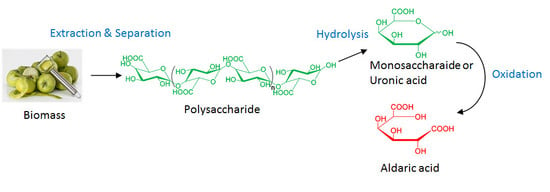
Production of Hexaric Acids from Biomass
Abstract
:1. Introduction
2. Classification of Aldohexoses and Hexaric Acids
3. Applications of Hexaric Acids
3.1. Monomers
3.2. Application of Hexaric Acid Polymers: An Example
3.3. Macromolecules
3.4. Chelating Agents, Coordination Polymers and Metal–Organic Frameworks
3.5. Other Platform Chemicals
4. Hexaric Acid Production Using Inorganic Catalysts
5. Hexaric Acid Production Using Biocatalysts
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| DOE | The US Department of Energy |
| PEIs | Polyethylene imines |
| MOF | Metal–organic framework |
| UDP | Uridine diphosphate |
| GDP | Guanosine diphosphate |
| UDH | Uronate dehydrogenase |
| PQQ | Pyrroloquinolone quinone |
| GDH | Glucose dehydrogenase |
| BOD | Bilirubin oxidase |
| DHPDH | PQQ domain of pyranose dehydrogenase from Coprinopsis cinerea |
References
- Cherubini, F. The biorefinery concept: Using biomass instead of oil for producing energy and chemicals. Energy Convers. Manag. 2010, 51, 1412–1421. [Google Scholar] [CrossRef]
- de Jong, E.; Higson, A.; Walsh, P.; Wellisch, M. Product developments in the bio-based chemicals arena. Biofuels Bioprod. Biorefin. 2012, 6, 606–624. [Google Scholar] [CrossRef]
- Werpy, T.; Peterson, G. Top Value Added Chemicals from Biomass: Volume I—Results of Screening for Potential Candidates from Sugars and Synthesis Gas; Technical Report Produced for the U.S. Department of Energy by the National Renewable Energy Laboratory: Washington, DC, USA, 2004. [Google Scholar]
- Climent, M.J.; Corma, A.; Iborra, S. Converting carbohydrates to bulk chemicals and fine chemicals over heterogeneous catalysts. Green Chem. 2011, 13, 520–540. [Google Scholar] [CrossRef]
- Isikgor, F.H.; Becer, C.R. Lignocellulosic biomass: A sustainable platform for the production of bio-based chemicals and polymers. Polym. Chem. 2015, 6, 4497–4559. [Google Scholar] [CrossRef]
- Mehtiö, T.; Toivari, M.; Wiebe, M.G.; Harlin, A.; Penttilä, M.; Koivula, A. Production and applications of carbohydrate-derived sugar acids as generic biobased chemicals. Crit. Rev. Biotechnol. 2016, 36, 904–916. [Google Scholar] [CrossRef]
- de Lederkremer, R.M.; Marino, C. Acids and their products of oxidation of sugars. Adv. Carbohydr. Chem. Biochem. 2003, 58, 199–306. [Google Scholar]
- Alterman, M.; Björsne, M.; Mühlman, A.; Classon, B.; Kvarnström, I.; Danielson, H.; Markgren, P.-O.; Nillroth, U.; Unge, T.; Hallberg, A.; et al. Design and synthesis of new potent C2-symmetric HIV-1 protease inhibitors. Use of l-mannaric acid as a peptidomimetic scaffold. J. Med. Chem. 1998, 41, 3782–3792. [Google Scholar] [CrossRef]
- Wachtmeister, J.; Mühlman, A.; Classon, B.; Kvarnström, I.; Hallberg, A.; Samuelsson, B. Impact of the central hydroxyl groups on the activity of symmetrical HIV-1 protease inhibitors derived from l-mannaric acid. Tetrahedron 2000, 56, 3219–3225. [Google Scholar] [CrossRef]
- Chakraborty, T.K.; Ghosh, S.; Jayaprakash, S.; Sharma, J.A.R.P.; Ravikanth, V.; Diwan, P.V.; Nagaraj, R.; Kunwar, A.C. Synthesis and conformational studies of peptidomimetics containing furanoid sugar amino acids and a sugar diacid. J. Org. Chem. 2000, 65, 6441–6457. [Google Scholar] [CrossRef]
- Chakraborty, T.K.; Ghosh, S.; Rao, M.H.V.R.; Kunwar, A.C.; Cho, H.; Ghosh, A.K. 2, 5-Anhydro sugar diacid and 2, 5-anhydro sugar diamine based C2 symmetric peptidomimetics as potential HIV-1 protease inhibitors. Tetrahedron Lett. 2000, 41, 10121–10125. [Google Scholar] [CrossRef]
- Mehltretter, C.L.; Alexander, B.H.; Rist, C.E. Sequestration by sugar acids. Ind. Eng. Chem. 1953, 45, 2782–2784. [Google Scholar] [CrossRef]
- Gónzalez-Portal, A.; Baluja-Santos, C.; Bermejo-Mártinez, F. Spectrophotometric determination of bismuth in pharmaceutical preparations using mucic acid. Analyst 1986, 111, 547–549. [Google Scholar] [CrossRef] [PubMed]
- Ramos, M.L.; Caldeira, M.M.; Gil, V.M.S. Multinuclear NMR study of the complexation of d-glucaric acid with molybdenum(VI) and tungsten(VI). Inorg. Chim. Acta 1991, 180, 219–224. [Google Scholar] [CrossRef]
