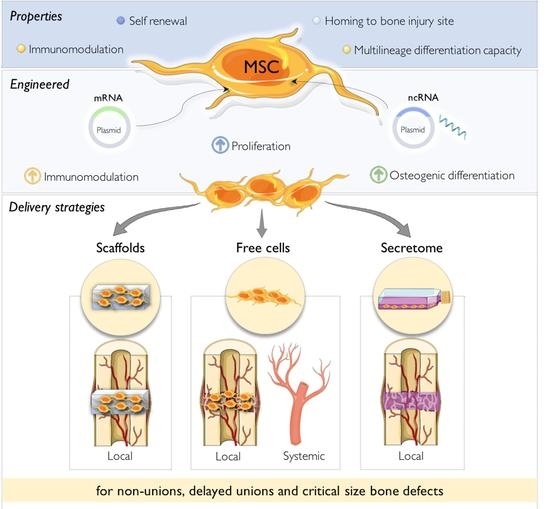
Genetically Engineered-MSC Therapies for Non-unions, Delayed Unions and Critical-size Bone Defects
Abstract
:1. Introduction
2. Molecular Candidates and Common Methods to Genetically-Engineer MSC to Promote Bone Repair
2.1. Genes of Interest to Promote Bone Repair in vivo: Promising Candidates
2.2. MSCs Engineering Strategies to Express/Inhibit Genes of Interest
2.3. Tracking MSC for in vivo Studies
3. MSC Delivery to Promote Bone Regeneration
3.1. Local Delivery of MSC: The Combined Effect with Biomaterial Scaffolds
3.2. Systemic Delivery of MSC for Bone Repair
4. Use of MSC-Secretome for Repair of Long Bone Fractures
4.1. Advantages and Limitations
4.2. Protein Content of MSC-Secretome
4.3. Animal Models/Clinical Testing Using MSC Secretome
5. Potential of MSCs Therapies for Non-Union Bone Defects in the Clinic
6. Conclusions and Future Prospects: The Advantages and Limitations of Using MSC in Bone Regeneration
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Deschaseaux, F.; Sensébé, L.; Heymann, D. Mechanisms of bone repair and regeneration. Trends Mol. Med. 2009, 15, 417–429. [Google Scholar] [CrossRef] [PubMed]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Mackie, E.; Ahmed, Y.; Tatarczuch, L.; Chen, K.-S.; Mirams, M.; Mackie, E.; Ahmed, Y. Endochondral ossification: How cartilage is converted into bone in the developing skeleton. Int. J. Biochem. Cell Boil. 2008, 40, 46–62. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Ren, H.; Han, Z. Mesenchymal stem cells: Immunomodulatory capability and clinical potential in immune diseases. J. Cell. Immunother. 2016, 2, 3–20. [Google Scholar] [CrossRef] [Green Version]
- Amini, A.R.; Laurencin, C.T.; Nukavarapu, S.P. Bone Tissue Engineering: Recent Advances and Challenges. Crit. Rev. Biomed. Eng. 2012, 40, 363–408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hak, D.J.; Fitzpatrick, D.; Bishop, J.A.; Marsh, J.L.; Tilp, S.; Schnettler, R.; Simpson, H.; Alt, V. Delayed union and nonunions: Epidemiology, clinical issues, and financial aspects. Injury 2014, 45, S3–S7. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Barrena, E.; Rosset, P.; Lozano, D.; Stanovici, J.; Ermthaller, C.; Gerbhard, F. Bone fracture healing: Cell therapy in delayed unions and nonunions. Bone 2015, 70, 93–101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schlundt, C.; Bucher, C.H.; Tsitsilonis, S.; Schell, H.; Duda, G.N.; Schmidt-Bleek, K. Clinical and Research Approaches to Treat Non-union Fracture. Curr. Osteoporos. Rep. 2018, 16, 155–168. [Google Scholar] [CrossRef]
- Klingemann, H.; Matzilevich, D.; Marchand, J. Mesenchymal Stem Cells–Sources and Clinical Applications. Transfus. Med. Hemotherapy 2008, 35, 272–277. [Google Scholar] [CrossRef]
- Chalisserry, E.P.; Nam, S.Y.; Park, S.H.; Anil, S. Therapeutic potential of dental stem cells. J. Tissue Eng. 2017, 8, 2041731417702531. [Google Scholar] [CrossRef]
- Ledesma-Martínez, E.; Mendoza-Núñez, V.M.; Santiago-Osorio, E. Mesenchymal Stem Cells Derived from Dental Pulp: A Review. Stem Cells Int. 2016, 2016, 4709572. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.T.-J.; Gronthos, S.; Shi, S. Mesenchymal stem cells derived from dental tissues vs. those from other sources: Their biology and role in regenerative medicine. J. Dent. Res. 2009, 88, 792–806. [Google Scholar] [CrossRef] [PubMed]
- Undale, A.; Fraser, D.; Hefferan, T.; Kopher, R.A.; Herrick, J.; Evans, G.L.; Li, X.; Kakar, S.; Hayes, M.; Atkinson, E.; et al. Induction of Fracture Repair by Mesenchymal Cells Derived from Human Embryonic Stem Cells or Bone Marrow. J. Orthop. Res. 2011, 29, 1804–1811. [Google Scholar] [CrossRef]
- Kargozar, S.; Mozafari, M.; Hashemian, S.J.; Brouki Milan, P.; Hamzehlou, S.; Soleimani, M.; Joghataei, M.T.; Gholipourmalekabadi, M.; Korourian, A.; Mousavizadeh, K.; et al. Osteogenic potential of stem cells-seeded bioactive nanocomposite scaffolds: A comparative study between human mesenchymal stem cells derived from bone, umbilical cord Wharton’s jelly, and adipose tissue. J. Biomed. Mater. Res. B Appl. Biomater. 2018, 106, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Zou, L.; Chen, Q.; Quanbeck, Z.; Bechtold, J.E.; Kaufman, D.S. Angiogenic activity mediates bone repair from human pluripotent stem cell-derived osteogenic cells. Sci. Rep. 2016, 6, 22868. [Google Scholar] [CrossRef] [Green Version]
- Shoji, T.; Ii, M.; Mifune, Y.; Matsumoto, T.; Kawamoto, A.; Kwon, S.-M.; Kuroda, T.; Kuroda, R.; Kurosaka, M.; Asahara, T. Local transplantation of human multipotent adipose-derived stem cells accelerates fracture healing via enhanced osteogenesis and angiogenesis. Lab. Investig. 2010, 90, 637–664. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Zhang, X.-L.; Li, L.; Shen, F.-M.; Zhong, M.-K. Stem cell therapy for bone repair: A systematic review and meta-analysis of preclinical studies with large animal models. Br. J. Clin. Pharmacol. 2014, 78, 718–726. [Google Scholar] [CrossRef]
