Combination with l-Menthol Enhances Transdermal Penetration of Indomethacin Solid Nanoparticles
Abstract
:1. Introduction
2. Results
2.1. Evaluation of Transdermal Formulations Containing IMC SNPs and l-Menthol
2.2. Behavior of IMC Release from IMC Transdermal Formulations with or without l-Menthol
2.3. IMC Penetration into the Rat Skin when IMC Transdermal Formulations with or without l-Menthol Were Applied
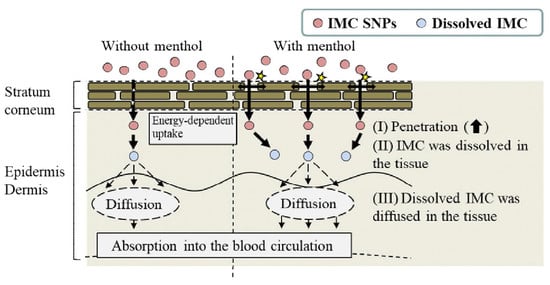
3. Discussion
4. Materials and Methods
4.1. Animals and Reagents
4.2. Design of IMC SNPs-based Transdermal Formulations with or without l-Menthol
4.3. Particle Characteristics of the IMC Transdermal Formulations
4.4. Measurement of IMC
4.5. Evaluation of Drug Release from IMC Transdermal Formulations
4.6. Evaluation of Skin Penetration of IMC Transdermal Formulations
4.7. Evaluation of Percutaneous Absorption from IMC Transdermal Formulations
4.8. Characterization of IMC
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AFM | atomic force microscope |
| AUC | area under the indomethacin concentration-time curve |
| carbopol | Carbopol® 934 |
| COX | cyclooxygenase |
| D | diffusion constant within the skin |
| HPβCD | 2-hydroxypropyl-β-cyclodextrin |
| IMC | indomethacin |
| P-IMC gel | transdermal formulations containing indomethacin solid microparticles |
| N-IMC gel | transdermal formulations containing indomethacin solid nanoparticles |
| Jc | penetration rate |
| ka | apparent absorption rate constant |
| ke | elimination rate constant |
| Km | skin/preparation partition coefficient |
| Kp | penetration coefficient through the skin |
| kr | drug release rate constant |
| MC | methylcellulose |
| P-IMC/MT gel | transdermal formulations containing indomethacin solid microparticles and l-menthol |
| N-IMC/MT gel | transdermal formulations containing indomethacin solid nanoparticles and l-menthol |
| NSAIDs | non-steroidal anti-inflammatory drug |
| SC | stratum corneum |
| SNPs | solid nanoparticles |
| TDD | transdermal drug delivery |
| tlag | lag time |
| XRD | powder X-ray diffraction |
References
- Sostres, C.; Gargallo, C.J.; Arroyo, M.T.; Lanas, A. Adverse effects of non-steroidal anti-inflammatory drugs (NSAIDs, aspirin and coxibs) on upper gastrointestinal tract. Best Pract. Res. Clin. Gastroenterol. 2010, 24, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Bateman, D.N. Non-steroidal anti-inflammatory drugs. Medicine (Baltimore) 2012, 40, 140. [Google Scholar] [CrossRef]
- Kim, S.J.; Flach, A.J.; Jampol, L.M. Nonsteroidal anti-inflammatory drugs in ophthalmology. Surv. Ophthalmol. 2010, 5, 108–133. [Google Scholar] [CrossRef] [PubMed]
- Nagai, N.; Fukuhata, T.; Ito, Y.; Usui, S.; Hirano, K. Involvement of interleukin 18 in indomethacin-induced lesions of the gastric mucosa in adjuvant-induced arthritis rat. Toxicology 2009, 255, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Kato, S.; Takeuchi, K. Alteration of gastric ulcerogenic and healing responses in rats with adjuvant-induced arthritis. Jpn. J. Pharmacol. 2002, 89, 1–6. [Google Scholar] [CrossRef]