- Ramos, M.L.; Caldeira, M.M.; Gil, V.M.S.; van Bekkum, H.; Peters, J.A. Multinuclear NMR study of the complexation of d-galactaric and d-mannaric acids with molybdenum(VI). Polyhedron 1994, 13, 1825–1833. [Google Scholar] [CrossRef]
- Ramos, M.L.; Caldeira, M.M.; Gil, V.M.S.; van Bekkum, H.; Peters, J.A. Multinuclear NMR study of complexation of d-galactaric and d-mannaric acids with tungsten(VI) oxoions. J. Coord. Chem. 1994, 33, 319–329. [Google Scholar] [CrossRef]
- Abbadi, A.; Gotlieb, K.F.; Meiberg, J.B.M.; Peters, J.A.; van Bekkum, H. New Ca-sequestering materials. Based on the oxidation of the hydrolysis products of lactose. Green Chem. 1999, 1, 231–235. [Google Scholar] [CrossRef]
- Saladini, M.; Ferrari, E.; Menabue, L. Co-ordination of transition metal ions by galactaric acid: A potentiometric and spectroscopic study. J. Inorg. Biochem. 2002, 92, 121–127. [Google Scholar] [CrossRef]
- Lakatos, A.; Bertani, R.; Kiss, T.; Venzo, A.; Casarin, M.; Benetollo, F.; Ganis, P.; Favretto, D. AlIII ion complexes of saccharic acid and mucic acid: A solution and solid-state study. Chem. Eur. J. 2004, 10, 1281–1290. [Google Scholar] [CrossRef]
- Abrahams, B.F.; McCormick, L.J.; Moubaraki, B.; Murray, K.S.; Robson, R.; Waters, T. Two Cu21 clusters with pseudo-D3 symmetry derived from the d-saccharate pentaanion, C6H5O85−. Chem. Eur. J. 2011, 17, 7454–7459. [Google Scholar] [CrossRef]
- Koefod, R.S. Corrosion-inhibiting deicer composition. U.S. Patent 7658861B2, 9 February 2010. [Google Scholar]
- Wolfrom, M.L.; Toy, M.S.; Chaney, A. Condensation polymers from tetra-o-acetylgalactaroyl dichloride and diamines. J. Am. Chem. Soc. 1958, 80, 6328–6330. [Google Scholar] [CrossRef]
- Bird, T.P.; Black, W.A.P.; Dewar, E.T.; Hare, J.B. Polyamides containing carbohydrate residues. J. Chem. Soc. 1963, 1208–1212. [Google Scholar] [CrossRef]
- Bird, T.P.; Black, W.A.P.; Dewar, E.T.; Hare, J.B. Polyamides containing carbohydrate residues. Part II. Benzylidenedioxy-derivatives. J. Chem. Soc. 1963, 3389–3391. [Google Scholar] [CrossRef]
- Ogata, N.; Sanui, K.; Hosoda, Y.; Nakamura, H. Active polycondensation of diethyl 2, 3, 4, 5-tetrahydroxyadipate with diamines. J. Polym. Sci. Polym. Chem. Ed. 1976, 14, 783–792. [Google Scholar] [CrossRef]
- Ogata, N.; Sanui, K.; Kayama, Y. Copolycondensation of hydroxyl diesters and active diesters with hexamethylenediamine. J. Polym. Sci. Polym. Chem. Ed. 1977, 15, 1523–1526. [Google Scholar] [CrossRef]
- Ogata, N.; Sanui, K.; Hosoda, Y.; Nakamura, H.; Kishi, H. Polycondensation of diethyl mucate with hexamethylenediamine in the presence of poly(vinyl pyridine). J. Polym. Sci. Polym. Chem. Ed. 1980, 18, 933–938. [Google Scholar] [CrossRef]
- Kiely, D.E.; Chen, L.; Lin, T.-H. Hydroxylated nylons based on unprotected esterified d-glucaric acid by simple condensation reactions. J. Am. Chem. Soc. 1994, 116, 571–578. [Google Scholar] [CrossRef]
- Chen, L.; Kiely, D.E. Synthesis of stereoregular head, tail hydroxylated nylons derived from d-glucose. J. Org. Chem. 1996, 61, 5847–5851. [Google Scholar] [CrossRef]
- Morton, D.W.; Kiely, D.E. Evaluation of the film and adhesive properties of some block copolymer polyhydroxypolyamides from esterified aldaric acids and diamines. J. Appl. Polym. Sci. 2000, 77, 3085–3092. [Google Scholar] [CrossRef]
- Kiely, D.E.; Chen, L.; Lin, T.-H. Synthetic polyhydroxypolyamides from galactaric, xylaric, d-glucaric, and d-mannaric acids and alkylenediamine monomers—Some comparisons. J. Polym. Sci. A Polym. Chem. 2000, 38, 594–603. [Google Scholar] [CrossRef]
- Orgueira, H.A.; Varela, O. Synthesis and characterization of stereoregular AABB-type polymannaramides. J. Polym. Sci. A Polym. Chem. 2001, 39, 1024–1030. [Google Scholar] [CrossRef]
- Mancera, M.; Roffé, I.; Rivas, M.; Galbis, J.A. New derivatives of d-mannaric and galactaric acids: Synthesis of a new stereoregular Nylon 66 analog from carbohydrate-based monomers having the d-manno configuration. Carbohydr. Res. 2003, 338, 1115–1119. [Google Scholar] [CrossRef]
- Henkensmeier, D.; Abele, B.C.; Candussio, A.; Thiem, J. Synthesis, characterisation and degradability of polyamides derived from aldaric acids and chain end functionalised polydimethylsiloxanes. Polymer 2004, 45, 7053–7059. [Google Scholar] [CrossRef]