- Scarfì, S.; Scarfì, S. Use of bone morphogenetic proteins in mesenchymal stem cell stimulation of cartilage and bone repair. World J. Stem Cells 2016, 8, 1–12. [Google Scholar] [CrossRef]
- Zheng, H.; Guo, Z.; Ma, Q.; Jia, H.; Dang, G. Cbfa1/osf2 Transduced Bone Marrow Stromal Cells Facilitate Bone Formation In Vitro and In Vivo. Calcif. Tissue Int. 2004, 74, 194–203. [Google Scholar] [CrossRef]
- Tu, Q.; Valverde, P.; Li, S.; Zhang, J.; Yang, P.; Chen, J. Osterix Overexpression in Mesenchymal Stem cells Stimulates Healing of Critical-Sized Defects in Murine Calvarial Bone. Tissue Eng. 2007, 13, 2431–2440. [Google Scholar] [CrossRef]
- Zhang, W.; Zhu, C.; Wu, Y.; Ye, D.; Wang, S.; Zou, D.; Zhang, X.; Kaplan, D.; Jiang, X. VEGF and BMP-2 promote bone regeneration by facilitating bone marrow stem cell homing and differentiation. Eur. Cells Mater. 2014, 27, 1–12. [Google Scholar] [CrossRef]
- Lin, C.-Y.; Chang, Y.-H.; Lin, K.-J.; Yen, T.-C.; Tai, C.-L.; Chen, C.-Y.; Lo, W.-H.; Hsiao, I.-T.; Hu, Y.-C. The healing of critical-sized femoral segmental bone defects in rabbits using baculovirus-engineered mesenchymal stem cells. Biomaterials 2010, 31, 3222–3230. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Usas, A.; Hannallah, D.; Olshanski, A.; Cooper, G.M.; Huard, J. Noggin Improves Bone Healing Elicited by Muscle Stem Cells Expressing Inducible BMP4. Mol. Ther. 2005, 12, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Simonsen, J.L.; Rosada, C.; Serakinci, N.; Justesen, J.; Stenderup, K.; Rattan, S.I.; Jensen, T.G.; Kassem, M.; Kjeldsen, C.R.; Serakıncı, N. Telomerase expression extends the proliferative life-span and maintains the osteogenic potential of human bone marrow stromal cells. Nat. Biotechnol. 2002, 20, 592–596. [Google Scholar] [CrossRef] [PubMed]
- Teven, C.M.; Liu, X.; Hu, N.; Tang, N.; Kim, S.H.; Huang, E.; Yang, K.; Li, M.; Gao, J.-L.; Liu, H.; et al. Epigenetic Regulation of Mesenchymal Stem Cells: A Focus on Osteogenic and Adipogenic Differentiation. Stem Cells Int. 2011, 2011, 1–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paino, F.; La Noce, M.; Tirino, V.; Naddeo, P.; Desiderio, V.; Pirozzi, G.; De Rosa, A.; Laino, L.; Altucci, L.; Papaccio, G.; et al. Histone Deacetylase Inhibition with Valproic Acid Downregulates Osteocalcin Gene Expression in Human Dental Pulp Stem Cells and Osteoblasts: Evidence for HDAC2 Involvement. Stem Cells 2014, 32, 279–289. [Google Scholar] [CrossRef]
- La Noce, M.; Mele, L.; Laino, L.; Iolascon, G.; Pieretti, G.; Papaccio, G.; Desiderio, V.; Tirino, V.; Paino, F. Cytoplasmic Interactions between the Glucocorticoid Receptor and HDAC2 Regulate Osteocalcin Expression in VPA-Treated MSCs. Cells 2019, 8, 217. [Google Scholar] [CrossRef]
- Jing, S.; Ren, T.; Zhang, F.; Lin, J. microRNA-10b promotes the migration of mouse bone marrow-derived mesenchymal stem cells and downregulates the expression of E-cadherin. Mol. Med. Rep. 2013, 8, 1084–1088. [Google Scholar] [Green Version]
- Almeida, M.I.; Silva, A.M.; Vasconcelos, D.M.; Almeida, C.R.; Caires, H.; Pinto, M.T.; Calin, G.A.; Santos, S.G.; Barbosa, M.A. miR-195 in human primary mesenchymal stromal/stem cells regulates proliferation, osteogenesis and paracrine effect on angiogenesis. Oncotarget 2016, 7, 7–22. [Google Scholar] [CrossRef]
- Xu, C.; Ren, G.; Cao, G.; Chen, Q.; Shou, P.; Zheng, C.; Du, L.; Han, X.; Jiang, M.; Yang, Q.; et al. miR-155 Regulates Immune Modulatory Properties of Mesenchymal Stem Cells by Targeting TAK1-binding Protein 2. J. Boil. Chem. 2013, 288, 11074–11079. [Google Scholar] [CrossRef] [Green Version]
- Bartel, D.P. MicroRNAs: Target recognition and regulatory functions. Cell 2009, 136, 215–233. [Google Scholar] [CrossRef] [PubMed]
- Paul, P.; Chakraborty, A.; Sarkar, D.; Langthasa, M.; Rahman, M.; Bari, M.; Singha, R.S.; Malakar, A.K.; Chakraborty, S. Interplay between miRNAs and human diseases. J. Cell. Physiol. 2018, 233, 2007–2018. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Wang, G.; Hu, C.-H.; Shi, Y.; Liao, L.; Shi, S.; Cai, Y.; Cheng, S.; Wang, X.; Liu, Y.; et al. Tumor necrosis factor α suppresses the mesenchymal stem cell osteogenesis promoter miR-21 in estrogen deficiency-induced osteoporosis. J. Bone Miner. Res. 2013, 28, 559–573. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Xu, L.; Huang, S.; Hou, Y.; Liu, Y.; Chan, K.-M.; Pan, X.-H.; Li, G. mir-21 Overexpressing Mesenchymal Stem Cells Accelerate Fracture Healing in a Rat Closed Femur Fracture Model. BioMed Res. Int. 2015, 2015, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.M.; Almeida, M.I.; Teixeira, J.H.; Ivan, C.; Oliveira, J.; Vasconcelos, D.; Neves, N.; Ribeiro-Machado, C.; Cunha, C.; Barbosa, M.A.; et al. Profiling the circulating miRnome reveals a temporal regulation of the bone injury response. Theranostics 2018, 8, 3902–3917. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Liu, X.; Zhao, K.; Zhu, Y.; Hu, B.; Zhou, Y.; Wang, M.; Wu, Y.; Zhang, C.; Xu, J.; et al. miRNA-21 promotes osteogenesis via the PTEN/PI3K/Akt/HIF-1α pathway and enhances bone regeneration in critical size defects. Stem Cell Res. Ther. 2019, 10, 65. [Google Scholar] [CrossRef] [PubMed]
