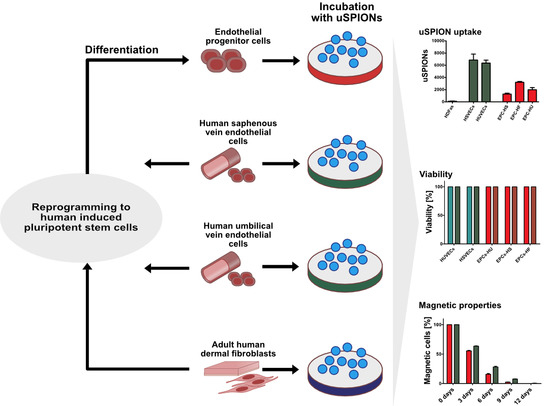
The Effect of Uncoated SPIONs on hiPSC-Differentiated Endothelial Cells
Abstract
:1. Introduction
2. Results
2.1. Uptake of uSPIONs Is Different between Cell Types
2.2. Biodistribution of the uSPIONs Observed by Transmission Electron Microscopy
2.3. ECs Show and Maintain Magnetic Properties after Labeling
2.4. Exposure to uSPIONs does not Affect Cell Survival of ECs and hiPSC-ECs
2.5. Labelled ECs and EPCs Retain Endothelial Markers and Angiogenic Properties
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Reprogramming and Differentiation of HUVECs, HSVECs and hDFs
4.3. Preparation and Analysis of uSPIONs
4.4. Prussian Blue Staining
4.5. MTT Assay
4.6. Annexin Assay
4.7. Transmission Electron Microscopy
4.8. Study of Magnetic Properties
4.9. Flow-Cytometry Analysis of Endothelial Characteristics
4.10. Image and Statistical Data Analysis
4.11. Ethics Statement
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ECs | endothelial cells |
| EPCs | endothelial progenitor cells |
| SPIONs | superparamagnetic iron-oxide nanoparticles |
| uSPIONs | uncoated superparamagnetic iron-oxide nanoparticles |
| hiPSCs | human induced pluripotent stem cells |
| hiPSC-ECs | hiPSC-derived endothelial cells |
| HUVECs | human umbilical vein endothelial cells |
| hiPSC-HUs | hiPSC derived from human umbilical vein endothelial cells |
| ECs-HU | endothelial cells differentiated from hiPSC-HU |
| hDF | human dermal fibroblasts |
| hiPSC-HF | hiPSC derived from human dermal fibroblasts |
| ECs-HF | endothelial cells differentiated from hiPSC-HF |
| HSVECs | human saphena vein endothelial cells |
| hiPSC-HSs | hiPSC derived from human saphena vein endothelial cells |
| ECs-HS | endothelial cells differentiated from hiPSC-HSs |
| ROS | reactive oxygen species |
| TEM | transmission electron microscopy |
References
- Chong, M.S.K.; Ng, W.K.; Chan, J.K.Y. Concise Review: Endothelial Progenitor Cells in Regenerative Medicine: Applications and Challenges. Stem Cells Transl. Med. 2016, 5, 530–538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asahara, T.; Kawamoto, A.; Masuda, H. Concise Review: Circulating Endothelial Progenitor Cells for Vascular Medicine. Stem Cells 2011, 29, 1650–1655. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, E.B.; Walenta, K.; Scharlau, J.; Nickenig, G.; Werner, N. CD34-/CD133+/VEGFR-2+ endothelial progenitor cell subpopulation with potent vasoregenerative capacities. Circ. Res. 2006, 98, e20–e25. [Google Scholar] [CrossRef] [PubMed]
- Hirata, K.; Li, T.S.; Nishida, M.; Ito, H.; Matsuzaki, M.; Kasaoka, S.; Hamano, K. Autologous bone marrow cell implantation as therapeutic angiogenesis for ischemic hindlimb in diabetic rat model. Am. J. Physiol. Heart Circ. Physiol. 2003, 284, H66–H70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Polyak, B.; Fishbein, I.; Chorny, M.; Alferiev, I.; Williams, D.; Yellen, B.; Friedman, G.; Levy, R.J. High field gradient targeting of magnetic nanoparticle-loaded endothelial cells to the surfaces of steel stents. Proc. Natl. Acad. Sci. USA 2008, 105, 698–703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fadini, G.P.; Miorin, M.; Facco, M.; Bonamico, S.; Baesso, I.; Grego, F.; Menegolo, M.; de Kreutzenberg, S.V.; Tiengo, A.; Agostini, C.; et al. Circulating Endothelial Progenitor Cells Are Reduced in Peripheral Vascular Complications of Type 2 Diabetes Mellitus. J. Am. Coll. Cardiol. 2005, 45, 1449–1457. [Google Scholar] [CrossRef] [PubMed]
