAC1 | Voltage-dependent anion channel 1 |
References
- Thompson, C.B. Rethinking the Regulation of Cellular Metabolism. Cold Spring Harb. Symp. Quant. Biol. 2012, 76, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Grassian, A.R.; Coloff, J.L.; Brugge, J.S. Extracellular matrix regulation of metabolism and implications for tumorigenesis extracellular matrix regulation of metabolism and implications for tumorigenesis. In Cold Spring Harbor Symposia on Quantitative Biology; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2011; pp. 313–324. [Google Scholar]
- Schafer, Z.T.; Grassian, A.R.; Song, L.; Jiang, Z.; Gerhart-Hines, Z.; Irie, H.Y.; Gao, S.; Puigserver, P.; Brugge, J.S. Antioxidant and Oncogene Rescue of Metabolic Defects Caused by Loss of Matrix Attachment. Nature 2009, 461, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. The Hallmarks of Cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef] [Green Version]
- Vogelstein, B.; Kinzler, K.W. Cancer genes and the pathways they control. Nat. Med. 2004, 10, 789. [Google Scholar] [CrossRef] [PubMed]
- Carter, L.G.; D’Orazio, J.A.; Pearson, K.J. Resveratrol and cancer: Focus on in vivo evidence. Endocr. Relat. Cancer 2014, 21, R209–R225. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, T.; Wu, J.M. Resveratrol: Biological and pharmaceutical properties as anticancer molecule. Biofactors 2010, 36, 360–369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalra, N.; Roy, P.; Prasad, S.; Shukla, Y. Resveratrol induces apoptosis involving mitochondrial pathways in mouse skin tumorigenesis. Life Sci. 2008, 82, 348–358. [Google Scholar] [CrossRef] [PubMed]
- Chin, Y.-T.; Hsieh, M.-T.; Yang, S.-H.; Tsai, P.-W.; Wang, S.-H.; Wang, C.-C.; Lee, Y.-S.; Cheng, G.-Y.; HuangFu, W.-C.; London, D.; et al. Anti-proliferative and gene expression actions of resveratrol in breast cancer cells in vitro. Oncotarget 2015, 5, 12891–12907. [Google Scholar] [CrossRef] [PubMed]
- De la Lastra, C.A.; Villegas, I. Resveratrol as an antioxidant and pro-oxidant agent: Mechanisms and clinical implications. Biochem. Soc. Trans. 2007, 35, 1156–1160. [Google Scholar] [CrossRef]
- Arcanjo, N.M.O.; Luna, C.; Madruga, M.S.; Estévez, M. Antioxidant and pro-oxidant actions of resveratrol on human serum albumin in the presence of toxic diabetes metabolites: Glyoxal and methyl-glyoxal. Biochim. Biophys. Acta—Gen. Subj. 2018, 1862, 1938–1947. [Google Scholar] [CrossRef]
- Yamamoto, T.; Seino, Y.; Fukumoto, H.; Koh, G.; Yano, H.; Inagaki, N.; Yamada, Y.; Inoue, K.; Manabe, T.; Imura, H. Over-expression of facilitative glucose transporter genes in human cancer. Biochem. Biophys. Res. Commun. 1990, 170, 223–230. [Google Scholar] [CrossRef]
- Nishioka, T.; Oda, Y.; Seino, Y.; Yamamoto, T.; Inagaki, N.; Yano, H.; Imura, H.; Shigemoto, R.; Kikuchi, H. Distribution of the Glucose Transporters in Human Brain Tumors. Cancer Res. 1992, 52, 3972–3979. [Google Scholar] [PubMed]
- Brown, R.S.; Wahl, R.L. Overexpression of glut-1 glucose transporter in human breast cancer an immunohistochemical study. Cancer 2018, 72, 2979–2985. [Google Scholar] [CrossRef]
- Cantuaria, G.; Fagotti, A.; Ferrandina, G.; Magalhaes, A.; Nadji, M.; Angioli, R.; Penalver, M.; Mancuso, S.; Scambia, G. GLUT-1 expression in ovarian carcinoma. Cancer 2001, 92, 1144–1150. [Google Scholar] [CrossRef]
- Som, P.; Atkins, H.L.; Bandoypadhyay, D.; Fowler, J.S.; Macgregor, R.R.; Matsui, K.; Oster, Z.H.; Sacker, D.F.; Shiue, C.Y.; Turner, H.; et al. A FluorinatedGlucose Analog, 2-fluoro-2-deoxy-D-glucose (F-18): Nontoxic Tracer for RapidTumor Detection ofacute orchronic. J. Nucl. Med. 1980, 21, 670–675. [Google Scholar] [PubMed]
- Almuhaideb, A.; Papathanasiou, N.; Bomanji, J. (18)F-FDG PET/CT Imaging In Oncology. Ann. Saudi Med. 2011, 31, 3–13. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, W.; Cao, Y.; Liu, Y.; Bergmeier, S.; Chen, X. Small compound inhibitors of basal glucose transport inhibit cell proliferation and induce apoptosis in cancer cells via glucose-deprivation-like mechanisms. Cancer Lett. 2010, 298, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Cao, Y.; Zhang, W.; Bergmeier, S.; Qian, Y.; Akbar, H.; Colvin, R.; Ding, J.; Tong, L.; Wu, S.; et al. A Small-Molecule Inhibitor of Glucose Transporter 1 Downregulates Glycolysis, Induces Cell-Cycle Arrest, and Inhibits Cancer Cell Growth In Vitro and In Vivo. Mol. Cancer Ther. 2012, 11, 1672–1682. [Google Scholar] [CrossRef]
- Rolfe, D.F.; Brown, G.C. Cellular energy utilization and molecular origin of standard metabolic rate in mammals. Physiol. Rev. 1997, 77, 731–758. [Google Scholar] [CrossRef] [Green Version]
- Eschbach, W. Über den Stoffwechsel der Ektopie. Arch. Gynakol. 1956, 188, 81–83. [Google Scholar] [CrossRef]
- Warburg, O.; Wind, F.; Negelein, E. The Metabolism of Tumors in the Body. J. Gen. Physiol. 1927, 8, 519–530. [Google Scholar] [CrossRef] [PubMed]
- Vander Heiden, M.G.; Cantley, L.C.; Thompson, C.B. Understanding the Warburg Effect: The Metabolic Requirements of Cell Proliferation. Science 2009, 324, 1029–1033. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gu, J.J.; Singh, A.; Xue, K.; Mavis, C.; Barth, M.; Yanamadala, V.; Lenz, P.; Grau, M.; Lenz, G.; Czuczman, M.S.; et al. Up-regulation of hexokinase II contributes to rituximab-chemotherapy resistance and is a clinically relevant target for therapeutic development. Oncotarget 2018, 9, 4020–4033. [Google Scholar] [CrossRef] [PubMed]
- Christlieb, S.B.; Strandholdt, C.N.; Olsen, B.B.; Mylam, K.J.; Larsen, T.S.; Nielsen, A.L.; Rohde, M.; Gerke, O.; Olsen, K.E.; Møller, M.B.; et al. Dual time-point FDG PET/CT and FDG uptake and related enzymes in lymphadenopathies: Preliminary results. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 1824–1836. [Google Scholar] [CrossRef] [PubMed]
- Fantin, V.R.; St-Pierre, J.; Leder, P. Attenuation of LDH-A expression uncovers a link between glycolysis, mitochondrial physiology, and tumor maintenance. Cancer Cell 2006, 9, 425–434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shim, H.; Chun, Y.S.; Lewis, B.C.; Dang, C.V. A unique glucose-dependent apoptotic pathway induced by c-Myc. Proc. Natl. Acad. Sci. USA 1998, 95, 1511–1516. [Google Scholar] [CrossRef]