- Liu, Y.; Reineke, T.M. Hydroxyl stereochemistry and amine number within poly(glycoamidoamine)s affect intracellular DNA delivery. J. Am. Chem. Soc. 2005, 127, 3004–3015. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Guerra, S. Carbohydrate-based polyamides and polyesters: An overview illustrated with two selected examples. High Perform. Polym. 2012, 24, 9–23. [Google Scholar] [CrossRef]
- Black, W.A.P.; Dewar, E.T.; Hare, J.B. Polyesters containing carbohydrate residues. J. Chem. Soc. 1963, 5724–5727. [Google Scholar] [CrossRef]
- Zamora, F.; Hakkou, K.; Alla, A.; Rivas, M.; de Ilarduya, A.M.; Muñoz-Guerra, S.; Galbis, J.A. Butylene copolyesters based on aldaric and terephthalic acids. Synthesis and characterization. J. Polym. Sci. Part A Polym. Chem. 2009, 47, 1168–1177. [Google Scholar] [CrossRef]
- Lavilla, C.; Alla, A.; de Ilarduya, A.M.; Benito, E.; García-Martín, M.G.; Galbis, J.A.; Muñoz-Guerra, S. Carbohydrate-based polyesters made from bicyclic acetalized galactaric acid. Biomacromolecules 2011, 12, 2642–2652. [Google Scholar] [CrossRef]
- Lavilla, C.; Alla, A.; de Ilarduya, A.M.; Benito, E.; García-Martín, M.G.; Galbis, J.A.; Muñoz-Guerra, S. Bio-based poly(butylene terephthalate) copolyesters containing bicyclic diacetalized galactitol and galactaric acid: Influence of composition on properties. Polymer 2012, 53, 3432–3445. [Google Scholar] [CrossRef]
- Lavilla, C.; Alla, A.; de Ilarduya, A.M.; Benito, E.; García-Martín, M.G.; Galbis, J.A.; Muñoz-Guerra, S. Carbohydrate-based copolyesters made from bicyclic acetalized galactaric acid. J. Polym. Sci. Part A Polym. Chem. 2012, 50, 1591–1604. [Google Scholar] [CrossRef]
- Mehtiö, T.; Nurmi, L.; Rämö, V.; Mikkonen, H.; Harlin, A. Synthesis and characterization of copolyanhydrides of carbohydrate-based galactaric acid and adipic acid. Carbohydr. Res. 2015, 402, 102–110. [Google Scholar] [CrossRef]
- Pan, D.W.; Davis, M.E. Cationic mucic acid polymer-based siRNA delivery systems. Bioconjug. Chem. 2015, 26, 1791–1803. [Google Scholar] [CrossRef]
- Abrahams, B.F.; Moylan, M.; Orchard, S.D.; Robson, R. Zinc Saccharate: A Robust, 3D coordination network with two types of isolated, parallel channels, one hydrophilic and the other hydrophobic. Angew. Chem. Int. Ed. 2003, 42, 1848–1851. [Google Scholar] [CrossRef]
- Abrahams, B.F.; Moylan, M.; Orchard, S.D.; Robson, R. Channel-containing lanthanide mucate structures. CrystEngComm 2003, 5, 313–317. [Google Scholar] [CrossRef]
- Wong, K.-L.; Law, G.-L.; Yang, Y.-Y.; Wong, W.-T. A highly porous luminescent terbium–organic framework for reversible anion sensing. Adv. Mater. 2006, 18, 1051–1054. [Google Scholar] [CrossRef]
- Abrahams, B.F.; Grannas, M.J.; McCormick, L.J.; Robson, R.; Thistlethwaite, P.J. Chiral and achiral linear coordination polymers from aldaric acids. CrystEngComm 2010, 12, 2885–2895. [Google Scholar] [CrossRef]
- Busschaert, N.; Caltagirone, C.; van Rossom, W.; Gale, P.A. Applications of Supramolecular Anion Recognition. Chem. Rev. 2015, 115, 8038–8155. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, A.W.; Okawa, H.; Hashimoto, K. Synthesis of glycopolymers bearing mannaric pendants as inhibitors on the β-glucuronidase activity: The inhibition mechanisms of mannaric-and glucaric-compounds. J. Polym. Sci. Part A Polym. Chem. 2009, 47, 2032–2042. [Google Scholar] [CrossRef]
- Tian, L.; Yam, L.; Zhou, N.; Tat, H.; Uhrich, K.E. Amphiphilic Scorpion-like Macromolecules: Design, Synthesis, and Characterization. Macromolecules 2004, 37, 538–543. [Google Scholar] [CrossRef]
- Gu, L.; Faig, A.; Abdelhamid, D.; Uhrich, K. Sugar-based amphiphilic polymers for biomedical applications: From nanocarriers to therapeutics. Acc. Chem. Res. 2014, 47, 2867–2877. [Google Scholar] [CrossRef]
- Pohjanlehto, H.; Setälä, H.; Kammiovirta, K.; Harlin, A. The use of N, N′-diallylaldardiamides as cross-linkers in xylan derivatives-based hydrogels. Carbohydr. Res. 2011, 346, 2736–2745. [Google Scholar] [CrossRef]
- Calcium-D-Glucarate. Available online: https://www.ncbi.nlm.nih.gov/pubmed/12197785 (accessed on 25 July 2019).