- Ren, C.; Fang, L.; Ling, L.; Wang, Q.; Liu, S.; Zhao, L.; He, Z. Design and in vivo evaluation of an indapamide transdermal patch. Int. J. Pharm. 2009, 370, 129–135. [Google Scholar] [CrossRef]
- Shinkai, N.; Korenaga, K.; Mizu, H.; Yamauchi, H. Intra-articular penetration of ketoprofen and analgesic effects after topical patch application in rats. J. Control. Release 2008, 131, 107–112. [Google Scholar] [CrossRef]
- So, J.W.; Park, H.H.; Lee, S.S.; Kim, D.C.; Shin, S.C.; Cho, C.W. Effect of microneedle on the pharmacokinetics of ketoprofen from its transdermal formulations. Drug Deliv. 2009, 16, 52–56. [Google Scholar] [CrossRef]
- Williams, A.C.; Barry, B.W. Penetration enhancers. Adv. Drug Deliv. Rev. 2004, 56, 603–618. [Google Scholar] [CrossRef]
- Djordjevic, L.; Primorac, M.; Stupar, M. In vitro release of diclofenac diethylamine from caprylocaproyl macrogolglycerides based microemulsions. Int. J. Pharm. 2005, 296, 73–79. [Google Scholar] [CrossRef]
- Podlogar, F.; Bester Rogac, M.; Gasperlin, M. The effect of internal structure of selected water—Tween 40®—Imwitor 308®—IPM microemulsions on ketoprofene release. Int. J. Pharm. 2005, 302, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Soma, D.; Attari, Z.; Reddy, M.S.; Damodaram, A.; Koteshwara, K.B.G. Solid lipid nanoparticles of irbesartan: Preparation, characterization, optimization and pharmacokinetic studies. Braz. J. Pharm. Sci. 2017, 53, 1–10. [Google Scholar] [CrossRef]
- Wissing, A.S.; Müller, R.H. Cosmetic applications for solid lipid nanoparticles (SLN). Int. J. Pharm. 2003, 254, 65–68. [Google Scholar] [CrossRef]
- Montenegro, L.; Lai, F.; Offera, A.; Sarpietro, M.G.; Micicche, L.; Maccioni, A.M.; Valenti, D.; Fadda, A.M. From nanoemulsions to nanostructured lipid carriers: A relevant development in dermal delivery of drugs and cosmetics. J. Drug Deliv. Sci. Technol. 2016, 32, 100–112. [Google Scholar] [CrossRef]
- Nagai, N.; Ogata, F.; Ishii, M.; Fukuoka, Y.; Otake, H.; Nakazawa, Y.; Kawasaki, N. Involvement of Endocytosis in the Transdermal Penetration Mechanism of Ketoprofen Nanoparticles. Int. J. Mol. Sci. 2018, 19, 2138. [Google Scholar] [CrossRef] [PubMed]
- Nagai, N.; Iwamae, A.; Tanimoto, S.; Yoshioka, C.; Ito, Y. Pharmacokinetics and Antiinflammatory Effect of a Novel Gel System Containing Ketoprofen Solid Nanoparticles. Biol. Pharm. Bull. 2015, 38, 1918–1924. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagai, N.; Ito, Y. Therapeutic Effects of Gel Ointments containing Tranilast Nanoparticles on Paw Edema in Adjuvant-Induced Arthritis Rats. Biol. Pharm. Bull. 2014, 37, 96–104. [Google Scholar] [CrossRef]