- Zu, X.L.; Guppy, M. Cancer metabolism: Facts, fantasy, and fiction. Biochem. Biophys. Res. Commun. 2004, 313, 459–465. [Google Scholar] [CrossRef]
- Rodríguez-Enríquez, S.; Carreño-Fuentes, L.; Gallardo-Pérez, J.C.; Saavedra, E.; Quezada, H.; Vega, A.; Marín-Hernández, A.; Olín-Sandoval, V.; Torres-Márquez, M.E.; Moreno-Sánchez, R. Oxidative phosphorylation is impaired by prolonged hypoxia in breast and possibly in cervix carcinoma. Int. J. Biochem. Cell Biol. 2010, 42, 1744–1751. [Google Scholar] [CrossRef]
- Elwood, J.C.; Lin, Y.-C.; Cristofalo, V.J.; Weinhouse, S.; Morris, H.P. Glucose Utilization in Homogenates of the Morris Hepatoma 5123 and Related Tumors. Cancer Res. 1963, 23, 906–913. [Google Scholar]
- Kallinowski, F.; Schlenger, K.H.; Kloes, M.; Stohrer, M.; Vaupel, P. Tumor blood flow: The principal modulator of oxidative and glycolytic metabolism, and of the metabolic micromilieu of human tumor xenografts in vivo. Int. J. Cancer 2018, 44, 266–272. [Google Scholar] [CrossRef]
- Balaban, R.S.; Bader, J.P. Studies on the relationship between glycolysis and (Na++K+)-ATPase in cultured cells. Biochim. Biophys. Acta—Mol. Cell Res. 1984, 804, 419–426. [Google Scholar] [CrossRef]
- Griguer, C.E.; Oliva, C.R.; Gillespie, G.Y. Glucose metabolism heterogeneity in human and mouse malignant glioma cell lines. J. Neurooncol. 2005, 74, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Bouzier, A.-K.; Voisin, P.; Goodwin, R.; Canioni, P.; Merle, M. Glucose and Lactate Metabolism in C6 Glioma Cells: Evidence for the Preferential Utilization of Lactate for Cell Oxidative Metabolism. Dev. Neurosci. 1998, 20, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Rossignol, R.; Gilkerson, R.; Aggeler, R.; Yamagata, K.; Remington, S.J.; Capaldi, R.A. Energy Substrate Modulates Mitochondrial Structure and Oxidative Capacity in Cancer Cells. Cancer Res. 2004, 64, 985–993. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smolková, K.; Bellance, N.; Scandurra, F.; Génot, E.; Gnaiger, E.; Plecitá-Hlavatá, L.; Ježek, P.; Rossignol, R. Mitochondrial bioenergetic adaptations of breast cancer cells to aglycemia and hypoxia. J. Bioenerg. Biomembr. 2010, 42, 55–67. [Google Scholar] [CrossRef]
- Moreno-Sánchez, R.; Rodríguez-Enríquez, S.; Marín-Hernández, A.; Saavedra, E. Energy metabolism in tumor cells. FEBS J. 2007, 274, 1393–1418. [Google Scholar] [CrossRef] [PubMed]
- Samudio, I.; Fiegl, M.; McQueen, T.; Clise-Dwyer, K.; Andreeff, M. The Warburg effect in leukemia-stroma cocultures is mediated by mitochondrial uncoupling associated with uncoupling protein 2 activation. Cancer Res. 2008, 68, 5198–5205. [Google Scholar] [CrossRef]
- Samudio, I.; Harmancey, R.; Fiegl, M.; Kantarjian, H.; Konopleva, M.; Korchin, B.; Kaluarachchi, K.; Bornmann, W.; Duvvuri, S.; Taegtmeyer, H.; et al. Pharmacologic inhibition of fatty acid oxidation sensitizes human leukemia cells to apoptosis induction. J. Clin. Invest. 2010, 120, 142–156. [Google Scholar] [CrossRef] [Green Version]
- Vélez, J.; Hail, N.; Konopleva, M.; Zeng, Z.; Kojima, K.; Samudio, I.; Andreeff, M. Mitochondrial Uncoupling and the Reprograming of Intermediary Metabolism in Leukemia Cells. Front. Oncol. 2013, 3, 67. [Google Scholar] [CrossRef] [Green Version]
- Baffy, G. Mitochondrial uncoupling in cancer cells: Liabilities and opportunities. Biochim. Biophys. Acta. Bioenerg. 2017, 1858, 655–664. [Google Scholar] [CrossRef]
- Esteves, P.; Pecqueur, C.; Alves-Guerra, M.-C. UCP2 induces metabolic reprogramming to inhibit proliferation of cancer cells. Mol. Cell. Oncol. 2015, 2, e975024. [Google Scholar] [CrossRef] [PubMed]
- Wuest, M.; Hamann, I.; Bouvet, V.; Glubrecht, D.; Marshall, A.; Trayner, B.; Soueidan, O.-M.; Krys, D.; Wagner, M.; Cheeseman, C.; et al. Molecular Imaging of GLUT1 and GLUT5 in Breast Cancer: A Multitracer Positron Emission Tomography Imaging Study in Mice. Mol. Pharmacol. 2018, 93, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Liberti, M.V.; Locasale, J.W. The Warburg Effect: How Does it Benefit Cancer Cells? Trends Biochem. Sci. 2016, 41, 211–218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mueckler, M.; Thorens, B. The SLC2 (GLUT) family of membrane transporters. Mol. Aspects Med. 2013, 34, 121–138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, L.-Q.; Cheung, L.S.; Feng, L.; Tanner, W.; Frommer, W.B. Transport of Sugars. Annu. Rev. Biochem. 2015, 84, 865–894. [Google Scholar] [CrossRef]
- Feng, L.; Frommer, W.B. Structure and function of SemiSWEET and SWEET sugar transporters. Trends Biochem. Sci. 2015, 40, 480–486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, D.; Yan, N. GLUT, SGLT, and SWEET: Structural and mechanistic investigations of the glucose transporters. Protein Sci. 2015, 25, 546–558. [Google Scholar] [CrossRef] [Green Version]
- Wright, E.M.; Ghezzi, C.; Loo, D.D.F. Novel and Unexpected Functions of SGLTs. Physiology 2017, 32, 435–443. [Google Scholar] [CrossRef]
- Sosso, D.; Luo, D.; Li, Q.-B.; Sasse, J.; Yang, J.; Gendrot, G.; Suzuki, M.; Koch, K.E.; McCarty, D.R.; Chourey, P.S.; et al. Seed filling in domesticated maize and rice depends on SWEET-mediated hexose transport. Nat. Genet. 2015, 47, 1489. [Google Scholar] [CrossRef]
- Lin, I.W.; Sosso, D.; Chen, L.-Q.; Gase, K.; Kim, S.-G.; Kessler, D.; Klinkenberg, P.M.; Gorder, M.K.; Hou, B.-H.; Qu, X.-Q.; et al. Nectar secretion requires sucrose phosphate synthases and the sugar transporter SWEET9. Nature 2014, 508, 546. [Google Scholar] [CrossRef]
- Chen, L.-Q.; Hou, B.-H.; Lalonde, S.; Takanaga, H.; Hartung, M.L.; Qu, X.-Q.; Guo, W.-J.; Kim, J.-G.; Underwood, W.; Chaudhuri, B.; et al. Sugar transporters for intercellular exchange and nutrition of pathogens. Nature 2010, 468, 527. [Google Scholar] [CrossRef] [PubMed]
- Thorens, B.; Mueckler, M. Glucose transporters in the 21st Century. Am. J. Physiol. Metab. 2009, 298, E141–E145. [Google Scholar] [CrossRef] [PubMed]