- Butler, C.L.; Cretcher, L.H. The preparation of allomucic acid and certain of its derivatives. J. Am. Chem. Soc. 1929, 51, 2167–2170. [Google Scholar] [CrossRef]
- Williams, S.R.; Maynard, H.D.; Chmelka, B.F. Synthesis of 13C-enriched pyrrole from 2-13C d-galactose. J. Label. Compd. Radiopharm. 1999, 42, 927–936. [Google Scholar] [CrossRef]
- Pellissier, H. Syntheses of 1-induronyl synthons. A review. Org. Prep. Proced. Int. 2002, 34, 441–465. [Google Scholar] [CrossRef]
- Taguchi, Y.; Oishi, A.; Iida, H. One-step synthesis of dibutyl furandicarboxylates from galactaric acid. Chem. Lett. 2008, 37, 50–51. [Google Scholar] [CrossRef]
- Thiyagarajan, S.; Pukin, A.; van Haveren, J.; Lutz, M.; van Es, D.S. Concurrent formation of furan-2, 5-and furan-2, 4-dicarboxylic acid: Unexpected aspects of the Henkel reaction. RSC Adv. 2013, 3, 15678–15686. [Google Scholar] [CrossRef]
- Li, X.; Wu, D.; Lu, T.; Yi, G.; Su, H.; Zhang, Y. Highly efficient chemical process to convert mucic acid into adipic acid and DFT studies of the mechanism of the rhenium-catalyzed deoxydehydration. Angew. Chem. Int. Ed. 2014, 53, 4200–4204. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, X.; Su, X.; Ang, E.L.; Zhang, Y.; Zhao, H. Production of adipic acid from sugar beet residue by combined biological and chemical catalysis. ChemCatChem 2016, 8, 1500–1506. [Google Scholar] [CrossRef]
- Shin, N.; Kwon, S.; Moon, S.; Hong, C.H.; Kim, Y.G. Ionic liquid-mediated deoxydehydration reactions: Green synthetic process for bio-based adipic acid. Tetrahedron 2017, 73, 4758–4765. [Google Scholar] [CrossRef]
- Larson, R.T.; Samant, A.; Chen, J.; Lee, W.; Bohn, M.A.; Ohlmann, D.M.; Zuend, S.J.; Toste, F.D. Hydrogen gas-mediated deoxydehydration/hydrogenation of sugar acids: Catalytic conversion of glucarates to adipates. J. Am. Chem. Soc. 2017, 139, 14001–14004. [Google Scholar] [CrossRef]
- Jeffery, G.A.; Wood, R.A. The crystal structure of galactaric acid (mucic acid) at−147°: An unusually dense, hydrogen-bonded structure. Carbohydr. Res. 1982, 108, 205–211. [Google Scholar] [CrossRef]
- Taga, T.; Kuroda, Y.; Osaki, K. Interactions of calcium ions with carbohydrates: X-ray diffraction and NMR spectroscopic studies on the potassium salt and the calcium salt of d-glucaric acid. Bull. Chem. Soc. Jpn. 1977, 50, 3079–3083. [Google Scholar] [CrossRef]
- Sohst, O.; Tollens, B. Über krystallisirte Zuckersäure (Zuckerlactonsäure). Justus Liebig’s Ann. Chem. 1888, 245, 1–27. [Google Scholar] [CrossRef]
- Easterfield, T.H. XXXIV.—The oxidation of mannitol by nitric acid. d.-Mannosaccharic acid. J. Chem. Soc. Trans. 1891, 59, 306–310. [Google Scholar] [CrossRef]
- Smith, T.N.; Hash, K.; Davey, C.-L.; Mills, H.; Williams, H.; Kiely, D.E. Modifications in the nitric acid oxidation of d-glucose. Carbohydr. Res. 2012, 350, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Rautiainen, S.; Lehtinen, P.; Chen, J.; Leskelä, M.; Niemelä, K.; Leskelä, M.; Repo, T. Selective oxidation of uronic acids into aldaric acids over gold catalyst. RSC Adv. 2015, 5, 19502–19507. [Google Scholar] [CrossRef] [Green Version]
- Carpenter, C.A.; Hardcastle, K.I.; Kiely, D.E. Modifications in the nitric acid oxidation of d-mannose: X-ray crystal structure of N, N′-dimethyl d-mannaramide. Carbohydr. Res. 2013, 376, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Fauvarque, J.-F.; Mestre, M.; Trévin, S.; Marzouk, H.; Jud, J.-M. Recent industrial experiences with electroorganic synthesis-industrial electrosynthesis of an insoluble compound-galactaric acid. Actual. Chim. 1998, 214, 48–50. [Google Scholar]
- Rangappa, K.S.; Anitha, N.; Nikath, M.A.; Rai, K.M.L.; Gowda, N.M.M. Oxidation of uronic acids by manganese(III) sulfate in acid solution: A kinetic and mechanistic study. Synth. React. Inorg. Met. Org. Chem. 2001, 31, 713–723. [Google Scholar] [CrossRef]
- Merbouh, N.; Bobbitt, J.M.; Brückner, C. 4-AcNH-tempo-catalyzed oxidation of aldoses to aldaric acids using chlorine or bromine as terminal oxidants. J. Carbohydr. Chem. 2002, 21, 65–77. [Google Scholar] [CrossRef]