- Lv, C.; Hao, Y.; Han, Y.; Zhang, W.; Cong, L.; Shi, Y.; Tu, G. Role and mechanism of microRNA-21 in H2O2-induced apoptosis in bone marrow mesenchymal stem cells. J. Clin. Neurosci. 2016, 27, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Feng, L.; Liu, Y.; Duan, J.-Q.; Lin, W.-P.; Zhang, J.-F.; Li, G. MicroRNA-218 Promotes Osteogenic Differentiation of Mesenchymal Stem Cells and Accelerates Bone Fracture Healing. Calcif. Tissue Int. 2018, 103, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, M.; Bakhshandeh, B.; Dehghan, M.M.; Mehrnia, M.R.; Khojasteh, A. Functional synergy of anti-mir221 and nanohydroxyapatite scaffold in bone tissue engineering of rat skull. J. Mater. Sci. Mater. Electron. 2016, 27, 132. [Google Scholar] [CrossRef] [PubMed]
- Yoshizuka, M.; Nakasa, T.; Kawanishi, Y.; Hachisuka, S.; Furuta, T.; Miyaki, S.; Adachi, N.; Ochi, M. Inhibition of microRNA-222 expression accelerates bone healing with enhancement of osteogenesis, chondrogenesis, and angiogenesis in a rat refractory fracture model. J. Orthop. Sci. 2016, 21, 852–858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liao, Y.-H.; Chang, Y.-H.; Sung, L.-Y.; Li, K.-C.; Yeh, C.-L.; Yen, T.-C.; Hwang, S.-M.; Lin, K.-J.; Hu, Y.-C. Osteogenic differentiation of adipose-derived stem cells and calvarial defect repair using baculovirus-mediated co-expression of BMP-2 and miR-148b. Biomaterials 2014, 35, 4901–4910. [Google Scholar] [CrossRef] [PubMed]
- Li, K.-C.; Chang, Y.-H.; Yeh, C.-L.; Hu, Y.-C. Healing of osteoporotic bone defects by baculovirus-engineered bone marrow-derived MSCs expressing MicroRNA sponges. Biomaterials 2016, 74, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Xie, Q.; Wang, Z.; Zhou, H.; Yu, Z.; Huang, Y.; Sun, H.; Bi, X.; Wang, Y.; Shi, W.; Gu, P.; et al. The role of miR-135-modified adipose-derived mesenchymal stem cells in bone regeneration. Biomaterials 2016, 75, 279–294. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Zhou, H.; Zou, D.; Xie, Q.; Bi, X.; Gu, P.; Fan, X. The role of miR-31-modified adipose tissue-derived stem cells in repairing rat critical-sized calvarial defects. Biomaterials 2013, 34, 6717–6728. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.M.; Moura, S.R.; Teixeira, J.H.; Barbosa, M.A.; Santos, S.G.; Almeida, M.I. Long noncoding RNAs: A missing link in osteoporosis. Bone Res. 2019, 7, 10. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.; Cao, L.; He, S.; Zhong, Y.; Ma, H.; Zhang, Y.; Shuai, C. An Overview of Long Noncoding RNAs Involved in Bone Regeneration from Mesenchymal Stem Cells. Stem Cells Int. 2018, 2018, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.-Y.; Li, X.; Miao, Z.-N.; Ye, J.-X.; Wang, B.; Zhang, F.; Xu, R.-S.; Jiang, D.-L.; Zhao, M.-D.; Yuan, F.L. Long Non-coding RNAs: A New Regulatory Code for Osteoporosis. Front. Endocrinol. 2018, 9, 587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.; Huang, L. Nonviral gene therapy: Promises and challenges. Gene Ther. 2000, 7, 31–34. [Google Scholar] [CrossRef]
- Li, W.; Wei, H.; Xia, C.; Zhu, X.; Hou, G.; Xu, F.; Song, X.; Zhan, Y. Gene gun transferring-bone morphogenetic protein 2 (BMP-2) gene enhanced bone fracture healing in rabbits. Int. J. Clin. Exp. Med. 2015, 8, 19982–19993. [Google Scholar]
- Rols, M.-P.; Delteil, C.; Golzio, M.; Dumond, P.; Cros, S.; Teissié, J. In vivo electrically mediated protein and gene transfer in murine melanoma. Nat. Biotechnol. 1998, 16, 168–171. [Google Scholar] [CrossRef]
- Santos-Carballal, B.; Fernández, E.F.; Goycoolea, F.M. Chitosan in Non-Viral Gene Delivery: Role of Structure, Characterization Methods, and Insights in Cancer and Rare Diseases Therapies. Polymers 2018, 10, 444. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, E.; Potier, E.; Logeart-Avramoglou, D.; Salomskaite-Davalgiene, S.; Mir, L.M.; Petite, H. Optimization of a gene electrotransfer method for mesenchymal stem cell transfection. Gene Ther. 2008, 15, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Rieß, J.; Gelse, K.; Kloss, F.; Von Der Mark, K.; Wiltfang, J.; Neukam, F.W.; Schneider, H. Bone regeneration in critical size defects by cell-mediated BMP-2 gene transfer: A comparison of adenoviral vectors and liposomes. Gene Ther. 2003, 10, 1089–1098. [Google Scholar] [CrossRef] [PubMed]
- Blum, J.S.; Barry, M.A.; Mikos, A.G.; Jansen, J.A. In Vivo Evaluation of Gene Therapy Vectors in Ex Vivo-Derived Marrow Stromal Cells for Bone Regeneration in a Rat Critical-Size Calvarial Defect Model. Hum. Gene Ther. 2003, 14, 1689–1701. [Google Scholar]