- Burant, C.F.; Bell, G.I. Mammalian facilitative glucose transporters: Evidence for similar substrate recognition sites in functionally monomeric proteins. Biochemistry 1992, 31, 10414–10420. [Google Scholar] [CrossRef] [PubMed]
- Ojeda, P.; Pérez, A.; Ojeda, L.; Vargas-Uribe, M.; Rivas, C.I.; Salas, M.; Vera, J.C.; Reyes, A.M. Noncompetitive blocking of human GLUT1 hexose transporter by methylxanthines reveals an exofacial regulatory binding site. Am. J. Physiol. Physiol. 2012, 303, C530–C539. [Google Scholar] [CrossRef] [PubMed]
- Leto, D.; Saltiel, A.R. Regulation of glucose transport by insulin: Traffic control of GLUT4. Nat. Rev. Mol. Cell Biol. 2012, 13, 383. [Google Scholar] [CrossRef] [PubMed]
- Yan, N. A Glimpse of Membrane Transport through Structures—Advances in the Structural Biology of the GLUT Glucose Transporters. J. Mol. Biol. 2017, 429, 2710–2725. [Google Scholar] [CrossRef] [PubMed]
- Deng, D.; Xu, C.; Sun, P.; Wu, J.; Yan, C.; Hu, M.; Yan, N. Crystal structure of the human glucose transporter GLUT1. Nature 2014, 510, 121. [Google Scholar] [CrossRef]
- Kapoor, K.; Finer-Moore, J.S.; Pedersen, B.P.; Caboni, L.; Waight, A.; Hillig, R.C.; Bringmann, P.; Heisler, I.; Müller, T.; Siebeneicher, H.; et al. Mechanism of inhibition of human glucose transporter GLUT1 is conserved between cytochalasin B and phenylalanine amides. Proc. Natl. Acad. Sci. USA 2016, 113, 4711–4716. [Google Scholar] [CrossRef] [Green Version]
- Nomura, N.; Verdon, G.; Kang, H.J.; Shimamura, T.; Nomura, Y.; Sonoda, Y.; Hussien, S.A.; Qureshi, A.A.; Coincon, M.; Sato, Y.; et al. Structure and mechanism of the mammalian fructose transporter GLUT5. Nature 2015, 526, 397. [Google Scholar] [CrossRef]
- Deng, D.; Sun, P.; Yan, C.; Ke, M.; Jiang, X.; Xiong, L.; Ren, W.; Hirata, K.; Yamamoto, M.; Fan, S.; et al. Molecular basis of ligand recognition and transport by glucose transporters. Nature 2015, 526, 391. [Google Scholar] [CrossRef]
- Kumar, S.; Nussinov, R. Close-Range Electrostatic Interactions in Proteins. ChemBioChem 2002, 3, 604–617. [Google Scholar] [CrossRef]
- Yan, N. Structural advances for the major facilitator superfamily (MFS) transporters. Trends Biochem. Sci. 2013, 38, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Quistgaard, E.M.; Löw, C.; Guettou, F.; Nordlund, P. Understanding transport by the major facilitator superfamily (MFS): Structures pave the way. Nat. Rev. Mol. Cell Biol. 2016, 17, 123. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, K.C.; Cunha, I.W.; Rocha, R.M.; Ayala, F.R.; Cajaíba, M.M.; Begnami, M.D.; Vilela, R.S.; Paiva, G.R.; Andrade, R.G.; Soares, F.A. GLUT1 expression in malignant tumors and its use as an immunodiagnostic marker. Clinics 2011, 66, 965–972. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parente, P.; Coli, A.; Massi, G.; Mangoni, A.; Fabrizi, M.M.; Bigotti, G. Immunohistochemical expression of the glucose transporters Glut-1 and Glut-3 in human malignant melanomas and benign melanocytic lesions. J. Exp. Clin. Cancer Res. 2008, 27, 34. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.; Kim, H.; Nam, K.; Shin, I. Silencing of Glut1 induces chemoresistance via modulation of Akt/GSK-3β/β-catenin/survivin signaling pathway in breast cancer cells. Arch. Biochem. Biophys. 2017, 636, 110–122. [Google Scholar] [CrossRef] [PubMed]
- Younes, M.; Brown, R.W.; Mody, D.R.; Fernandez, L.; Laucirica, R. GLUT1 expression in human breast carcinoma: Correlation with known prognostic markers. Anticancer Res. 1995, 15, 2895–2898. [Google Scholar]
- Cantuaria, G.; Magalhaes, A.; Penalver, M.; Angioli, R.; Braunschweiger, P.; Gomez-Marin, O.; Kanhoush, R.; Gomez-Fernandez, C.; Nadji, M. Expression of GLUT-1 Glucose Transporter in Borderline and Malignant Epithelial Tumors of the Ovary. Gynecol. Oncol. 2000, 79, 33–37. [Google Scholar] [CrossRef]
- Semaan, A.; Munkarah, A.R.; Arabi, H.; Bandyopadhyay, S.; Seward, S.; Kumar, S.; Qazi, A.; Hussein, Y.; Morris, R.T.; Ali-Fehmi, R. Expression of GLUT-1 in epithelial ovarian carcinoma: Correlation with tumor cell proliferation, angiogenesis, survival and ability to predict optimal cytoreduction. Gynecol. Oncol. 2011, 121, 181–186. [Google Scholar] [CrossRef]
- Chandler, J.D.; Williams, E.D.; Slavin, J.L.; Best, J.D.; Rogers, S. Expression and localization of GLUT1 and GLUT12 in prostate carcinoma. Cancer 2003, 97, 2035–2042. [Google Scholar] [CrossRef]
- Reinicke, K.; Sotomayor, P.; Cisterna, P.; Delgado, C.; Nualart, F.; Godoy, A. Cellular distribution of Glut-1 and Glut-5 in benign and malignant human prostate tissue. J. Cell. Biochem. 2011, 113, 553–562. [Google Scholar] [CrossRef] [PubMed]
- Grabellus, F.; Nagarajah, J.; Bockisch, A.; Schmid, K.W.; Sheu, S.-Y. Glucose Transporter 1 Expression, Tumor Proliferation, and Iodine/Glucose Uptake in Thyroid Cancer With Emphasis on Poorly Differentiated Thyroid Carcinoma. Clin. Nucl. Med. 2012, 37, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Cooper, R.; Sarioğlu, S.; Sökmen, S.; Füzün, M.; Küpelioğlu, A.; Valentine, H.; Görken, I.B.; Airley, R.; West, C. Glucose transporter-1 (GLUT-1): A potential marker of prognosis in rectal carcinoma? Br. J. Cancer 2003, 89, 870. [Google Scholar] [CrossRef] [PubMed]
- Abdou, A.G.; Eldien, M.M.S.; Elsakka, D. GLUT-1 Expression in Cutaneous Basal and Squamous Cell Carcinomas. Int. J. Surg. Pathol. 2015, 23, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Haber, R.S.; Rathan, A.; Weiser, K.R.; Pritsker, A.; Itzkowitz, S.H.; Bodian, C.; Slater, G.; Weiss, A.; Burstein, D.E. GLUT1 glucose transporter expression in colorectal carcinoma. Cancer 2000, 83, 34–40. [Google Scholar] [CrossRef]
- Jun, Y.J.; Jang, S.M.; Han, H.L.; Lee, K.H.; Jang, K.S.; Paik, S.S. Clinicopathologic signifcance of GULT1 expression and its correlation with Apaf-1 in colorectal adenocarcinomas. World J. Gastroenterol. 2011, 17, 1866–1873. [Google Scholar] [CrossRef] [PubMed]