- Nagai, N.; Yoshioka, C.; Ito, Y. Topical Therapies for Rheumatoid Arthritis by Gel Ointments containing Indomethacin Nanoparticles in Adjuvant-Induced Arthritis Rat. J. Oleo Sci. 2015, 64, 337–346. [Google Scholar] [CrossRef] [Green Version]
- Nagai, N.; Tanino, T.; Ito, Y. Pharmacokinetic Studies of Gel System Containing Ibuprofen Solid Nanoparticles. J. Oleo Sci. 2016, 65, 1045–1053. [Google Scholar] [CrossRef] [Green Version]
- Sinha, V.R.; Kaur, M.P. Permeation enhancers for transdermal drug delivery. Drug Dev. Ind. Pharm. 2000, 26, 1131–1140. [Google Scholar] [CrossRef]
- Kaplun-Frischoff, Y.; Touitou, J. Testosterone skin permeation enhancement by menthol through formation of eutectic with drug and interaction with skin lipids. Pharm. Sci. 1997, 86, 1394–1399. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Liu, K.; Manaloto, E.; Casey, A.; Cribaro, G.P.; Byrne, H.J.; Tian, F.; Barcia, C.; Conway, G.E.; Cullen, P.J.; et al. Cold Atmospheric Plasma Induces ATP-Dependent Endocytosis of Nanoparticles and Synergistic U373MG Cancer Cell Death. Sci. Rep. 2018, 8, 5298. [Google Scholar] [CrossRef] [PubMed]
- Rehman, K.; Zulfakar, M.H. Recent advances in gel technologies for topical and transdermal drug delivery. Drug Dev. Ind. Pharm. 2013, 40, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Brambilla, D.; Luciani, P.; Leroux, J. Breakthrough discoveries in drug delivery technologies: The next 30 years. J. Control. Release 2014, 190, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Han, T.; Das, D.B. Potential of combined ultrasound and microneedles for enhanced transdermal drug permeation: A review. Eur. J. Pharm. Biopharm. 2015, 89, 312–328. [Google Scholar] [CrossRef] [Green Version]
- Ahad, A.; Aqil, M.; Kohli, K.; Sultana, Y.; Mujeeb, M.; Ali, A. Transdermal drug delivery: The inherent challenges and technological advancements. Asian J. Pharm. Sci. 2010, 5, 276–288. [Google Scholar]
- Gao, H.; Shi, W.; Freund, L.B. Mechanics of receptor-mediated endocytosis. Proc. Natl. Acad. Sci. USA 2005, 102, 9469–9474. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Gao, H.; Bao, G. Physical Principles of Nanoparticle Cellular Endocytosis. ACS Nano 2015, 9, 8655–8671. [Google Scholar] [CrossRef] [Green Version]
- Chithrani, B.D.; Chan, W.C. Elucidating the mechanism of cellular uptake and removal of protein-coated gold nanoparticles of different sizes and shapes. Nano Lett. 2007, 7, 1542–1550. [Google Scholar] [CrossRef]
- Tanwar, H.; Sachdeva, R. Transdermal drug delivery system: A review. IJPSR 2016, 7, 2274–2290. [Google Scholar]
- Mbah, C.J.; Uzor, P.F.; Omeje, E.O. Perspective on transdermal drug delivery. J. Chem. Pharm. Res. 2011, 3, 680–700. [Google Scholar]
- Bartosova, L.; Bajgar, J. Transdermal drug delivery in vitro using diffusion cells. Curr. Med. Chem. 2012, 19, 4671–4677. [Google Scholar] [CrossRef] [PubMed]