- Peichev, M.; Naiyer, A.J.; Pereira, D.; Zhu, Z.; Lane, W.J.; Williams, M.; Oz, M.C.; Hicklin, D.J.; Witte, L.; Moore, M.A.; et al. Expression of VEGFR-2 and AC133 by circulating human CD34(+) cells identifies a population of functional endothelial precursors. Blood 2000, 95, 952–958. [Google Scholar]
- Hibino, N.; Duncan, D.R.; Nalbandian, A.; Yi, T.; Qyang, Y.; Shinoka, T.; Breuer, C.K. Evaluation of the use of an induced puripotent stem cell sheet for the construction of tissue-engineered vascular grafts. J. Thorac. Cardiovasc. Surg. 2012, 143, 696–703. [Google Scholar] [CrossRef] [Green Version]
- Simara, P.; Motl, J.A.; Kaufman, D.S. Pluripotent stem cells and gene therapy. Transl. Res. 2013, 161, 284–292. [Google Scholar] [CrossRef] [Green Version]
- Nakayama, K.H.; Joshi, P.A.; Lai, E.S.; Gujar, P.; Joubert, L.M.; Chen, B.; Huang, N.F. Bilayered vascular graft derived from human induced pluripotent stem cells with biomimetic structure and function. Regen. Med. 2015, 10, 745–755. [Google Scholar] [CrossRef]
- Bao, X.; Lian, X.; Dunn, K.K.; Shi, M.; Han, T.; Qian, T.; Bhute, V.J.; Canfield, S.G.; Palecek, S.P. Chemically-defined albumin-free differentiation of human pluripotent stem cells to endothelial progenitor cells. Stem Cell Res. 2015, 15, 122–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prasain, N.; Lee, M.R.; Vemula, S.; Meador, J.L.; Yoshimoto, M.; Ferkowicz, M.J.; Fett, A.; Gupta, M.; Rapp, B.M.; Saadatzadeh, M.R.; et al. Differentiation of human pluripotent stem cells to cells similar to cord-blood endothelial colony-forming cells. Nat. Biotechnol. 2014, 32, 1151–1157. [Google Scholar] [CrossRef] [PubMed]
- Simara, P.; Tesarova, L.; Rehakova, D.; Farkas, S.; Salingova, B.; Kutalkova, K.; Vavreckova, E.; Matula, P.; Matula, P.; Veverkova, L.; et al. Reprogramming of adult peripheral blood cells into human induced pluripotent stem cells as a safe and accessible source of endothelial cells. Stem Cells Dev. 2017, 27, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Orlova, V.V.; Drabsch, Y.; Freund, C.; Petrus-Reurer, S.; van den Hil, F.E.; Muenthaisong, S.; Dijke, P.T.; Mummery, C.L. Functionality of endothelial cells and pericytes from human pluripotent stem cells demonstrated in cultured vascular plexus and zebrafish xenografts. Arter. Thromb. Vasc. Biol. 2014, 34, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Samuel, R.; Daheron, L.; Liao, S.; Vardam, T.; Kamoun, W.S.; Batista, A.; Buecker, C.; Schäfer, R.; Han, X.; Au, P.; et al. Generation of functionally competent and durable engineered blood vessels from human induced pluripotent stem cells. Proc. Natl. Acad. Sci. USA 2013, 110, 12774–12779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ali, A.; Zafar, H.; Zia, M.; Ul Haq, I.; Phull, A.R.; Ali, J.S.; Hussain, A. Synthesis, characterization, applications, and challenges of iron oxide nanoparticles. Nanotechnol. Sci. Appl. 2016, 9, 49–67. [Google Scholar] [CrossRef] [PubMed]
- Anselmo, A.C.; Mitragotri, S. Nanoparticles in the clinic. Bioeng. Transl. Med. 2016, 1, 10–29. [Google Scholar] [CrossRef]