- Sakashita, M.; Aoyama, N.; Minami, R.; Maekawa, S.; Kuroda, K.; Shirasaka, D.; Ichihara, T.; Kuroda, Y.; Maeda, S.; Kasuga, M. Glut1 expression in T1 and T2 stage colorectal carcinomas: Its relationship to clinicopathological features. Eur. J. Cancer 2001, 37, 204–209. [Google Scholar] [CrossRef]
- Giatromanolaki, A.; Sivridis, E.; Arelaki, S.; Koukourakis, M.I. Expression of enzymes related to glucose metabolism in non-small cell lung cancer and prognosis. Exp. Lung Res. 2017, 43, 167–174. [Google Scholar] [CrossRef]
- Ayala, F.R.R.; Rocha, R.M.; Carvalho, K.C.; Carvalho, A.L.; Da Cunha, I.W.; Lourenço, S.V.; Soares, F.A. Glut1 and Glut3 as Potential Prognostic Markers for Oral Squamous Cell Carcinoma. Molecules 2010, 15, 2374–2387. [Google Scholar] [CrossRef]
- Oliver, R.J.; Woodwards, R.T.M.; Sloan, P.; Thakker, N.S.; Stratford, I.J.; Airley, R.E. Prognostic value of facilitative glucose transporter Glut-1 in oral squamous cell carcinomas treated by surgical resection: Results of EORTC Translational Research Fund studies. Eur. J. Cancer 2004, 40, 503–507. [Google Scholar] [CrossRef]
- Choi, Y.S.; Kim, S.J.; Kim, D.S.; Park, S.J.; Park, Y.; Shin, H.J.; Jung, K.-Y.; Baek, S.-K.; Shin, B.K.; Choi, J.W.; et al. Glucose Transporter-1 Expression in Squamous Cell Carcinoma of the Tongue. Cancer Res. Treat. 2007, 39, 109–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiba, I.; Ogawa, K.; Morioka, T.; Shimoji, H.; Sunagawa, N.A.O.; Iraha, S.; Nishimaki, T.; Yoshimi, N.; Murayama, S. Clinical significance of GLUT-1 expression in patients with esophageal cancer treated with concurrent chemoradiotherapy. Oncol. Lett. 2011, 2, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Kim, Y.-W.; Chang, S.-G. Glucose Transporter-1 Expression in Urothelial Papilloma of the Bladder. Urol. Int. 2005, 74, 268–271. [Google Scholar] [CrossRef] [PubMed]
- Nes, J.A.P.; Griewank, K.G.; Schmid, K.-W.; Grabellus, F. Immunocytochemical analysis of glucose transporter protein-1 (GLUT-1) in typical, brain invasive, atypical and anaplastic meningioma. Neuropathology 2014, 35, 24–36. [Google Scholar] [PubMed]
- Luo, X.-M.; Zhou, S.-H.; Fan, J. Glucose Transporter-1 as a New Therapeutic Target in Laryngeal Carcinoma. J. Int. Med. Res. 2010, 38, 1885–1892. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Luo, X.-M.; Yao, H.-T.; Zhou, S.-H.; Ruan, L.-X.; Yan, S.-X. Expression of Glucose Transporter-1, Hypoxia-Inducible Factor-1α, Phosphatidylinositol 3-Kinase and Protein Kinase B (Akt) in Relation to [18F]Fluorodeoxyglucose Uptake in Nasopharyngeal Diffuse Large B-Cell Lymphoma: A Case Report and Literature Review. J. Int. Med. Res. 2010, 38, 2160–2168. [Google Scholar] [CrossRef] [PubMed]
- Basturk, O.; Singh, R.; Kaygusuz, E.; Balci, S.; Dursun, N.; Culhaci, N.; Adsay, N.V. GLUT-1 Expression in Pancreatic Neoplasia: Implications in Pathogenesis, Diagnosis, and Prognosis. Pancreas 2011, 40, 187–192. [Google Scholar] [CrossRef]
- Chan, D.A.; Sutphin, P.D.; Nguyen, P.; Turcotte, S.; Lai, E.W.; Banh, A.; Reynolds, G.E.; Chi, J.-T.; Wu, J.; Solow-Cordero, D.E.; et al. Targeting GLUT1 and the Warburg Effect in Renal Cell Carcinoma by Chemical Synthetic Lethality. Sci. Transl. Med. 2011, 3, 94ra70. [Google Scholar] [CrossRef]
- Nagase, Y.; Takata, K.; Moriyama, N.; Aso, Y.; Murakami, T.; Hirano, H. Investigative Urology: Immunohistochemical Localization of Glucose Transporters in Human Renal Cell Carcinoma. J. Urol. 1995, 153, 798–801. [Google Scholar] [CrossRef]
- Amann, T.; Hellerbrand, C. GLUT1 as a therapeutic target in hepatocellular carcinoma. Expert Opin. Ther. Targets 2009, 13, 1411–1427. [Google Scholar] [CrossRef]
- Ogawa, J.; Inoue, H.; Koide, S. Glucose-transporter-type-I-gene amplification correlates with Sialyl-Lewis-X synthesis and proliferation in lung cancer. Int. J. Cancer 1998, 74, 189–192. [Google Scholar] [CrossRef]
- Rudlowski, C.; Becker, A.J.; Schroder, W.; Rath, W.; Büttner, R.; Moser, M. GLUT1 Messenger RNA and Protein Induction Relates to the Malignant Transformation of Cervical Cancer. Am. J. Clin. Pathol. 2003, 120, 691–698. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Yongzhi, H.; Chen, S.; Luo, X.; Lin, Y.; Zhou, Y.; Jin, H.; Hou, B.; Deng, Y.; Tu, L.; et al. The prognostic value of GLUT1 in cancers: A systematic review and meta-analysis. Oncotarget 2015, 8, 43356–43367. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ye, C.; Chen, C.; Xiong, H.; Xie, B.; Zhou, J.; Chen, Y.; Zheng, S.; Wang, L. Glucose transporter GLUT1 expression and clinical outcome in solid tumors: A systematic review and meta-analysis. Oncotarget 2017, 8, 16875–16886. [Google Scholar] [CrossRef] [PubMed]
- Okino, S.T.; Chichester, C.H.; Withlock, J.P. Hypoxia-indicible mammalian gene expression analysed in vivo at a TATA-driven promoter and at an-initiator-driven promoter. J. Biol. Chem. 1998, 273, 23837. [Google Scholar] [CrossRef] [PubMed]
- Zelzer, E.; Levy, Y.; Kahana, C.; Shilo, B.; Rubinstein, M.; Cohen, B. Insulin induces transcription of target genes through the hypoxia-inducible factor HIF-1α/ARNT. EMBO J. 1998, 17, 5085–5094. [Google Scholar] [CrossRef]
- Ebert, B.L.; Firth, J.D.; Peter, J.; Ebert, B.L.; Firth, J.D.; Ratcliffe, P.J. Cell Biology and Metabolism: Hypoxia and Mitochondrial Inhibitors Regulate Expression of Glucose Transporter-1 via Distinct Cis-acting Sequences Hypoxia and Mitochondrial Inhibitors Regulate Expression of Glucose Transporter-1 via Distinct Cis-acting Seq. J. Biol. Chem. 1995, 270, 29083–29089. [Google Scholar] [CrossRef] [PubMed]
- Osthus, R.C.; Shim, H.; Kim, S.; Li, Q.; Reddy, R.; Mukherjee, M.; Xu, Y.; Wonsey, D.; Lee, L.A.; Dang, C.V. Deregulation of glucose transporter 1 and glycolytic gene expression by c-Myc. J. Biol. Chem. 2000, 275, 21797–21800. [Google Scholar] [CrossRef]
- Chen, C.; Pore, N.; Behrooz, A.; Ismail-Beigi, F.; Maity, A. Regulation of glut1 mRNA by hypoxia-inducible factor-1. Interaction between H-ras and hypoxia. J. Biol. Chem. 2001, 276, 9519–9525. [Google Scholar] [CrossRef]
- Yun, J.; Rago, C.; Cheong, I.; Pagliarini, R.; Angenendt, P.; Rajagopalan, H.; Schmidt, K.; Willson, J.K.V.; Markowitz, S.; Zhou, S.; et al. Glucose Deprivation Contributes to the Development of KRAS Pathway Mutations in Tumor Cells. Science 2009, 325, 1555–1559. [Google Scholar]