- Ibert, M.; Marsais, F.; Merbouh, N.; Brückner, C. Determination of the side-products formed during the nitroxide-mediated bleach oxidation of glucose to glucaric acid. Carbohydr. Res. 2002, 337, 1059–1063. [Google Scholar] [CrossRef]
- Marcq, O.; Barbe, J.-M.; Trichet, A.; Guilard, R. Reaction pathways of glucose oxidation by ozone under acidic conditions. Carbohydr. Res. 2009, 344, 1303–1310. [Google Scholar] [CrossRef] [PubMed]
- Bonhoure, J.-P.; Abboud, H.; Aussenac, T.R.; Coste, C.U.; Hoang, L.; Ralainirina, R.; Rannou, A.C. New method for synthesising mucic acid using ozone. WIPO WO2013144483A1, 18 March 2013. [Google Scholar]
- Glattfeld, J.W.E.; Gershon, S. The Catalytic Dehydrogenation of Sugar Alcohols. J. Am. Chem. Soc. 1938, 60, 2013–2023. [Google Scholar] [CrossRef]
- Mehltretter, C.L.; Rist, C.E.; Alexander, B.H. Process for the preparation of D-glucosaccharic acid. U.S. Patent 2472168A, 7 June 1949. [Google Scholar]
- Boussie, T.R.; Dias, E.L.; Fresco, Z.M.; Murphy, V.J.; Shoemaker, J.; Archer, R.; Jiang, H. Production of adipic acid and derivatives from carbohydrate-containing materials. WIPO WO2010144862A2, 16 December 2010. [Google Scholar]
- van Es, D.S.; van Haveren, J.; Raaijmakers, H.W.C.; van der Klis, F.; van Engelen, G.P.F.M.; Frissen, A.E. Catalytic oxidation of uronic acids to aldaric acids. WIPO WO2013151428A1, 10 October 2013. [Google Scholar]
- Hong, C.H.; Kim, S.H.; Kim, Y.G.; Shin, N.R. Method for producing glucaric acid. U.S. Patent 9227904B1, 5 January 2016. [Google Scholar]
- Marion, P.; Derrien, E.; Pinel, C.; Besson, M. Process for the preparation of a mixture of aldaric acids or salts thereof. EP3088378A1, 2 November 2016. [Google Scholar]
- Dirkn, J.M.H.; van der Baan, H.S.; van den Broen, J.M.A.J.J. The preparation of d-glucaric acid by the oxidation of d-gluconic acid catalysed by platinum on carbon. Carbohydr. Res. 1977, 59, 63–72. [Google Scholar] [CrossRef]
- Dirkn, J.M.H.; van der Baan, H.S. The oxidation of gluconic acid with platinum on carbon as catalyst. J. Catal. 1981, 67, 14–20. [Google Scholar]
- Besson, M.; Flèche, G.; Fuertes, P.; Gallezot, P.; Lahmer, F. Oxidation of glucose and gluconate on Pt, Pt Bi, and Pt Au catalysts. Recl. Trav. Chim. Pays Bas 1996, 115, 217–221. [Google Scholar] [CrossRef]
- Parpot, P.; Nunes, N.; Bettencourt, A.P. Electrocatalytic oxidation of monosaccharides on gold electrode in alkaline medium: Structure–reactivity relationship. J. Electroanal. Chem. 2006, 596, 65–73. [Google Scholar] [CrossRef]
- Bujak, P.; Bartczak, P.; Polanski, J. Highly efficient room-temperature oxidation of cyclohexene and d-glucose over nanogold Au/SiO2 in water. J. Catal. 2012, 295, 15–21. [Google Scholar] [CrossRef]
- Colmenares, J.C.; Magdziarz, A. Room temperature versatile conversion of biomass-derived compounds by means of supported TiO2 photocatalysts. J. Mol. Catal. A Chem. 2013, 366, 156–162. [Google Scholar] [CrossRef]
- Colmenares, J.C.; Magdziarz, A.; Chernyayeva, O.; Lisovytskiy, D.; Kurzydłowski, K.; Grzonka, J. Sonication-Assisted Low-Temperature Routes for the Synthesis of Supported Fe–TiO2 Econanomaterials: Partial Photooxidation of Glucose and Phenol Aqueous Degradation. ChemCatChem 2013, 5, 2270–2277. [Google Scholar] [CrossRef]
- van der Klis, F.; Frissen, A.E.; van Haveren, J.; van Es, D.S. Waste not, want not: Mild and selective catalytic oxidation of uronic acids. ChemSusChem 2013, 6, 1640–1645. [Google Scholar] [CrossRef]
- Bin, D.; Wang, H.; Li, J.; Wang, H.; Yin, Z.; Kang, J.; He, B.; Li, Z. Controllable oxidation of glucose to gluconic acid and glucaric acid using an electrocatalytic reactor. Electrochim. Acta 2014, 130, 170–178. [Google Scholar] [CrossRef]
- Jin, X.; Zhao, M.; Vora, M.; Shen, J.; Zeng, C.; Yan, W.; Thapa, P.S.; Subramaniam, B.; Chaudhari, R.V. Synergistic effects of bimetallic PtPd/TiO2 nanocatalysts in oxidation of glucose to glucaric acid: Structure dependent activity and selectivity. Ind. Eng. Chem. Res. 2016, 55, 2932–2945. [Google Scholar] [CrossRef]
- Lee, J.; Saha, B.; Vlachos, D.G. Pt catalysts for efficient aerobic oxidation of glucose to glucaric acid in water. Green Chem. 2016, 18, 3815–3822. [Google Scholar] [CrossRef]