- Chen, R.; Yu, H.; Jia, Z.Y.; Yao, Q.L.; Teng, G.J. Efficient nano iron particle-labeling and noninvasive MR imaging of mouse bone marrow-derived endothelial progenitor cells. Int. J. Nanomed. 2011, 6, 511–519. [Google Scholar] [CrossRef] [Green Version]
- Soenen, S.J.; Himmelreich, U.; Nuytten, N.; De Cuyper, M. Cytotoxic effects of iron oxide nanoparticles and implications for safety in cell labelling. Biomaterials 2011, 32, 195–205. [Google Scholar] [CrossRef]
- Castaneda, R.T.; Khurana, A.; Khan, R.; Daldrup-Link, H.E. Labeling stem cells with ferumoxytol, an FDA-approved iron oxide nanoparticle. J. Vis. Exp. Jove 2011, 57, e3482. [Google Scholar] [CrossRef]
- Bogart, L.K.; Pourroy, G.; Murphy, C.J.; Puntes, V.; Pellegrino, T.; Rosenblum, D.; Peer, D.; Lévy, R. Nanoparticles for imaging, sensing, and therapeutic intervention. ACS Nano 2014, 8, 3107–3122. [Google Scholar] [CrossRef]
- Rejman, J.; Oberle, V.; Zuhorn, I.S.; Hoekstra, D. Size-dependent internalization of particles via the pathways of clathrin- and caveolae-mediated endocytosis. Biochem. J. 2004, 377, 159–169. [Google Scholar] [CrossRef]
- Calero, M.; Gutiérrez, L.; Salas, G.; Luengo, Y.; Lázaro, A.; Acedo, P.; Morales, M.P.; Miranda, R.; Villanueva, A. Efficient and safe internalization of magnetic iron oxide nanoparticles: two fundamental requirements for biomedical applications. Nanomedicine 2014, 10, 733–743. [Google Scholar] [CrossRef]
- Kettler, K.; Veltman, K.; van de Meent, D.; van Wezel, A.; Hendriks, A.J. Cellular uptake of nanoparticles as determined by particle properties, experimental conditions, and cell type. Environ. Toxicol. Chem. 2014, 33, 481–492. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, M.; Simchi, A.; Imani, M.; Shokrgozar, M.A.; Milani, A.S.; Häfeli, U.O.; Stroeve, P. A new approach for the in vitro identification of the cytotoxicity of superparamagnetic iron oxide nanoparticles. Colloids Surf. B Biointerfaces 2010, 75, 300–309. [Google Scholar] [CrossRef]
- Bashir, M.R.; Bhatti, L.; Marin, D.; Nelson, R.C. Emerging applications for ferumoxytol as a contrast agent in MRI. J. Magn. Reson. Imaging 2015, 41, 884–898. [Google Scholar] [CrossRef]
- Shahnaz, G.; Kremser, C.; Reinisch, A.; Vetter, A.; Laffleur, F.; Rahmat, D.; Iqbal, J.; Dünnhaupt, S.; Salvenmoser, W.; Tessadri, R.; et al. Efficient MRI labeling of endothelial progenitor cells: design of thiolated surface stabilized superparamagnetic iron oxide nanoparticles. Eur. J. Pharm. Biopharm. 2013, 85, 346–355. [Google Scholar] [CrossRef]
- Wei, M.Q.; Wen, D.D.; Wang, X.Y.; Huan, Y.; Yang, Y.; Xu, J.; Cheng, K.; Zheng, M.W. Experimental study of endothelial progenitor cells labeled with superparamagnetic iron oxide in vitro. Mol. Med. Rep. 2015, 11, 3814–3819. [Google Scholar] [CrossRef]
- Nguyen, V.H.; Lee, B.-J. Protein corona: A new approach for nanomedicine design. Int. J. Nanomed. 2017, 12, 3137–3151. [Google Scholar] [CrossRef]
- Carril, M.; Padro, D.; del Pino, P.; Carrillo-Carrion, C.; Gallego, M.; Parak, W.J. In situ detection of the protein corona in complex environments. Nat. Commun. 2017, 8, 1542. [Google Scholar] [CrossRef]
- Wang, F.; Yu, L.; Monopoli, M.P.; Sandin, P.; Mahon, E.; Salvati, A.; Dawson, K.A. The biomolecular corona is retained during nanoparticle uptake and protects the cells from the damage induced by cationic nanoparticles until degraded in the lysosomes. Nanomed. Nanotechnol. Biol. Med. 2013, 9, 1159–1168. [Google Scholar] [CrossRef] [PubMed]