- Jacobs, S.R.; Herman, C.E.; MacIver, N.J.; Wofford, J.A.; Wieman, H.L.; Hammen, J.J.; Rathmell, J.C. Glucose Uptake Is Limiting in T Cell Activation and Requires CD28-Mediated Akt-Dependent and Independent Pathways. J. Immunol. 2008, 180, 4476–4486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wofford, J.A.; Wieman, H.L.; Jacobs, S.R.; Zhao, Y.; Rathmell, J.C. IL-7 promotes Glut1 trafficking and glucose uptake via STAT5-mediated activation of Akt to support T-cell survival. Blood 2008, 111, 2101–2111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barthel, A.; Okino, S.T.; Liao, J.; Nakatani, K.; Li, J.; Whitlock, J.P.; Roth, R.A. Regulation of GLUT1 gene transcription by the serine/threonine kinase Akt1. J. Biol. Chem. 1999, 274, 20281–20286. [Google Scholar] [CrossRef] [PubMed]
- Qi, C.; Pekala, P.H. The Influence of mRNA Stability on Glucose Transporter (GLUT1) Gene Expression. Biochem. Biophys. Res. Commun. 1999, 263, 265–269. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; Fan, Q.; Liang, Y.; Xiaodong, Z.; Ma, Y.; Zhihong, Z.; Hua, X.; Su, D.; Sun, H.; Li, H.; et al. Promotion of glycolysis by HOTAIR through GLUT1 upregulation via mTOR signaling. Oncol. Rep. 2017, 38, 1902–1908. [Google Scholar] [CrossRef] [Green Version]
- Yuan, G.; Zhao, Y.; Wu, D.; Gao, C. Mir-150 Up-Regulates Glut1 and Increases Glycolysis in Osteosarcoma Cells. Asian Pac. J. Cancer Prev. 2017, 18, 1127–1131. [Google Scholar] [PubMed]
- Zambrano, A.; Jara, E.; Murgas, P.; Jara, C.; Castro, M.A.; Angulo, C.; Concha, I.I. Cytokine stimulation promotes increased glucose uptake via translocation at the plasma membrane of GLUT1 in HEK293 cells. J. Cell. Biochem. 2010, 110, 1471–1480. [Google Scholar] [CrossRef]
- Wieman, H.L.; Wofford, J.A.; Rathmell, J.C. Cytokine Stimulation Promotes Glucose Uptake via Phosphatidylinositol-3 Kinase/Akt Regulation of Glut1 Activity and Trafficking. Mol. Biol. Cell 2007, 18, 1437–1446. [Google Scholar] [CrossRef] [Green Version]
- Melstrom, L.G.; Salabat, M.R.; Ding, X.-Z.; Milam, B.M.; Strouch, M.; Pelling, J.C.; Bentrem, D.J. Apigenin Inhibits the GLUT-1 Glucose Transporter and the Phosphoinositide 3-Kinase/Akt Pathway in Human Pancreatic Cancer Cells. Pancreas 2008, 37, 426–431. [Google Scholar] [CrossRef]
- Bishayee, A. Cancer Prevention and Treatment with Resveratrol: From Rodent Studies to Clinical Trials. Cancer Prev. Res. 2009, 2, 409–418. [Google Scholar] [CrossRef] [Green Version]
- Pirola, L.; Fröjdö, S. Resveratrol: One molecule, many targets. IUBMB Life 2008, 60, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, L.; Youssef, S.; Bhattacharya, S.; Kenealey, J.; Polans, A.S.; van Ginkel, P.R. Resveratrol: Challenges in Translation to the Clinic—A Critical Discussion. Clin. Cancer Res. 2010, 16, 5942–5948. [Google Scholar] [CrossRef] [PubMed]
- Vera, J.C.; Reyes, A.M.; Velásquez, F.V.; Rivas, C.I.; Zhang, R.H.; Strobel, P.; Slebe, J.C.; Núñez-Alarcón, J.; Golde, D.W. Direct Inhibition of the Hexose Transporter GLUT1 by Tyrosine Kinase Inhibitors. Biochemistry 2001, 40, 777–790. [Google Scholar] [CrossRef] [PubMed]
- Vera, J.C.; Reyes, A.M.; Cárcamo, J.G.; Velásquez, F.V.; Rivas, C.I.; Zhang, R.H.; Strobel, P.; Iribarren, R.; Scher, H.I.; Slebe, J.C.; et al. Genistein is a natural inhibitor of hexose and dehydroascorbic acid transport through the glucose transporter, GLUT1. J. Biol. Chem. 1996, 271, 8719–8724. [Google Scholar] [CrossRef] [PubMed]
- Salas, M.; Obando, P.; Ojeda, L.; Ojeda, P.; Pérez, A.; Vargas-Uribe, M.; Rivas, C.I.; Vera, J.C.; Reyes, A.M. Resolution of the direct interaction with and inhibition of the human GLUT1 hexose transporter by resveratrol from its effect on glucose accumulation. Am. J. Physiol. Physiol. 2013, 305, C90–C99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kueck, A.; Opipari, A.W.; Griffith, K.A.; Tan, L.; Choi, M.; Huang, J.; Wahl, H.; Liu, J.R. Resveratrol inhibits glucose metabolism in human ovarian cancer cells. Gynecol. Oncol. 2007, 107, 450–457. [Google Scholar] [CrossRef]
- Opipari, A.W.; Tan, L.; Boitano, A.E.; Sorenson, D.R.; Aurora, A.; Liu, J.R. Resveratrol-induced Autophagocytosis in Ovarian Cancer Cells. Cancer Res. 2004, 64, 696–703. [Google Scholar] [CrossRef]
- Liu, Y.; Tong, L.; Luo, Y.; Li, X.; Chen, G.; Wang, Y. Resveratrol inhibits the proliferation and induces the apoptosis in ovarian cancer cells via inhibiting glycolysis and targeting AMPK/mTOR signaling pathway. J. Cell. Biochem. 2018, 119, 6162–6172. [Google Scholar] [CrossRef]
- Chen, S.; Zhao, Z.; Ke, L.; Li, Z.; Li, W.; Zhang, Z.; Zhou, Y.; Feng, X.; Zhu, W. Resveratrol improves glucose uptake in insulin-resistant adipocytes via Sirt1. J. Nutr. Biochem. 2018, 55, 209–218. [Google Scholar] [CrossRef]
- Varshney, P.; Dey, C.S. Resveratrol regulates neuronal glucose uptake and insulin sensitivity via P21-activated kinase 2 (PAK2). Biochem. Biophys. Res. Commun. 2017, 485, 372–378. [Google Scholar] [CrossRef]
- Delmas, D.; Lancon, A.; Colin, D.; Jannin, B.; Latruffe, N. Resveratrol as a Chemopreventive Agent: A Promising Molecule for Fighting Cancer. Curr. Drug Targets 2006, 7, 423–442. [Google Scholar] [CrossRef] [PubMed]
- Baur, J.A.; Sinclair, D.A. Therapeutic potential of resveratrol: The in vivo evidence. Nat. Rev. Drug Discov. 2006, 5, 493. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-J.; Ahmad, F.; Philp, A.; Baar, K.; Williams, T.; Luo, H.; Ke, H.; Rehmann, H.; Taussig, R.; Brown, A.L.; et al. Resveratrol ameliorates aging-related metabolic phenotypes by inhibiting cAMP phosphodiesterases. Cell 2012, 148, 421–433. [Google Scholar] [CrossRef] [PubMed]
- Alarcón de la Lastra, C.; Villegas, I. Resveratrol as an anti-inflammatory and anti-aging agent: Mechanisms and clinical implications. Mol. Nutr. Food Res. 2005, 49, 405–430. [Google Scholar] [CrossRef] [PubMed]
- Meeran, S.M.; Katiyar, S.K. Cell cycle control as a basis for cancer chemoprevention through dietary agents. Front. Biosci. 2008, 13, 2191–2202. [Google Scholar] [CrossRef] [PubMed]