- Megías-Sayago, C.; Carrasco, C.J.; Ivanova, S.; Montilla, F.J.; Galindo, A.; Odriozola, J.A. Influence of the ionic liquid presence on the selective oxidation of glucose over molybdenum based catalysts. Catal. Today 2016, 278, 82–90. [Google Scholar] [CrossRef]
- Solmi, S.; Morreale, C.; Ospitali, F.; Agnoli, S.; Cavani, F. Oxidation of d-glucose to glucaric acid using Au/C catalysts. ChemCatChem 2017, 9, 2797–2806. [Google Scholar] [CrossRef]
- Derrien, E.; Mounguengui-Diallo, M.; Perret, N.; Marion, P.; Pinel, C.; Besson, M. Aerobic oxidation of glucose to glucaric acid under alkaline-free conditions: Au-based bimetallic catalysts and the effect of residues in a hemicellulose hydrolysate. Ind. Eng. Chem. Res. 2017, 56, 13175–13189. [Google Scholar] [CrossRef]
- Purushothaman, R.K.P.; van der Klis, F.; Frissen, A.E.; van Haveren, J.; Mayoral, A.; van der Bent, A.; van Es, D.S. Base-free selective oxidation of pectin derived galacturonic acid to galactaric acid using supported gold catalysts. Green Chem. 2018, 20, 2763–2774. [Google Scholar] [CrossRef]
- Armstrong, R.D.; Hirayama, J.; Knight, D.W.; Hutchings, G.J. Quantitative Determination of Pt-Catalyzed d-Glucose Oxidation Products Using 2D NMR. ACS Catal. 2019, 9, 325–335. [Google Scholar] [CrossRef]
- Chen, R.; Yang, C.; Zhang, Q.; Zhang, B.; Deng, K. Visible-light-driven selective oxidation of glucose in water with H-ZSM-5 zeolite supported biomimetic photocatalyst. J. Catal. 2019, 374, 297–305. [Google Scholar] [CrossRef]
- Besson, M.; Lahmer, F.; Gallezot, P.; Fuertes, P.; Fleche, G. Catalytic Oxidation of Glucose on Bismuth-Promoted Palladium Catalysts. J. Catal. 1995, 152, 116–121. [Google Scholar] [CrossRef]
- Mojzita, D.; Wiebe, M.; Hilditch, S.; Boer, H.; Penttilä, M.; Richard, P. Metabolic engineering of fungal strains for conversion of d-galacturonate to meso-galactarate. Appl. Environ. Microbiol. 2010, 76, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Hassid, W.Z.; Putman, E.W. Transformation of sugars in plants. Annu. Rev. Plant Biol. 1950, 1, 109–124. [Google Scholar] [CrossRef]
- Lin, T.-Y.; Hassid, W.Z. Pathway of alginic acid synthesis in the marine brown alga, Fucus gardneri silva. J. Biol. Chem. 1966, 241, 5284–5297. [Google Scholar] [PubMed]
- Nyvall, P.; Corre, E.; Boisset, C.; Barbeyron, T.; Rousvoal, S.; Scornet, D.; Kloareg, B.; Boyen, C. Characterization of mannuronan C-5-epimerase genes from the brown alga Laminaria digitata. Plant Physiol. 2003, 133, 726–735. [Google Scholar] [CrossRef] [PubMed]
- Rehm, B.H.A. Bacterial polymers: Biosynthesis, modifications and applications. Nat. Rev. Microbiol. 2010, 8, 578–592. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Shao, Z.; Jin, W.; Duan, D. Comparative characterization of two GDP-mannose dehydrogenase genes from Saccharina japonica (Laminariales, Phaeophyceae). BMC Plant Biol. 2016, 16, 62. [Google Scholar] [CrossRef] [PubMed]
- Moon, T.S.; Yoon, S.-H.; Lanza, A.M.; Roy-Mayhew, J.D.; Prather, K.L.J. Production of glucaric acid from a synthetic pathway in recombinant Escherichia coli. Appl. Environ. Microbiol. 2009, 75, 589–595. [Google Scholar] [CrossRef] [PubMed]
- Lippow, S.M.; Moon, T.S.; Basu, S.; Yoon, S.-H.; Li, X.; Chapman, X.; Robison, K.; Lipovšek, D.; Prather, K.L.J. Engineering enzyme specificity using computational design of a defined-sequence library. Chem. Biol. 2010, 17, 1306–1315. [Google Scholar] [CrossRef] [PubMed]
- Shiue, E.; Prather, K.L.J. Improving d-glucaric acid production from myo-inositol in E. coli by increasing MIOX stability and myo-inositol transport. Metab. Eng. 2014, 22, 22–31. [Google Scholar] [CrossRef]
- Shiue, E.; Brockman, I.M.; Prather, K.L.J. Improving product yields on D-glucose in Escherichia coli via knockout of pgi and zwf and feeding of supplemental carbon sources. Biotechnol. Bioeng. 2015, 112, 579–587. [Google Scholar] [CrossRef]
- Reizman, I.M.B.; Stenger, A.R.; Reisch, C.R.; Gupta, A.; Connors, N.C.; Prather, K.L.J. Improvement of glucaric acid production in E. coli via dynamic control of metabolic fluxes. Metab. Eng. Commun. 2015, 2, 109–116. [Google Scholar] [CrossRef]