- Conner, S.D.; Schmid, S.L. Regulated portals of entry into the cell. Nature 2003, 422, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Behzadi, S.; Serpooshan, V.; Tao, W.; Hamaly, M.A.; Alkawareek, M.Y.; Dreaden, E.C.; Brown, D.; Alkilany, A.M.; Farokhzad, O.C.; Mahmoudi, M. Cellular uptake of nanoparticles: Journey inside the cell. Chem. Soc. Rev. 2017, 46, 4218–4244. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Li, J.; Lykotrafitis, G.; Bao, G.; Suresh, S. Size-Dependent Endocytosis of Nanoparticles. Adv Mater. 2009, 21, 419–424. [Google Scholar] [CrossRef] [Green Version]
- Roth, T.F.; Porter, K.R. Yolk protein uptake in the oocyte of the mosquito Aedes aegypti. L. J. Cell Biol. 1964, 20, 313–332. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Gao, H.; Bao, G. Physical Principles of Nanoparticle Cellular Endocytosis. ACS Nano 2015, 9, 8655–8671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanini, A.; Schmitt, A.; Kacem, K.; Chau, F.; Ammar, S.; Gavard, J. Evaluation of iron oxide nanoparticle biocompatibility. Int. J. Nanomed. 2011, 6, 787–794. [Google Scholar] [Green Version]
- Singh, N.; Jenkins, G.J.; Asadi, R.; Doak, S.H. Potential toxicity of superparamagnetic iron oxide nanoparticles (SPION). Nano Rev. 2010, 1. [Google Scholar] [CrossRef] [Green Version]
- Buyukhatipoglu, K.; Clyne, A.M. Superparamagnetic iron oxide nanoparticles change endothelial cell morphology and mechanics via reactive oxygen species formation. J. Biomed. Mater. Res. A 2011, 96, 186–195. [Google Scholar] [CrossRef]
- Pongrac, I.M.; Pavičić, I.; Milić, M.; Brkić Ahmed, L.; Babič, M.; Horák, D.; Vinković Vrček, I.; Gajović, S. Oxidative stress response in neural stem cells exposed to different superparamagnetic iron oxide nanoparticles. Int. J. Nanomed. 2016, 11, 1701–1715. [Google Scholar] [Green Version]
- Zhu, X.M.; Wang, Y.X.; Leung, K.C.; Lee, S.F.; Zhao, F.; Wang, D.W.; Lai, J.M.; Wan, C.; Cheng, C.H.; Ahuja, A.T. Enhanced cellular uptake of aminosilane-coated superparamagnetic iron oxide nanoparticles in mammalian cell lines. Int. J. Nanomed. 2012, 7, 953–964. [Google Scholar] [Green Version]
- Clift, M.J.D.; Bhattacharjee, S.; Brown, D.M.; Stone, V. The effects of serum on the toxicity of manufactured nanoparticles. Toxicol. Lett. 2010, 198, 358–365. [Google Scholar] [CrossRef] [PubMed]
- Arbab, A.S.; Yocum, G.T.; Rad, A.M.; Khakoo, A.Y.; Fellowes, V.; Read, E.J.; Frank, J.A. Labeling of cells with ferumoxides-protamine sulfate complexes does not inhibit function or differentiation capacity of hematopoietic or mesenchymal stem cells. NMR Biomed. 2005, 18, 553–559. [Google Scholar] [CrossRef] [PubMed]
- Kostura, L.; Kraitchman, D.L.; Mackay, A.M.; Pittenger, M.F.; Bulte, J.W. Feridex labeling of mesenchymal stem cells inhibits chondrogenesis but not adipogenesis or osteogenesis. NMR Biomed. 2004, 17, 513–517. [Google Scholar] [CrossRef] [PubMed]