- Van Ginkel, P.R.; Sareen, D.; Subramanian, L.; Walker, Q.; Darjatmoko, S.R.; Lindstrom, M.J.; Kulkarni, A.; Albert, D.M.; Polans, A.S. Resveratrol Inhibits Tumor Growth of Human Neuroblastoma and Mediates Apoptosis by Directly Targeting Mitochondria. Clin. Cancer Res. 2007, 13, 5162–5169. [Google Scholar] [CrossRef] [PubMed]
- Dai, H.; Deng, H.-B.; Wang, Y.-H.; Guo, J.-J. Resveratrol inhibits the growth of gastric cancer via the Wnt/β-catenin pathway. Oncol. Lett. 2018, 16, 1579–1583. [Google Scholar] [CrossRef] [PubMed]
- Kleszcz, R.; Paluszczak, J.; Krajka-Kuźniak, V.; Baer-Dubowska, W. The inhibition of c-MYC transcription factor modulates the expression of glycolytic and glutaminolytic enzymes in FaDu hypopharyngeal carcinoma cells. Adv. Clin. Exp. Med. 2018, 27, 735–742. [Google Scholar] [CrossRef] [PubMed]
- Puissant, A.; Auberger, P. AMPK- and p62/SQSTM1-dependent autophagy mediate Resveratrol-induced cell death in chronic myelogenous leukemia. Autophagy 2010, 6, 655–657. [Google Scholar] [CrossRef] [Green Version]
- Puissant, A.; Robert, G.; Fenouille, N.; Luciano, F.; Cassuto, J.-P.; Raynaud, S.; Auberger, P. Resveratrol Promotes Autophagic Cell Death in Chronic Myelogenous Leukemia Cells via JNK-Mediated p62/SQSTM1 Expression and AMPK Activation. Cancer Res. 2010, 70, 1042–1052. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Tian, F.; Xiao, Q.; Hu, Y.; Li, J.; Jiang, F.; Liu, Y. Exploiting the Role of Resveratrol in Rat Mitochondrial Permeability Transition. J. Membr. Biol. 2013, 246, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Yang, B.; Jia, X.; Li, M.; Tan, W.; Ma, S.; Shi, X.; Feng, L. Distinctive Roles of Sirtuins on Diabetes, Protective or Detrimental? Front. Endocrinol. 2018, 9, 724. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.; Mostoslavsky, R. SIRT6: A master epigenetic gatekeeper of glucose metabolism. Transcription 2010, 1, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Dembic, M.; Andersen, H.S.; Bastin, J.; Doktor, T.K.; Corydon, T.J.; Sass, J.O.; Costa, A.L.; Djouadi, F.; Andresen, B.S. Next generation sequencing of RNA reveals novel targets of resveratrol with possible implications for Canavan disease. Mol. Genet. Metab. 2019, 126, 64–76. [Google Scholar] [CrossRef] [Green Version]
- Song, R.; Xu, W.; Chen, Y.; Li, Z.; Zeng, Y.; Fu, Y. The expression of Sirtuins 1 and 4 in peripheral blood leukocytes from patients with type 2 diabetes. Eur. J. Histochem. 2011, 55, e10. [Google Scholar] [CrossRef] [PubMed]
- Gomes, B.A.Q.; Silva, J.P.B.; Romeiro, C.F.R.; Dos Santos, S.M.; Rodrigues, C.A.; Gonçalves, P.R.; Sakai, J.T.; Mendes, P.F.S.; Varela, E.L.P.; Monteiro, M.C. Neuroprotective Mechanisms of Resveratrol in Alzheimer’s Disease: Role of SIRT1. Oxid. Med. Cell. Longev. 2018, 2018, 8152373. [Google Scholar] [CrossRef]
- Palsamy, P.; Subramanian, S. Resveratrol, a natural phytoalexin, normalizes hyperglycemia in streptozotocin-nicotinamide induced experimental diabetic rats. Biomed. Pharmacother. 2008, 62, 598–605. [Google Scholar] [CrossRef] [PubMed]
- Palsamy, P.; Subramanian, S. Modulatory effects of resveratrol on attenuating the key enzymes activities of carbohydrate metabolism in streptozotocin–nicotinamide-induced diabetic rats. Chem. Biol. Interact. 2009, 179, 356–362. [Google Scholar] [CrossRef]
- Iqbal, M.A.; Gupta, V.; Gopinath, P.; Mazurek, S.; Bamezai, R.N.K. Pyruvate kinase M2 and cancer: An updated assessment. FEBS Lett. 2014, 588, 2685–2692. [Google Scholar] [CrossRef]
- Zhao, H.; Han, L.; Jian, Y.; Ma, Y.; Yan, W.; Chen, X.; Xu, H.; Li, L. Resveratrol induces apoptosis in human melanoma cell through negatively regulating Erk/PKM2/Bcl-2 axis. Onco. Targets. Ther. 2018, 11, 8995–9006. [Google Scholar] [CrossRef]
- Christofk, H.R.; Vander Heiden, M.G.; Harris, M.H.; Ramanathan, A.; Gerszten, R.E.; Wei, R.; Fleming, M.D.; Schreiber, S.L.; Cantley, L.C. The M2 splice isoform of pyruvate kinase is important for cancer metabolism and tumour growth. Nature 2008, 452, 230. [Google Scholar] [CrossRef] [PubMed]
- Wenner, C.E. Cell signaling and cancer-possible targets for therapy. J. Cell. Physiol. 2010, 223, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Tian, M.; Xie, Y.; Meng, Y.; Ma, W.; Tong, Z.; Yang, X.; Lai, S.; Zhou, Y.; He, M.; Liao, Z. Resveratrol protects cardiomyocytes against anoxia/reoxygenation via dephosphorylation of VDAC1 by Akt-GSK3 β pathway. Eur. J. Pharmacol. 2019, 843, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Yip, Y.M.; Li, L. In silico construction of HK2-VDAC1 complex and investigating the HK2 binding-induced molecular gating mechanism of VDAC1. Mitochondrion 2016, 30, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.-H.; Duan, R.; Xia, Y.-T.; Xiong, Q.-P.; Wang, H.-Y.; Chan, G.K.-L.; Liu, S.-Y.; Dong, T.T.-X.; Qin, Q.-W.; Tsim, K.W.-K. Binding of Resveratrol to Vascular Endothelial Growth Factor Suppresses Angiogenesis by Inhibiting the Receptor Signaling. J. Agric. Food Chem. 2019, 67, 1127–1137. [Google Scholar] [CrossRef] [PubMed]
- Gwak, H.; Haegeman, G.; Tsang, B.K.; Song, Y.S. Cancer-specific interruption of glucose metabolism by resveratrol is mediated through inhibition of Akt/GLUT1 axis in ovarian cancer cells. Mol. Carcinog. 2015, 54, 1529–1540. [Google Scholar] [CrossRef] [PubMed]
- Warnke, I.; Jocken, J.W.E.; Schoop, R.; Toepfer, C.; Goralczyk, R.; Schwager, J. Combinations of bio-active dietary constituents affect human white adipocyte function in-vitro. Nutr. Metab. (Lond) 2016, 13, 84. [Google Scholar] [CrossRef]
- Wu, H.; He, L.; Shi, J.; Hou, X.; Zhang, H.; Zhang, X.; An, Q.; Fan, F. Resveratrol inhibits VEGF-induced angiogenesis in human endothelial cells associated with suppression of aerobic glycolysis via modulation of PKM2 nuclear translocation. Clin. Exp. Pharmacol. Physiol. 2018, 45, 1265–1273. [Google Scholar] [CrossRef]
- Lappas, M.; Andrikopoulos, S.; Permezel, M. Hypoxanthine–xanthine oxidase down-regulates GLUT1 transcription via SIRT1 resulting in decreased glucose uptake in human placenta. J. Endocrinol. 2012, 213, 49–57. [Google Scholar] [CrossRef]