- Gupta, A.; Hicks, M.A.; Manchester, S.P.; Prather, K.L.J. Porting the synthetic d-glucaric acid pathway from Escherichia coli to Saccharomyces cerevisiae. Biotechnol. J. 2016, 11, 1201–1208. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Reizman, I.M.B.; Reisch, C.R.; Prather, K.L.J. Dynamic regulation of metabolic flux in engineered bacteria using a pathway-independent quorum-sensing circuit. Nat. Biotechnol. 2017, 35, 273–279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schoevaart, R.; Kieboom, T. Galactose dialdehyde: The forgotten candidate for a protein cross-linker? Carbohydr. Res. 2001, 334, 1–6. [Google Scholar] [CrossRef]
- Schoevaart, R.; Kieboom, T. Galactose dialdehyde as potential protein cross-linker: Proof of principle. Carbohydr. Res. 2002, 337, 899–904. [Google Scholar] [CrossRef]
- Schoevaart, R.; Kieboom, T. Application of galactose oxidase in chemoenzymatic one-pot cascade reactions without intermediate recovery steps. Top. Catal. 2004, 27, 3–9. [Google Scholar] [CrossRef]
- Sun, L.; Bulter, T.; Alcalde, M.; Petrounia, I.P.; Arnold, F.H. Modification of galactose oxidase to introduce glucose 6-oxidase activity. ChemBioChem 2002, 3, 781–783. [Google Scholar] [CrossRef]
- Moon, T.S.; Nielsen, D.R.; Prather, K.L.J. Sensitivity analysis of a proposed model mechanism for newly created glucose-6-oxidases. AIChE J. 2012, 58, 2303–2308. [Google Scholar] [CrossRef]
- Richard, P.; Hilditch, S. d-galacturonic acid catabolism in microorganisms and its biotechnological relevance. Appl. Microbiol. Biotechnol. 2009, 2, 597–604. [Google Scholar] [CrossRef]
- Pedrolli, D.B.; Monteiro, A.C.; Gomes, E.; Carmona, E.C. Pectin and pectinases: Production, characterization and industrial application of microbial pectinolytic enzymes. Open Biotechnol. J. 2009, 3, 9–18. [Google Scholar] [CrossRef]
- Voragen, A.G.J.; Coenen, G.-J.; Verhoef, R.P.; Schols, H.A. Pectin, a versatile polysaccharide present in plant cell walls. Struct. Chem. 2009, 20, 263–275. [Google Scholar] [CrossRef] [Green Version]
- Babbar, N.; Dejonghe, W.; Gatti, M.; Sforza, S.; Elst, K. Pectic oligosaccharides from agricultural by-products: Production, characterization and health benefits. Crit. Rev. Biotechnol. 2016, 36, 594–606. [Google Scholar] [CrossRef] [PubMed]
- D’Ayala, G.G.; Malinconico, M.; Laurienzo, P. Marine derived polysaccharides for biomedical applications: Chemical modification approaches. Molecules 2008, 13, 2069–2106. [Google Scholar] [CrossRef] [PubMed]
- Vera, J.; Castro, J.; Gonzalez, A.; Moenne, A. Seaweed polysaccharides and derived oligosaccharides stimulate defense responses and protection against pathogens in plants. Mar. Drugs 2011, 9, 2514–2525. [Google Scholar] [CrossRef] [PubMed]
- Foley, P.M.; Beach, E.S.; Zimmerman, J.B. Algae as a source of renewable chemicals: Opportunities and challenges. Green Chem. 2011, 13, 1399–1405. [Google Scholar] [CrossRef]
- Kerton, F.M.; Liu, Y.; Omari, K.W.; Hawboldt, K. Green chemistry and the ocean-based biorefinery. Green Chem. 2013, 15, 860–871. [Google Scholar] [CrossRef] [Green Version]
- Michalak, I.; Chojnacka, K. Algae as production systems of bioactive compounds. Eng. Life Sci. 2015, 15, 160–176. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saha, B.C. Hemicellulose bioconversion. J. Ind. Microbiol. Biotechnol. 2003, 30, 279–291. [Google Scholar] [CrossRef] [PubMed]
- Scheller, H.V.; Ulvskov, P. Hemicelluloses. Annu. Rev. Plant Biol. 2010, 61, 263–289. [Google Scholar] [CrossRef]
- Lahaye, M.; Robic, A. Structure and functional properties of ulvan, a polysaccharide from green seaweeds. Biomacromolecules 2007, 8, 1765–1774. [Google Scholar] [CrossRef] [PubMed]
- Benz, J.P.; Protzko, R.J.; Andrich, J.M.S.; Bauer, S.; Dueber, J.E.; Somerville, C.R. Identification and characterization of a galacturonic acid transporter from Neurospora crassa and its application for Saccharomyces cerevisiae fermentation processes. Biotechnol. Biofuels 2014, 7, 20. [Google Scholar] [CrossRef] [PubMed]