- Nagai, N.; Ogata, F.; Otake, H.; Nakazawa, Y.; Kawasaki, N. Design of a transdermal formulation containing raloxifene nanoparticles for osteoporosis treatment. Int. J. Nanomed. 2018, 13, 5215–5229. [Google Scholar] [CrossRef] [PubMed]
- Hufnagel, H.; Hakim, P.; Lima, A.; Hollfelder, F. Fluid phase endocytosis contributes to transfection of DNA by PEI-25. Mol. Ther. 2009, 17, 1411–1417. [Google Scholar] [CrossRef] [PubMed]
- Malomouzh, A.I.; Mukhitov, A.R.; Proskurina, S.E.; Vyskocil, F.; Nikolsky, E.E. The effect of dynasore, a blocker of dynamin-dependent endocytosis, on spontaneous quantal and non-quantal release of acetylcholine in murine neuromuscular junctions. Dokl. Biol. Sci. 2014, 459, 330–333. [Google Scholar] [CrossRef] [PubMed]
- Mäger, I.; Langel, K.; Lehto, T.; Eiríksdóttir, E.; Langel, U. The role of endocytosis on the uptake kinetics of luciferin-conjugated cell-penetrating peptides. Biochim. Biophys. Acta Biomembr. 2012, 1818, 502–511. [Google Scholar] [CrossRef] [PubMed] [Green Version]







| Formulation | Jc (nmol/cm2/h) | Kp (×10−4 cm/h) | Km (×10−2) | τ (h) | D (×10−4 cm2/h) |
|---|---|---|---|---|---|
| P-IMC gel | 18.6 ± 1.8 #,$ | 0.66 ± 0.06 #,$ | 0.92 ± 0.09 #,$ | 1.65 ± 0.03 #,$ | 5.10 ± 0.11#,$ |
| P-IMC/MT gel | 43.1 ± 3.1 * | 1.52 ± 0.11 * | 2.68 ± 0.28 * | 2.03 ± 0.07 * | 4.10 ± 0.11 * |
| N-IMC gel | 41.6 ± 4.6 * | 1.49 ± 0.17 * | 2.51 ± 0.22 * | 2.00 ± 0.06 * | 4.20 ± 0.12 * |
| N-IMC/MT gel | 158.1 ± 4.2 *,#,$ | 5.65 ± 0.15 *,#,$ | 10.8 ± 0.29 *,#,$ | 2.27 ± 0.02 *,#,$ | 3.71 ± 0.04 *,#,$ |
| Parameter | P-IMC Gel | P-IMC/MT Gel | N-IMC Gel | N-IMC/MT Gel |
|---|---|---|---|---|
| ka (h−1) | 0.18 ± 0.06 #,$ | 0.37 ± 0.05 * | 0.34 ± 0.04 * | 0.83 ± 0.03 *,#,$ |
| F (×10−3) | 0.14 ± 0.03 | 0.16 ± 0.02 | 0.15 ± 0.02 | 0.21 ± 0.01 *,#,$ |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nagai, N.; Ogata, F.; Yamaguchi, M.; Fukuoka, Y.; Otake, H.; Nakazawa, Y.; Kawasaki, N. Combination with l-Menthol Enhances Transdermal Penetration of Indomethacin Solid Nanoparticles. Int. J. Mol. Sci. 2019, 20, 3644. https://doi.org/10.3390/ijms20153644
Nagai N, Ogata F, Yamaguchi M, Fukuoka Y, Otake H, Nakazawa Y, Kawasaki N. Combination with l-Menthol Enhances Transdermal Penetration of Indomethacin Solid Nanoparticles. International Journal of Molecular Sciences. 2019; 20(15):3644. https://doi.org/10.3390/ijms20153644
Chicago/Turabian StyleNagai, Noriaki, Fumihiko Ogata, Mizuki Yamaguchi, Yuya Fukuoka, Hiroko Otake, Yosuke Nakazawa, and Naohito Kawasaki. 2019. "Combination with l-Menthol Enhances Transdermal Penetration of Indomethacin Solid Nanoparticles" International Journal of Molecular Sciences 20, no. 15: 3644. https://doi.org/10.3390/ijms20153644
APA StyleNagai, N., Ogata, F., Yamaguchi, M., Fukuoka, Y., Otake, H., Nakazawa, Y., & Kawasaki, N. (2019). Combination with l-Menthol Enhances Transdermal Penetration of Indomethacin Solid Nanoparticles. International Journal of Molecular Sciences, 20(15), 3644. https://doi.org/10.3390/ijms20153644