- Jung, K.-H.; Lee, J.H.; Quach, C.H.T.; Paik, J.-Y.; Oh, H.; Park, J.W.; Lee, E.J.; Moon, S.-H.; Lee, K.-H. Resveratrol Suppresses Cancer Cell Glucose Uptake by Targeting Reactive Oxygen Species–Mediated Hypoxia-Inducible Factor-1α Activation. J. Nucl. Med. 2013, 54, 2161–2167. [Google Scholar] [CrossRef]
- Vislovukh, A.; Kratassiouk, G.; Porto, E.; Gralievska, N.; Beldiman, C.; Pinna, G.; El’skaya, A.; Harel-Bellan, A.; Negrutskii, B.; Groisman, I. Proto-oncogenic isoform A2 of eukaryotic translation elongation factor eEF1 is a target of miR-663 and miR-744. Br. J. Cancer 2013, 108, 2304. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.-H.; Chen, H.-A.; Chen, P.-S.; Cheng, Y.-J.; Hsu, W.-H.; Chang, Y.-W.; Chen, Y.-H.; Jan, Y.; Hsiao, M.; Chang, T.-Y.; et al. MiR-520h-mediated FOXC2 regulation is critical for inhibition of lung cancer progression by resveratrol. Oncogene 2012, 32, 431. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Liang, H.; Xia, Q.; Li, P.; Kong, H.; Lei, P.; Wang, S.; Tu, Z. Resveratrol induces apoptosis of pancreatic cancers cells by inhibiting miR-21 regulation of BCL-2 expression. Clin. Transl. Oncol. 2013, 15, 741–746. [Google Scholar] [CrossRef] [PubMed]
- Tili, E.; Michaille, J.-J.; Alder, H.; Volinia, S.; Delmas, D.; Latruffe, N.; Croce, C.M. Resveratrol modulates the levels of microRNAs targeting genes encoding tumor-suppressors and effectors of TGFβ signaling pathway in SW480 cells. Biochem. Pharmacol. 2010, 80, 2057–2065. [Google Scholar] [CrossRef] [PubMed]
- Kumazaki, M.; Noguchi, S.; Yasui, Y.; Iwasaki, J.; Shinohara, H.; Yamada, N.; Akao, Y. Anti-cancer effects of naturally occurring compounds through modulation of signal transduction and miRNA expression in human colon cancer cells. J. Nutr. Biochem. 2013, 24, 1849–1858. [Google Scholar] [CrossRef] [PubMed]
- Pandima Devi, K.; Rajavel, T.; Daglia, M.; Nabavi, S.F.; Bishayee, A.; Nabavi, S.M. Targeting miRNAs by polyphenols: Novel therapeutic strategy for cancer. Semin. Cancer Biol. 2017, 46, 146–157. [Google Scholar] [CrossRef] [PubMed]
- Dhar, S.; Hicks, C.; Levenson, A.S. Resveratrol and prostate cancer: Promising role for microRNAs. Mol. Nutr. Food Res. 2011, 55, 1219–1229. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, K.; Yamamoto, Y.; Ochiya, T. Regulatory role of resveratrol, a microRNA-controlling compound, in HNRNPA1 expression, which is associated with poor prognosis in breast cancer. Oncotarget 2018, 9, 24718–24730. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheth, S.; Jajoo, S.; Kaur, T.; Mukherjea, D.; Sheehan, K.; Rybak, L.P.; Ramkumar, V. Resveratrol reduces prostate cancer growth and metastasis by inhibiting the Akt/MicroRNA-21 pathway. PLoS ONE 2012, 7, e51655. [Google Scholar] [CrossRef]
- Yang, S.-F.; Lee, W.-J.; Tan, P.; Tang, C.-H.; Hsiao, M.; Hsieh, F.-K.; Chien, M.-H. Upregulation of miR-328 and inhibition of CREB-DNA-binding activity are critical for resveratrol-mediated suppression of matrix metalloproteinase-2 and subsequent metastatic ability in human osteosarcomas. Oncotarget 2014, 6, 2736–2753. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.; Chen, Y.; Liu, F.; Yin, M. Overexpression of miRNA-143 Inhibits Colon Cancer Cell Proliferation by Inhibiting Glucose Uptake. Arch. Med. Res. 2018, 49, 497–503. [Google Scholar] [CrossRef] [PubMed]
- Esteves, J.V.; Enguita, F.J.; Machado, U.F. MicroRNAs-Mediated Regulation of Skeletal Muscle GLUT4 Expression and Translocation in Insulin Resistance. J. Diabetes Res. 2017, 2017, 7267910. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Wang, Y.; Wu, C.; Yang, P.; Li, H.; Li, Z. Resveratrol Induces Cancer Cell Apoptosis through MiR-326/PKM2-Mediated ER Stress and Mitochondrial Fission. J. Agric. Food Chem. 2016, 64, 9356–9367. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Zuo, S.; Xin, W. miR-27b overexpression improves mitochondrial function in a Sirt1-dependent manner. J. Physiol. Biochem. 2015, 71, 753–762. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Wang, W.; He, G.; Kuick, R.D.; Gossner, G.; Kueck, A.S.; Wahl, H.; Opipari, A.W.; Liu, J.R. Resveratrol inhibits ovarian tumor growth in an in vivo mouse model. Cancer 2016, 122, 722–729. [Google Scholar] [CrossRef] [PubMed]
- Breen, D.M.; Sanli, T.; Giacca, A.; Tsiani, E. Stimulation of muscle cell glucose uptake by resveratrol through sirtuins and AMPK. Biochem. Biophys. Res. Commun. 2008, 374, 117–122. [Google Scholar] [CrossRef]
- Araújo, J.R.; Pereira, A.C.; Correia-Branco, A.; Keating, E.; Martel, F. Oxidative stress induced by tert-butylhydroperoxide interferes with the placental transport of glucose: In vitro studies with BeWo cells. Eur. J. Pharmacol. 2013, 720, 218–226. [Google Scholar] [CrossRef]
- Zhao, Y.; Butler, E.B.; Tan, M. Targeting cellular metabolism to improve cancer therapeutics. Cell Death Dis. 2013, 4, e532. [Google Scholar] [CrossRef]
- Afzal, I.; Cunningham, P.; Naftalin, R.J. Interactions of ATP, oestradiol, genistein and the anti-oestrogens, faslodex (ICI 182780) and tamoxifen, with the human erythrocyte glucose transporter, GLUT1. Biochem. J. 2002, 365, 707–719. [Google Scholar] [CrossRef] [Green Version]
- Pérez, A.; Ojeda, P.; Ojeda, L.; Salas, M.; Rivas, C.I.; Vera, J.C.; Reyes, A.M. Hexose Transporter GLUT1 Harbors Several Distinct Regulatory Binding Sites for Flavones and Tyrphostins. Biochemistry 2011, 50, 8834–8845. [Google Scholar] [CrossRef]
- Leon, D.; Parada, D.; Vargas-Uribe, M.; Perez, A.A.; Ojeda, L.; Zambrano, A.; Reyes, A.M.; Salas, M. Effect of nordihydroguaiaretic acid on cell viability and glucose transport in human leukemic cell lines. FEBS Open Bio. 2016, 6, 1000–1007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pérez, A.; Ojeda, P.; Valenzuela, X.; Ortega, M.; Sánchez, C.; Ojeda, L.; Castro, M.; Cárcamo, J.G.; Rauch, M.C.; Concha, I.I.; et al. Endofacial competitive inhibition of the glucose transporter 1 activity by gossypol. Am. J. Physiol. Physiol. 2009, 297, C86–C93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azevedo, C.; Correia-Branco, A.; Araújo, J.R.; Guimarães, J.T.; Keating, E.; Martel, F. The Chemopreventive Effect of the Dietary Compound Kaempferol on the MCF-7 Human Breast Cancer Cell Line Is Dependent on Inhibition of Glucose Cellular Uptake. Nutr. Cancer 2015, 67, 504–513. [Google Scholar] [CrossRef]