- Raftery, R.M.; Mencía-Castaño, I.; Sperger, S.; Chen, G.; Cavanagh, B.; Feichtinger, G.A.; Redl, H.; Hacobian, A.; O’Brien, F.J. Delivery of the improved BMP-2-Advanced plasmid DNA within a gene-activated scaffold accelerates mesenchymal stem cell osteogenesis and critical size defect repair. J. Control. Release 2018, 283, 20–31. [Google Scholar] [CrossRef] [PubMed]
- Umebayashi, M.; Sumita, Y.; Kawai, Y.; Watanabe, S.; Asahina, I. Gene-Activated Matrix Comprised of Atelocollagen and Plasmid DNA Encoding BMP4 or Runx2 Promotes Rat Cranial Bone Augmentation. BioRes. Open Access 2015, 4, 164–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raftery, R.M.; Castaño, I.M.; Chen, G.; Cavanagh, B.; Quinn, B.; Curtin, C.M.; Cryan, S.A.; O’Brien, F.J. Translating the role of osteogenic-angiogenic coupling in bone formation: Highly efficient chitosan-pDNA activated scaffolds can accelerate bone regeneration in critical-sized bone defects. Biomaterials 2017, 149, 116–127. [Google Scholar] [CrossRef]
- Kawai, M.; Bessho, K.; Maruyama, H.; Miyazaki, J.-I.; Yamamoto, T. Simultaneous gene transfer of bone morphogenetic protein (BMP) -2 and BMP-7 by in vivo electroporation induces rapid bone formation and BMP-4 expression. BMC Musculoskelet. Disord. 2006, 7, 62. [Google Scholar] [CrossRef]
- Truong, V.A.; Hsu, M.-N.; Nguyen, N.T.K.; Lin, M.-W.; Shen, C.-C.; Lin, C.-Y.; Hu, Y.-C. CRISPRai for simultaneous gene activation and inhibition to promote stem cell chondrogenesis and calvarial bone regeneration. Nucleic Acids Res. 2019, 1–14. [Google Scholar] [CrossRef]
- Dégano, I.R.; Vilalta, M.; Bagó, J.R.; Matthies, A.M.; Hubbell, J.A.; Dimitriou, H.; Bianco, P.; Rubio, N.; Blanco, J. Bioluminescence imaging of calvarial bone repair using bone marrow and adipose tissue-derived mesenchymal stem cells. Biomaterials 2008, 29, 427–437. [Google Scholar] [CrossRef]
- Corn, D.J.; Kim, Y.; Krebs, M.D.; Mounts, T.; Molter, J.; Gerson, S.; Alsberg, E.; Dennis, J.E.; Lee, Z. Imaging early stage osteogenic differentiation of mesenchymal stem cells. J. Orthop. Res. 2013, 31, 871–879. [Google Scholar] [CrossRef] [PubMed]
- Zong, C.; Xue, D.; Yuan, W.; Wang, W.; Shen, D.; Tong, X.; Shi, D.; Liu, L.; Zheng, Q.; Gao, C.; et al. Reconstruction of rat calvarial defects with human mesenchymal stem cells and osteoblast-like cells in poly-lactic-co-glycolic acid scaffolds. Eur. Cells Mater. 2010, 20, 109–120. [Google Scholar] [CrossRef]
- Dupont, K.M.; Sharma, K.; Stevens, H.Y.; Boerckel, J.D.; García, A.J.; Guldberg, R.E. Human stem cell delivery for treatment of large segmental bone defects. Proc. Natl. Acad. Sci. USA 2010, 107, 3305–3310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.M.; Kim, B.-S.; Lee, H.; Im, G.-I. In Vivo Tracking of Mesechymal Stem Cells Using Fluorescent Nanoparticles in an Osteochondral Repair Model. Mol. Ther. 2012, 20, 1434–1442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arrington, E.D.; Smith, W.J.; Chambers, H.G.; Bucknell, A.L.; Davino, N.A. Complications of Iliac Crest Bone Graft Harvesting. Clin. Orthop. Relat. Res. 1996, 329, 300–309. [Google Scholar] [CrossRef] [PubMed]
- Hinsenkamp, M.; Muylle, L.; Eastlund, T.; Fehily, D.; Noel, L.; Strong, D.M. Adverse reactions and events related to musculoskeletal allografts: Reviewed by the World Health Organisation Project NOTIFY. Int. Orthop. 2012, 36, 633–641. [Google Scholar] [CrossRef] [PubMed]
- De Witte, T.-M.; E Fratila-Apachitei, L.; A Zadpoor, A.; A Peppas, N. Bone tissue engineering via growth factor delivery: From scaffolds to complex matrices. Regen. Biomater. 2018, 5, 197–211. [Google Scholar] [CrossRef] [PubMed]
- Hirasawa, M.; Mure, H.; Toi, H.; Nagahiro, S. Surgical Results of Lumbar Interbody Fusion Using Calcium Phosphate Cement. Neurol. Medico-Chirurgica 2014, 54, 722–726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, H.; Yang, H.-L. Calcium Phosphate Scaffolds Combined with Bone Morphogenetic Proteins or Mesenchymal Stem Cells in Bone Tissue Engineering. Chin. Med. J. 2015, 128, 1121–1127. [Google Scholar]
- Maiti, S.K.; Ninu, A.R.; Sangeetha, P.; Mathew, D.D.; Tamilmahan, P.; Kritaniya, D.; Kumar, N.; Hescheler, J. Mesenchymal stem cells-seeded bio-ceramic construct for bone regeneration in large critical-size bone defect in rabbit. J. Stem Cells Regen. Med. 2016, 12, 87–99. [Google Scholar]
- Marcacci, M.; Kon, E.; Moukhachev, V.; Lavroukov, A.; Kutepov, S.; Quarto, R.; Mastrogiacomo, M.; Cancedda, R. Stem Cells Associated with Macroporous Bioceramics for Long Bone Repair: 6-to 7-Year Outcome of a Pilot Clinical Study. Tissue Eng. 2007, 13, 947–955. [Google Scholar] [CrossRef] [PubMed]
- Asti, A.; Gioglio, L. Natural and synthetic biodegradable polymers: Different scaffolds for cell expansion and tissue formation. Int. J. Artif. Organs 2014, 37, 187–205. [Google Scholar] [PubMed]