- Zeng, G.; Wang, G.; Guan, F.; Chang, K.; Jiao, H.; Gao, W.; Xi, S.; Yang, B. Human amniotic membrane-derived mesenchymal stem cells labeled with superparamagnetic iron oxide nanoparticles: The effect on neuron-like differentiation in vitro. Mol. Cell. Biochem. 2011, 357, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Hsiao, J.K.; Liu, H.M.; Lai, I.Y.; Yao, M.; Hsu, S.C.; Ko, B.S.; Yang, C.S.; Huang, D.M. The inhibitory effect of superparamagnetic iron oxide nanoparticle (Ferucarbotran) on osteogenic differentiation and its signaling mechanism in human mesenchymal stem cells. Toxicol. Appl. Pharm. 2010, 245, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Au, K.W.; Liao, S.Y.; Lee, Y.K.; Lai, W.H.; Ng, K.M.; Chan, Y.C.; Yip, M.C.; Ho, C.Y.; Wu, E.X.; Li, R.A.; et al. Effects of iron oxide nanoparticles on cardiac differentiation of embryonic stem cells. Biochem. Biophys. Res. Commun. 2009, 379, 898–903. [Google Scholar] [CrossRef]
- Šimara, P.; Tesařová, L.; Padourová, S.; Koutná, I. Generation of human induced pluripotent stem cells using genome integrating or non-integrating methods. Folia Biol. Praha 2014, 60 (Suppl. S1), 85–89. [Google Scholar]
- Simara, P.; Tesarova, L.; Rehakova, D.; Matula, P.; Stejskal, S.; Hampl, A.; Koutna, I. DNA double-strand breaks in human induced pluripotent stem cell reprogramming and long-term in vitro culturing. Stem Cell Res. 2017, 8, 73. [Google Scholar] [CrossRef]
- Orlova, V.V.; van den Hil, F.E.; Petrus-Reurer, S.; Drabsch, Y.; Ten Dijke, P.; Mummery, C.L. Generation, expansion and functional analysis of endothelial cells and pericytes derived from human pluripotent stem cells. Nat. Protoc. 2014, 9, 1514–1531. [Google Scholar] [CrossRef] [Green Version]
- Synek, P.; Jasek, O.; Zajickova, L. Study of Microwave Torch Plasmachemical Synthesis of Iron Oxide Nanoparticles Focused on the Analysis of Phase Composition. Plasma Chem. Plasma Process. 2014, 34, 327–341. [Google Scholar] [CrossRef]
- Synek, P.; Jasek, O.; Zajickova, L.; Kudrle, V.; Pizurova, N. Plasmachemical synthesis of maghemite nanoparticles in atmospheric pressure microwave torch. Mater. Lett. 2011, 65, 982–984. [Google Scholar] [CrossRef]
- de Faria, D.L.A.; Venâncio, S.S.; de Oliveira, M.T. Raman microspectroscopy of some iron oxides and oxyhydroxides. J. Rom. Spectrosc. 1997, 28, 873–878. [Google Scholar] [CrossRef]






© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salingova, B.; Simara, P.; Matula, P.; Zajickova, L.; Synek, P.; Jasek, O.; Veverkova, L.; Sedlackova, M.; Nichtova, Z.; Koutna, I. The Effect of Uncoated SPIONs on hiPSC-Differentiated Endothelial Cells. Int. J. Mol. Sci. 2019, 20, 3536. https://doi.org/10.3390/ijms20143536
Salingova B, Simara P, Matula P, Zajickova L, Synek P, Jasek O, Veverkova L, Sedlackova M, Nichtova Z, Koutna I. The Effect of Uncoated SPIONs on hiPSC-Differentiated Endothelial Cells. International Journal of Molecular Sciences. 2019; 20(14):3536. https://doi.org/10.3390/ijms20143536
Chicago/Turabian StyleSalingova, Barbara, Pavel Simara, Pavel Matula, Lenka Zajickova, Petr Synek, Ondrej Jasek, Lenka Veverkova, Miroslava Sedlackova, Zuzana Nichtova, and Irena Koutna. 2019. "The Effect of Uncoated SPIONs on hiPSC-Differentiated Endothelial Cells" International Journal of Molecular Sciences 20, no. 14: 3536. https://doi.org/10.3390/ijms20143536
APA StyleSalingova, B., Simara, P., Matula, P., Zajickova, L., Synek, P., Jasek, O., Veverkova, L., Sedlackova, M., Nichtova, Z., & Koutna, I. (2019). The Effect of Uncoated SPIONs on hiPSC-Differentiated Endothelial Cells. International Journal of Molecular Sciences, 20(14), 3536. https://doi.org/10.3390/ijms20143536