- Gunnink, L.K.; Alabi, O.D.; Kuiper, B.D.; Gunnink, S.M.; Schuiteman, S.J.; Strohbehn, L.E.; Hamilton, K.E.; Wrobel, K.E.; Louters, L.L. Curcumin directly inhibits the transport activity of GLUT1. Biochimie 2016, 125, 179–185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- León, D.; Uribe, E.; Zambrano, A.; Salas, M. Implications of resveratrol on glucose uptake and metabolism. Molecules 2017, 22, 398. [Google Scholar] [CrossRef]

| Cancer Type | References |
|---|---|
| Breast cancer carcinoma and adenocarcinoma | [14,65,68] |
| Ovarian carcinoma | [15,69,70] |
| Prostate carcinoma and adenocarcinoma | [65,71,72] |
| Thyroid carcinoma and adenocarcinoma | [65,73] |
| Gastric adenocarcinoma | [65] |
| Rectal carcinoma | [74] |
| Squamous cell carcinoma of the head and neck | [65,75] |
| Uterine cervix squamous cell carcinomas | [65] |
| Glioblastomas | [65] |
| Retinoblastomas | [65] |
| Colorectal carcinoma and adenocarcinomas. | [76,77,78] |
| Nonsmall cell lung carcinoma | [68,79] |
| Oral squamous cell carcinoma | [80,81] |
| Squamous cell carcinoma of the tongue | [82] |
| Esophageal cancer | [83] |
| Urothelial papilloma | [84] |
| Meningioma | [85] |
| Brain tumors | [13] |
| Laryngeal carcinoma | [86] |
| Nasopharyngeal diffuse large b-cell lymphoma | [87] |
| Pancreatic neoplasia | [88] |
| Renal cell carcinoma | [89,90] |
| Hepatocellular carcinoma | [91] |
| Lung cancer | [92] |
| Cervical cancer | [93] |
| Glucose Uptake | Glucose Analog Used | Cell Type | Reference |
|---|---|---|---|
| inhibit (in vivo) | 2-deoxy-2-[18F]fludeoxyglucose ([18F]FDG) uptake | A2780, SKOV3 (injected in female nu/nu mice). | [166] |
| inhibit (in vivo) | 2-deoxy-2-[18F]fludeoxyglucose ([18F]FDG) uptake | LLC (injected in BALB/c-n mice) | [151] |
| inhibit (in vitro) | 3-O-methyl-D-glucose (OMG) or 2-deoxy glucose (2-DG) uptake | HL60, U937, RBC | [116] |
| inhibit (in vitro) | (2-[N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)amino]-2-deoxyglucose) (2-NBDG) uptake | PA-1, OVCAR3, MDAH2774 | [147] |
| inhibit (in vitro) | [3H]-2-DG uptake | A2780, MDAH-2774, HOC-1, HOC-8, OVCA 429, and OVCA 432 SKOV3 | [117] |
| inhibit (in vitro) | Glucose Oxidase Assay Kit | A2780, SKOV3 | [119] |
| Increase (in vitro) | 2-deoxy-D-[3H] glucose uptake | 3T3-L1 | [120] |
| Inhibit (in vitro) | Glucose (hexokinase) assay kit | HUVEC | [149] |
| inhibit (in vitro) | (2-[N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)amino]-2-deoxyglucose) (2-NBDG) uptake | Neuro-2a (N2A) | [121] |
| Increase (in vitro) | 2-doxy-D-glucose (2DG) | L6 | [167] |
| Increase (in vitro) | [3H] DG | BeWo | [168] |
| Increase (in vitro) | 2-deoxy-D-glucose (2DG) | Placental lobules | [150] |
| Inhibitor | IC50 (Ki), µM * | Type of Inhibition | Cell Type | Reference |
|---|---|---|---|---|
| Flavones and Isoflavones | ||||
| Genistein | 10–15 mM (4–15) | competitive | HL60, CHO, RBC | [114,115,171] |
| Myricetin | (23) | competitive | HL60, CHO, RBC | [114] |
| Quercetin | (8–16) | competitive | HL60, CHO, RBC | [114,171] |
| Morin | (105) | competitive | HL60, CHO, RBC | [114] |
| Rhamnetin | (20) | competitive | HL60, CHO, RBC | [114] |
| Isorhamnetin | (5) | competitive | HL60, CHO, RBC | [114] |
| Biochanin A | (17) | competitive | HL60, CHO, RBC | [114] |
| Lavendustin and Tyrphostins | ||||
| Lavendustin A | (10) | competitive | HL60, CHO, RBC | [114] |
| Lavendustin B | (15) | competitive | HL60, CHO, RBC | [114] |
| Tyrphostin B44 | (90) | competitive | HL60, CHO, RBC | [114] |
| Tyrphostin B46 | (20–45) | competitive | HL60, CHO, RBC | [114,171] |
| Tyrphostin B48 | (50) | competitive | HL60, CHO, RBC | [114] |
| Tyrphostin B50 | (45) | competitive | HL60, CHO, RBC | [114] |
| Tyrphostin B56 | (170) | competitive | HL60, CHO, RBC | [114] |
| Tyrphostin AG879 | (85) | competitive | HL60, CHO, RBC | [114] |
| Tyrphostin A47 | (115–160) | noncompetitive | HL60, CHO, RBC | [114,171] |
| Other Tyrosine Kinase Inhibitors | ||||
| Methyl 2,5- dihydroxycinnamate | (150) | noncompetitive | HL60, CHO, RBC | [114] |
| Gossypol | 30 (7) | noncompetitive | HL60, CHO, RBC | [173] |
| Methylxanthines | ||||
| Pentoxifylline | 4.7 mM (2.8) | uncompetitive | RBC | [55] |
| Caffeine | 10 mM (4.5) | uncompetitive | RBC | [55] |
| Theophylline | 14.4 mM (5.3) | uncompetitive | RBC | [55] |
| Phloretin | 40 | RBC | [55] | |
| Other Polyphenols | ||||
| Resveratrol | 30 (122) | noncompetitive | HL60, U937, RBC | [116] |
| NDGA | 53–85 mM (4.5) | noncompetitive | HL60, U937, RBC | [172] |
| Gossypol | 30 (7) | noncompetitive | HL60, CHO, RBC | [173] |
| Kaempferol | 4 | mixed | MCF-7 | [174] |
| Curcumin | 19 | mixed | L929 | [175] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zambrano, A.; Molt, M.; Uribe, E.; Salas, M. Glut 1 in Cancer Cells and the Inhibitory Action of Resveratrol as A Potential Therapeutic Strategy. Int. J. Mol. Sci. 2019, 20, 3374. https://doi.org/10.3390/ijms20133374
Zambrano A, Molt M, Uribe E, Salas M. Glut 1 in Cancer Cells and the Inhibitory Action of Resveratrol as A Potential Therapeutic Strategy. International Journal of Molecular Sciences. 2019; 20(13):3374. https://doi.org/10.3390/ijms20133374
Chicago/Turabian StyleZambrano, Angara, Matías Molt, Elena Uribe, and Mónica Salas. 2019. "Glut 1 in Cancer Cells and the Inhibitory Action of Resveratrol as A Potential Therapeutic Strategy" International Journal of Molecular Sciences 20, no. 13: 3374. https://doi.org/10.3390/ijms20133374
APA StyleZambrano, A., Molt, M., Uribe, E., & Salas, M. (2019). Glut 1 in Cancer Cells and the Inhibitory Action of Resveratrol as A Potential Therapeutic Strategy. International Journal of Molecular Sciences, 20(13), 3374. https://doi.org/10.3390/ijms20133374