- Thrivikraman, G.; Athirasala, A.; Twohig, C.; Boda, S.K.; Bertassoni, L.E. Biomaterials for Craniofacial Bone Regeneration. Dent. Clin. N. Am. 2017, 61, 835–856. [Google Scholar] [CrossRef] [PubMed]
- De Kok, I.J.; Jere, D.; Padilla, R.J.; Cooper, L.F. Evaluation of a Collagen Scaffold for Cell-Based Bone Repair. Int. J. Oral Maxillofac. Implant. 2014, 29, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Costa-Pinto, A.R.; Reis, R.L.; Neves, N.M. Scaffolds Based Bone Tissue Engineering: The Role of Chitosan. Tissue Eng. Part B Rev. 2011, 17, 331–347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costa-Pinto, A.R.; Correlo, V.M.; Sol, P.C.; Bhattacharya, M.; Srouji, S.; Livne, E.; Reis, R.L.; Neves, N.M. Chitosan-poly (butylene succinate) scaffolds and human bone marrow stromal cells induce bone repair in a mouse calvaria model. J. Tissue Eng. Regen. Med. 2012, 6, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Desai, P.; Hasan, S.M.; Zambrana, L.; Hegde, V.; Saleh, A.; Cohn, M.R.; Lane, J.M. Bone Mesenchymal Stem Cells with Growth Factors Successfully Treat Nonunions and Delayed Unions. HSS J. 2015, 11, 104–111. [Google Scholar] [CrossRef] [Green Version]
- Ali Akbari Ghavimi, S.; Ebrahimzadeh, M.H.; Solati-Hashjin, M.; Abu Osman, N.A. Polycaprolactone/starch composite: Fabrication, structure, properties, and applications. J. Biomed. Mater. Res. A 2015, 103, 2482–2498. [Google Scholar] [CrossRef]
- Yang, X.; Roach, H.; Clarke, N.; Howdle, S.; Quirk, R.; Shakesheff, K.; Oreffo, R.; Shakesheff, K.; Oreffo, R. Human osteoprogenitor growth and differentiation on synthetic biodegradable structures after surface modification. Bone 2001, 29, 523–531. [Google Scholar] [CrossRef]
- Montjovent, M.-O.; Mathieu, L.; Schmoekel, H.; Mark, S.; Bourban, P.-E.; Zambelli, P.-Y.; Pioletti, D.P.; Laurent-Applegate, L.A.; Laurent-Applegate, L.A. Repair of critical size defects in the rat cranium using ceramic-reinforced PLA scaffolds obtained by supercritical gas foaming. J. Biomed. Mater. Res. Part A 2007, 83, 41–51. [Google Scholar] [CrossRef]
- Gentile, P.; Chiono, V.; Carmagnola, I.; Hatton, P.V. An Overview of Poly(lactic-co-glycolic) Acid (PLGA)-Based Biomaterials for Bone Tissue Engineering. Int. J. Mol. Sci. 2014, 15, 3640–3659. [Google Scholar] [CrossRef] [PubMed]
- Harada, N.; Watanabe, Y.; Sato, K.; Abe, S.; Yamanaka, K.; Sakai, Y.; Kaneko, T.; Matsushita, T. Bone regeneration in a massive rat femur defect through endochondral ossification achieved with chondrogenically differentiated MSCs in a degradable scaffold. Biomaterials 2014, 35, 7800–7810. [Google Scholar] [CrossRef] [PubMed]
- Woodruff, M.A.; Hutmacher, D.W. The return of a forgotten polymer—Polycaprolactone in the 21st century. Prog. Polym. Sci. 2010, 35, 1217–1256. [Google Scholar] [CrossRef]
- Kim, M.S.; Ahn, H.H.; Na Shin, Y.; Cho, M.H.; Khang, G.; Lee, H.B. An in vivo study of the host tissue response to subcutaneous implantation of PLGA- and/or porcine small intestinal submucosa-based scaffolds. Biomaterials 2007, 28, 5137–5143. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Kwon, J.; Kim, B.; Jeon, Y.; Khang, G.; Lee, D. Physicobiological properties and biocompatibility of biodegradable poly(oxalate-co-oxamide). J. Biomed. Mater. Res. Part A 2011, 98, 517–526. [Google Scholar] [CrossRef]
- Westhauser, F.; Weis, C.; Prokscha, M.; Bittrich, L.A.; Li, W.; Xiao, K.; Kneser, U.; Kauczor, H.-U.; Schmidmaier, G.; Boccaccini, A.R.; et al. Three-dimensional polymer coated 45S5-type bioactive glass scaffolds seeded with human mesenchymal stem cells show bone formation In Vivo. J. Mater. Sci. Mater. Electron. 2016, 27, 119. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.-Y.; Kim, H.-E.; Kim, M.-J.; Kim, U.-C.; Kim, J.-H.; Koh, Y.-H. Production and characterization of calcium phosphate (CaP) whisker-reinforced poly(ε-caprolactone) composites as bone regenerative. Mater. Sci. Eng. C 2010, 30, 1280–1284. [Google Scholar] [CrossRef]
- Meseguer-Olmo, L.; Vicente-Ortega, V.; Alcaraz-Baños, M.; Calvo-Guirado, J.L.; Vallet-Regí, M.; Arcos, D.; Baeza, A. In-Vivo behavior of Si-hydroxyapatite/polycaprolactone/DMB scaffolds fabricated by 3D printing. J. Biomed. Mater. Res. A 2013, 101, 2038–2048. [Google Scholar] [CrossRef]
- Bassi, A.; Gough, J.; Downes, S. A novel phosphonate for the repair of critical size bone defects. J. Tissue Eng. Regen. Med. 2012, 6, 833–840. [Google Scholar] [CrossRef]
- Byers, B.A.; Guldberg, R.E.; Hutmacher, D.W.; García, A.J. Effects of Runx2 genetic engineering and in vitro maturation of tissue-engineered constructs on the repair of critical size bone defects. J. Biomed. Mater. Res. A 2006, 76, 646–655. [Google Scholar] [CrossRef]
- Niemeyer, P.; Krause, U.; Fellenberg, J.; Kasten, P.; Seckinger, A.; Ho, A.D.; Simank, H.G. Evaluation of mineralized collagen and alpha-tricalcium phosphate as scaffolds for tissue engineering of bone using human mesenchymal stem cells. Cells Tissues Organs 2004, 177, 68–78. [Google Scholar] [CrossRef] [PubMed]