- Biz, A.; Sugai-Guérios, M.H.; Kuivanen, J.; Maaheimo, H.; Krieger, N.; Mitchell, D.A.; Richard, P. The introduction of the fungal d-galacturonate pathway enables the consumption of d-galacturonic acid by Saccharomyces cerevisiae. Microb. Cell Fact. 2016, 15, 144. [Google Scholar] [CrossRef] [PubMed]
- Kuivanen, J.; Wang, Y.M.J.; Richard, P. Engineering Aspergillus niger for galactaric acid production: Elimination of galactaric acid catabolism by using RNA sequencing and CRISPR/Cas9. Microb. Cell Fact. 2016, 15, 210. [Google Scholar] [CrossRef] [PubMed]
- Barth, D.; Wiebe, M.G. Enhancing fungal production of galactaric acid. Appl. Microbiol. Biotechnol. 2017, 101, 4033–4040. [Google Scholar] [CrossRef] [PubMed]
- Leh, D.S.; Biz, A.; de Paula, D.H.F.; Richard, P.; Gonçalves, A.G.; Noseda, M.D.; Mitchell, D.A.; Krieger, N. Conversion of citric pectin into d-galacturonic acid with high substrate loading using a fermented solid with pectinolytic activity. Biocatal. Agric. Biotechnol. 2017, 11, 214–219. [Google Scholar] [CrossRef]
- Paasikallio, T.; Huuskonen, A.; Wiebe, M.G. Scaling up and scaling down the production of galactaric acid from pectin using Trichoderma reesei. Microb. Cell Fact. 2017, 16, 119. [Google Scholar] [CrossRef] [PubMed]
- Sakuta, R.; Takeda, K.; Igarashi, K.; Ohno, H.; Nakamura, N. Pyrroloquinoline quinone-dependent glucose dehydrogenase anode: d-Galacturonic acid oxidation and galactaric acid production. J. Mol. Catal. B Enzym. 2016, 133, S76–S79. [Google Scholar] [CrossRef]
- Sakuta, R.; Takeda, K.; Igarashi, K.; Ohno, H.; Nakamura, N. Enzymes suitable for biorefinery to coproduce hexaric acids and electricity from hexuronic acids derived from biomass. Energy Technol. 2018, 6, 273–279. [Google Scholar] [CrossRef]
- Sakuta, R. Simultaneous production of biomass-derived platform chemicals and electricity. Ph.D. Thesis, Tokyo University of Agriculture and Technology, Tokyo, Japan, 27 March 2018. [Google Scholar]
- Amaniampong, P.N.; Karam, A.; Trinh, Q.T.; Xu, K.; Hirao, H.; Jérôme, F.; Chatel, G. Selective and catalyst-free oxidation of d-glucose to d-glucuronic acid induced by high-frequency ultrasound. Sci. Rep. 2017, 7, 40650. [Google Scholar] [CrossRef] [PubMed]
- Wojcieszak, R.; Cuccovia, I.M.; Silva, M.A.; Rossi, L.M. Selective oxidation of glucose to glucuronic acid by cesium-promoted gold nanoparticle catalyst. J. Mol. Catal. A Chem. 2016, 422, 35–42. [Google Scholar] [CrossRef]









| Polymers | Yield | mp (°C) a | Water Solubility b | Mnc |
|---|---|---|---|---|
| Poly(ethylene d-glucaramide) | 93.3 | 185 | Yes | 2036 |
| Poly(tetramethylene d-glucaramide) | 88.3 | 192–195 | Yes | 2725 |
| Poly(hexamethylene d-glucaramide) | 89.4 | 192–194 | No | 2552 |
| Poly(octamethylene d-glucaramide) | 86.8 | 195–200 | No | 3562 |
| Poly(decamethylene d-glucaramide) | 89.8 | 200–205 | No | 3218 |
| Poly(dodecamethylene d-glucaramide) | 93.9 | 200–205 | No | 2730 |
| Poly(ethylene galactaramide) | 90.1 | 205 | VS | 702 |
| Poly(tetramethylene galactaramide) | 82.1 | 230 | SS | 1179 |
| Poly(hexamethylene galactaramide) | 85.2 | 228 | NS | 2320 |
| Poly(octamethylene galactaramide) | 79.4 | 230 | NS | 4920 |
| Poly(decamethylene galactaramide) | 82.2 | 230 | NS | 2560 |
| Poly(dodecamethylene galactaramide) | 87.4 | 225 | NS | 2356 |
| Poly(ethylene d-mannaramide) | 72 | 180–206 | VS | 1410 |
| Poly(tetramethylene d-mannaramide) | 77 | 194–201 | VS | 1249 |
| Poly(hexamethylene d-mannaramide) | 68 | 205–210 | NS | 1247 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sakuta, R.; Nakamura, N. Production of Hexaric Acids from Biomass. Int. J. Mol. Sci. 2019, 20, 3660. https://doi.org/10.3390/ijms20153660
Sakuta R, Nakamura N. Production of Hexaric Acids from Biomass. International Journal of Molecular Sciences. 2019; 20(15):3660. https://doi.org/10.3390/ijms20153660
Chicago/Turabian StyleSakuta, Riku, and Nobuhumi Nakamura. 2019. "Production of Hexaric Acids from Biomass" International Journal of Molecular Sciences 20, no. 15: 3660. https://doi.org/10.3390/ijms20153660
APA StyleSakuta, R., & Nakamura, N. (2019). Production of Hexaric Acids from Biomass. International Journal of Molecular Sciences, 20(15), 3660. https://doi.org/10.3390/ijms20153660