- D’Agostino, A.; Maniero, S.; Puozzo, A.; Bassi, E.; Marsico, S.; Fortini, C.; Patergnani, S.; Tognon, M.; Mazzoni, E.; Manfrini, M.; et al. Human adipose stem cells induced to osteogenic differentiation by an innovative collagen/hydroxylapatite hybrid scaffold. FASEB J. 2017, 31, 4555–4565. [Google Scholar] [Green Version]
- Calabrese, G.; Giuffrida, R.; Forte, S.; Salvatorelli, L.; Fabbi, C.; Figallo, E.; Gulisano, M.; Parenti, R.; Magro, G.; Colarossi, C.; et al. Bone augmentation after ectopic implantation of a cell-free collagen-hydroxyapatite scaffold in the mouse. Sci. Rep. 2016, 6, 36399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.-L.; Chen, Q.; Du, B.-B.; Cao, L.; Lin, H.; Fan, Z.-Y.; Dong, J. Enhanced bone regeneration composite scaffolds of PLLA/β-TCP matrix grafted with gelatin and HAp. Mater. Sci. Eng. C 2018, 87, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Arafat, M.T.; Lam, C.X.; Ekaputra, A.K.; Wong, S.Y.; Li, X.; Gibson, I. Biomimetic composite coating on rapid prototyped scaffolds for bone tissue engineering. Acta Biomater. 2011, 7, 809–820. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Bai, Y.; Shih, M.S.; Hoffmann, C.; Peters, F.; Waldner, C.; Hübner, W.D. Effect of a β-TCP collagen composite bone substitute on healing of drilled bone voids in the distal femoral condyle of rabbits. J. Biomed. Mater. Res. B Appl. Biomater. 2014, 102, 376–383. [Google Scholar] [CrossRef] [PubMed]
- Gong, T.; Xie, J.; Liao, J.; Zhang, T.; Lin, S.; Lin, Y. Nanomaterials and bone regeneration. Bone Res. 2015, 3, 15029. [Google Scholar] [CrossRef]
- Van Rijt, S.; Habibovic, P. Enhancing regenerative approaches with nanoparticles. J. R. Soc. Interface 2017, 14, 20170093. [Google Scholar] [CrossRef]
- Cao, L.; Wang, J.; Hou, J.; Xing, W.; Liu, C. Vascularization and bone regeneration in a critical sized defect using 2-N,6-O-sulfated chitosan nanoparticles incorporating BMP-2. Biomaterials 2014, 35, 684–698. [Google Scholar] [CrossRef]
- Li, L.; Zhou, G.; Wang, Y.; Yang, G.; Ding, S.; Zhou, S. Controlled dual delivery of BMP-2 and dexamethasone by nanoparticle-embedded electrospun nanofibers for the efficient repair of critical-sized rat calvarial defect. Biomaterials 2015, 37, 218–229. [Google Scholar] [CrossRef]
- Pizzicannella, J.; Gugliandolo, A.; Orsini, T.; Fontana, A.; Ventrella, A.; Mazzon, E.; Bramanti, P.; Diomede, F.; Trubiani, O. Engineered Extracellular Vesicles from Human Periodontal-Ligament Stem Cells Increase VEGF/VEGFR2 Expression During Bone Regeneration. Front. Physiol. 2019, 10, 512. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, A.T.; Doyle, A.; Chen, C.; Coulon, D.; Dasa, V.; Del Piero, F.; Levi, B.; Monroe, W.T.; Gimble, J.M.; Hayes, D.J. Photoactivated miR-148b-nanoparticle conjugates improve closure of critical size mouse calvarial defects. Acta Biomater. 2015, 12, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, Y.; Gou, W.; Lu, Q.; Peng, J.; Lu, S. Role of mesenchymal stem cells in bone regeneration and fracture repair: A review. Int. Orthop. 2013, 37, 2491–2498. [Google Scholar] [CrossRef] [PubMed]
- Granero-Moltó, F.; Weis, J.A.; Miga, M.I.; Landis, B.; Myers, T.J.; O’Rear, L.; Longobardi, L.; Jansen, E.D.; Mortlock, D.P.; Spagnoli, A. Regenerative Effects of Transplanted Mesenchymal Stem Cells in Fracture Healing. Stem Cells 2009, 27, 1887–1898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lien, C.-Y.; Ho, K.C.-Y.; Lee, O.K.; Blunn, G.W.; Su, Y. Restoration of Bone Mass and Strength in Glucocorticoid-Treated Mice by Systemic Transplantation of CXCR4 and Cbfa-1 Co-Expressing Mesenchymal Stem Cells. J. Bone Miner. Res. 2009, 24, 837–848. [Google Scholar] [CrossRef] [PubMed]
- Rapp, A.; Bindl, R.; Heilmann, A.; Erbacher, A.; Brenner, R.; Ignatius, A.; Müller, I. Systemic mesenchymal stem cell administration enhances bone formation in fracture repair but not load-induced bone formation. eCM 2015, 29, 22–34. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Xu, L.; Zhang, Y.; Sun, Y.; Li, G. Systemic and Local Administration of Allogeneic Bone Marrow-Derived Mesenchymal Stem Cells Promotes Fracture Healing in Rats. Cell Transplant. 2015, 24, 2643–2655. [Google Scholar] [CrossRef] [Green Version]
- Xu, L.; Huang, S.; Hou, Y.; Liu, Y.; Ni, M.; Meng, F.; Wang, K.; Rui, Y.; Jiang, X.; Li, G. Sox11-modified mesenchymal stem cells (MSCs) accelerate bone fracture healing: Sox11 regulates differentiation and migration of MSCs. FASEB J. 2015, 29, 1143–1152. [Google Scholar] [CrossRef] [PubMed]
- Horwitz, E.M.; Prockop, D.J.; Fitzpatrick, L.A.; Koo, W.W.K.; Gordon, P.L.; Neel, M.; Sussman, M.; Orchard, P.; Marx, J.C.; Pyeritz, R.E.; et al. Transplantability and therapeutic effects of bone marrow-derived mesenchymal cells in children with osteogenesis imperfecta. Nat. Med. 1999, 5, 309–313. [Google Scholar] [CrossRef]
- Castelo-Branco, M.T.L.; Soares, I.D.P.; Lopes, D.V.; Buongusto, F.; Martinusso, C.A.; Rosario, A.D.; Souza, S.A.L.; Gutfilen, B.; Fonseca, L.M.B.; Elia, C.; et al. Intraperitoneal but Not Intravenous Cryopreserved Mesenchymal Stromal Cells Home to the Inflamed Colon and Ameliorate Experimental Colitis. PLoS ONE 2012, 7, e33360. [Google Scholar] [CrossRef]
- Cui, L.-L.; Kerkelä, E.; Bakreen, A.; Nitzsche, F.; Andrzejewska, A.; Nowakowski, A.; Janowski, M.; Walczak, P.; Boltze, J.; Lukomska, B.; et al. The cerebral embolism evoked by intra-arterial delivery of allogeneic bone marrow mesenchymal stem cells in rats is related to cell dose and infusion velocity. Stem Cell Res. Ther. 2015, 6, 11. [Google Scholar] [CrossRef] [PubMed]
- Katagiri, W.; Osugi, M.; Kawai, T.; Ueda, M. Novel Cell-Free Regeneration of Bone Using Stem Cell–Derived Growth Factors. Int. J. Oral Maxillofac. Implant. 2013, 28, 1009–1016. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, J.R.; Teixeira, G.Q.; Santos, S.G.; Barbosa, M.A.; Almeida-Porada, G.; Gonçalves, R.M. Mesenchymal Stromal Cell Secretome: Influencing Therapeutic Potential by Cellular Pre-conditioning. Front. Immunol. 2018, 9, 2837. [Google Scholar] [CrossRef] [PubMed]
- Estrada, R.; Li, N.; Sarojini, H.; An, J.; Lee, M.-J.; Wang, E. Secretome From Mesenchymal Stem Cells Induces Angiogenesis Via Cyr61. J. Cell. Physiol. 2009, 219, 563–571. [Google Scholar] [CrossRef] [PubMed]
- Linero, I.; Chaparro, O. Paracrine Effect of Mesenchymal Stem Cells Derived from Human Adipose Tissue in Bone Regeneration. PLoS ONE 2014, 9, e107001. [Google Scholar] [CrossRef] [PubMed]
- Dufrane, D. Impact of Age on Human Adipose Stem Cells for Bone Tissue Engineering. Cell Transplant. 2017, 26, 1496–1504. [Google Scholar] [CrossRef] [PubMed]
- Maffioli, E.; Nonnis, S.; Angioni, R.; Santagata, F.; Calì, B.; Zanotti, L.; Negri, A.; Viola, A.; Tedeschi, G. Proteomic analysis of the secretome of human bone marrow-derived mesenchymal stem cells primed by pro-inflammatory cytokines. J. Proteom. 2017, 166, 115–126. [Google Scholar] [CrossRef]
- Ralphs, J.; Alini, M.; Stoddart, M. Enhancing inflammatory and chemotactic signals to regulate bone regeneration. eCM 2014, 28, 320–334. [Google Scholar]
- Miranda, J.P.; Camões, S.P.; Gaspar, M.M.; Rodrigues, J.S.; Carvalheiro, M.; Bárcia, R.N.; Cruz, P.; Cruz, H.; Simões, S.; Santos, J.M. The Secretome Derived From 3D-Cultured Umbilical Cord Tissue MSCs Counteracts Manifestations Typifying Rheumatoid Arthritis. Front. Immunol. 2019, 10, 18. [Google Scholar] [CrossRef]
- Osugi, M.; Katagiri, W.; Yoshimi, R.; Inukai, T.; Hibi, H.; Ueda, M. Conditioned Media from Mesenchymal Stem Cells Enhanced Bone Regeneration in Rat Calvarial Bone Defects. Tissue Eng. Part A 2012, 18, 1479–1489. [Google Scholar] [CrossRef] [Green Version]
- Kawai, T.; Katagiri, W.; Osugi, M.; Sugimura, Y.; Hibi, H.; Ueda, M. Secretomes from bone marrow–derived mesenchymal stromal cells enhance periodontal tissue regeneration. Cytotherapy 2015, 17, 369–381. [Google Scholar] [CrossRef] [PubMed]
- Ogata, K.; Katagiri, W.; Osugi, M.; Kawai, T.; Sugimura, Y.; Hibi, H.; Nakamura, S.; Ueda, M. Evaluation of the therapeutic effects of conditioned media from mesenchymal stem cells in a rat bisphosphonate-related osteonecrosis of the jaw-like model. Bone 2015, 74, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Katagiri, W.; Osugi, M.; Kinoshita, K.; Hibi, H. Conditioned Medium from Mesenchymal Stem Cells Enhances Early Bone Regeneration After Maxillary Sinus Floor Elevation in Rabbits. Implant. Dent. 2015, 24, 1. [Google Scholar] [CrossRef] [PubMed]
- Inukai, T.; Katagiri, W.; Yoshimi, R.; Osugi, M.; Kawai, T.; Hibi, H.; Ueda, M. Novel application of stem cell-derived factors for periodontal regeneration. Biochem. Biophys. Res. Commun. 2013, 430, 763–768. [Google Scholar] [CrossRef] [PubMed]
- Ogata, K.; Katagiri, W.; Hibi, H. Secretomes from mesenchymal stem cells participate in the regulation of osteoclastogenesis in vitro. Clin. Oral Investig. 2017, 21, 1979–1988. [Google Scholar] [CrossRef] [PubMed]
- Katagiri, W.; Kawai, T.; Osugi, M.; Sugimura-Wakayama, Y.; Sakaguchi, K.; Kojima, T.; Kobayashi, T. Angiogenesis in newly regenerated bone by secretomes of human mesenchymal stem cells. Maxillofac. Plast. Reconstr. Surg. 2017, 39, 920. [Google Scholar] [CrossRef] [PubMed]
- Katagiri, W.; Sakaguchi, K.; Kawai, T.; Wakayama, Y.; Osugi, M.; Hibi, H. A defined mix of cytokines mimics conditioned medium from cultures of bone marrow-derived mesenchymal stem cells and elicits bone regeneration. Cell Prolif. 2017, 50, e12333. [Google Scholar] [CrossRef] [Green Version]
- Katagiri, W.; Osugi, M.; Kawai, T.; Hibi, H. First-in-human study and clinical case reports of the alveolar bone regeneration with the secretome from human mesenchymal stem cells. Head Face Med. 2016, 12, 300. [Google Scholar] [CrossRef]
- Katagiri, W.; Watanabe, J.; Toyama, N.; Osugi, M.; Sakaguchi, K.; Hibi, H. Clinical Study of Bone Regeneration by Conditioned Medium from Mesenchymal Stem Cells after Maxillary Sinus Floor Elevation. Implant. Dent. 2017, 26, 607–612. [Google Scholar] [CrossRef]
- Dufrane, D.; Docquier, P.L.; Delloye, C.; Poirel, H.A.; André, W.; Aouassar, N. Scaffold-free Three-dimensional Graft from Autologous Adipose-derived Stem Cells for Large Bone Defect Reconstruction: Clinical Proof of Concept. Medicine 2015, 94, e2220. [Google Scholar] [CrossRef]
- Vériter, S.; André, W.; Aouassar, N.; Poirel, H.A.; Lafosse, A.; Docquier, P.L.; Dufrane, D. Human Adipose-Derived Mesenchymal Stem Cells in Cell Therapy: Safety and Feasibility in Different ‘Hospital Exemption’ Clinical Applications. PLoS ONE 2015, 10, e0139566. [Google Scholar] [CrossRef] [PubMed]
- Flouzat-Lachaniette, C.H.; Heyberger, C.; Bouthors, C.; Roubineau, F.; Chevallier, N.; Rouard, H.; Hernigou, P. Osteogenic progenitors in bone marrow aspirates have clinical potential for tibial non-unions healing in diabetic patients. Int. Orthop. 2016, 40, 1375–1379. [Google Scholar] [CrossRef] [PubMed]
- Hernigou, P.; Guissou, I.; Homma, Y.; Poignard, A.; Chevallier, N.; Rouard, H.; Lachaniette, C.H.F. Percutaneous injection of bone marrow mesenchymal stem cells for ankle non-unions decreases complications in patients with diabetes. Int. Orthop. 2015, 39, 1639–1643. [Google Scholar] [CrossRef] [PubMed]
- Dilogo, I.H.; Primaputra, M.R.A.; Pawitan, J.A.; Liem, I.K. Modified Masquelet technique using allogeneic umbilical cord-derived mesenchymal stem cells for infected non-union femoral shaft fracture with a 12 cm bone defect: A case report. Int. J. Surg. Case Rep. 2017, 34, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chu, W.; Zhuang, Y.; Shi, D.; Tao, H.; Jin, C.; Dai, K.; Zhao, J.; Gan, Y. Bone Mesenchymal Stem Cell-Enriched β-Tricalcium Phosphate Scaffold Processed by the Screen-Enrich-Combine Circulating System Promotes Regeneration of Diaphyseal Bone Non-Union. Cell Transplant. 2019, 28, 212–223. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Xue, F.; Jun Xiao, H. Ilizarov method in combination with autologous mesenchymal stem cells from iliac crest shows improved outcome in tibial non-union. Saudi J. Biol. Sci. 2018, 25, 819–825. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Barrena, E.; Padilla-Eguiluz, N.G.; Avendaño-Solá, C.; Payares-Herrera, C.; Velasco-Iglesias, A.; Torres, F.; Rosset, P.; Gebhard, F.; Baldini, N.; Rubio-Suarez, J.C.; et al. A Multicentric, Open-Label, Randomized, Comparative Clinical Trial of Two Different Doses of Expanded hBM-MSCs Plus Biomaterial versus Iliac Crest Autograft, for Bone Healing in Nonunions after Long Bone Fractures: Study Protocol. Stem Cells Int. 2018, 2018, 6025918. [Google Scholar] [CrossRef]
- Gómez-Barrena, E.; Rosset, P.; Gebhard, F.; Hernigou, P.; Baldini, N.; Rouard, H.; Sensebé, L.; Gonzalo-Daganzo, R.M.; Giordano, R.; Padilla-Eguiluz, N.; et al. Feasibility and safety of treating non-unions in tibia, femur and humerus with autologous, expanded, bone marrow-derived mesenchymal stromal cells associated with biphasic calcium phosphate biomaterials in a multicentric, non-comparative trial. Biomaterials 2019, 196, 100–108. [Google Scholar] [CrossRef]
- Papaccio, F.; Paino, F.; Regad, T.; Papaccio, G.; Desiderio, V.; Tirino, V. Concise Review: Cancer Cells, Cancer Stem Cells, and Mesenchymal Stem Cells: Influence in Cancer Development. Stem Cells Transl. Med. 2017, 6, 2115–2125. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Freitas, J.; Santos, S.G.; Gonçalves, R.M.; Teixeira, J.H.; Barbosa, M.A.; Almeida, M.I. Genetically Engineered-MSC Therapies for Non-unions, Delayed Unions and Critical-size Bone Defects. Int. J. Mol. Sci. 2019, 20, 3430. https://doi.org/10.3390/ijms20143430
Freitas J, Santos SG, Gonçalves RM, Teixeira JH, Barbosa MA, Almeida MI. Genetically Engineered-MSC Therapies for Non-unions, Delayed Unions and Critical-size Bone Defects. International Journal of Molecular Sciences. 2019; 20(14):3430. https://doi.org/10.3390/ijms20143430
Chicago/Turabian StyleFreitas, Jaime, Susana Gomes Santos, Raquel Madeira Gonçalves, José Henrique Teixeira, Mário Adolfo Barbosa, and Maria Inês Almeida. 2019. "Genetically Engineered-MSC Therapies for Non-unions, Delayed Unions and Critical-size Bone Defects" International Journal of Molecular Sciences 20, no. 14: 3430. https://doi.org/10.3390/ijms20143430
APA StyleFreitas, J., Santos, S. G., Gonçalves, R. M., Teixeira, J. H., Barbosa, M. A., & Almeida, M. I. (2019). Genetically Engineered-MSC Therapies for Non-unions, Delayed Unions and Critical-size Bone Defects. International Journal of Molecular Sciences, 20(14), 3430. https://doi.org/10.3390/ijms20143430