Role of the Monocyte–Macrophage System in Normal Pregnancy and Preeclampsia
Abstract
:1. Introduction
2. Monocyte–Macrophage System during Pregnancy
2.1. Monocyte–Macrophage System: Developmental Origins and Cell Lineage
2.2. The Role of Monocytes in Pregnancy
2.3. The Role of Macrophages in Female Reproductive System Prior to and during Pregnancy
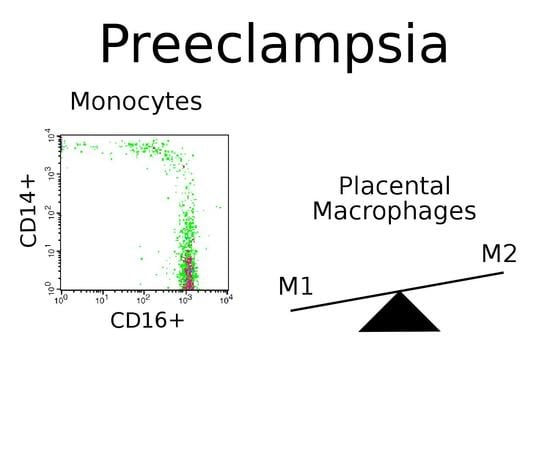
2.4. Monocytes in Preeclampsia
2.5. Macrophages in Preeclampsia
2.6. Potential Therapeutic Approaches
3. Summary and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| PE | Preeclampsia |
| IUGR | Intrauterine growth restriction |
| Arg1 | Arginase 1 |
| Treg | Regulatory T cells |
| Th2 | T helper 2 |
References
- World Health Organization WHO Recommendations for Prevention and Treatment of Pre-Eclampsia and Eclampsia. Available online: http://www.ncbi.nlm.nih.gov/books/NBK140561/ (accessed on 23 May 2016).
- Saito, S. Preeclampsia Basic, Genomic, and Clinical; Springer Nature Singapore Pte Ltd.: Singapore, 2018. [Google Scholar]
- Cornelius, D.C. Preeclampsia: From inflammation to immunoregulation. Clin. Med. Insights Blood Disord. 2018, 11. [Google Scholar] [CrossRef] [PubMed]
- Chazaud, B. Macrophages: Supportive cells for tissue repair and regeneration. Immunobiology 2014, 219, 172–178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perdiguero, E.G.; Geissmann, F. The development and maintenance of resident macrophages. Nat. Immunol. 2016, 17, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Hoeffel, G.; Chen, J.; Lavin, Y.; Low, D.; Almeida, F.F.; See, P.; Beaudin, A.E.; Lum, J.; Low, I.; Forsberg, E.C.; et al. C-Myb(+) erythro-myeloid progenitor-derived fetal monocytes give rise to adult tissue-resident macrophages. Immunity 2015, 42, 665–678. [Google Scholar] [CrossRef] [PubMed]
- Epelman, S.; Lavine, K.J.; Randolph, G.J. Origin and Functions of Tissue Macrophages. Immunity 2014, 41, 21–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ziegler-Heitbrock, L. Monocyte subsets in man and other species. Cell. Immunol. 2014, 289, 135–139. [Google Scholar] [CrossRef]
- Mildner, A.; Marinkovic, G.; Jung, S. Murine Monocytes: Origins, Subsets, Fates, and Functions. Microbiol. Spectr. 2016, 4. [Google Scholar] [Green Version]
- Wong, K.L.; Tai, J.J.; Wong, W.C.; Han, H.; Sem, X.; Yeap, W.H.; Kourilsky, P.; Wong, S.C. Gene expression profiling reveals the defining features of the classical, intermediate, and nonclassical human monocyte subsets. Blood 2011, 118, e16–e31. [Google Scholar] [CrossRef] [Green Version]
- Godin, I.; Cumano, A. The hare and the tortoise: An embryonic haematopoietic race. Nat. Rev. Immunol. 2002, 2, 593–604. [Google Scholar] [CrossRef]
- Röszer, T. Understanding the Biology of Self-Renewing Macrophages. Cells 2018, 7, 103. [Google Scholar] [CrossRef]
- Martinez, F.O.; Gordon, S. The M1 and M2 paradigm of macrophage activation: Time for reassessment. F1000Prime Rep. 2014, 6, 13. [Google Scholar] [CrossRef] [PubMed]
- Okizaki, S.; Ito, Y.; Hosono, K.; Oba, K.; Ohkubo, H.; Amano, H.; Shichiri, M.; Majima, M. Suppressed recruitment of alternatively activated macrophages reduces TGF-β1 and impairs wound healing in streptozotocin-induced diabetic mice. Biomed. Pharmacother. 2015, 70, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, H.; Uchida, K.; Guerrero, A.R.; Watanabe, S.; Sugita, D.; Takeura, N.; Yoshida, A.; Long, G.; Wright, K.T.; Johnson, W.E.B.; et al. Transplantation of Mesenchymal Stem Cells Promotes an Alternative Pathway of Macrophage Activation and Functional Recovery after Spinal Cord Injury. J. Neurotrauma 2012, 29, 1614–1625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singla, D.K.; Singla, R.D.; Abdelli, L.S.; Glass, C. Fibroblast Growth Factor-9 Enhances M2 Macrophage Differentiation and Attenuates Adverse Cardiac Remodeling in the Infarcted Diabetic Heart. PLoS ONE 2015, 10, e0120739. [Google Scholar] [CrossRef] [PubMed]
- Murray, P.J.; Wynn, T.A. Obstacles and opportunities for understanding macrophage polarization. J. Leukoc. Biol. 2011, 89, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Kiguchi, N.; Kobayashi, Y.; Saika, F.; Sakaguchi, H.; Maeda, T.; Kishioka, S. Peripheral interleukin-4 ameliorates inflammatory macrophage-dependent neuropathic pain. Pain 2015, 156, 684–693. [Google Scholar] [CrossRef]
- Malyshev, I.; Malyshev, Y. Current Concept and Update of the Macrophage Plasticity Concept: Intracellular Mechanisms of Reprogramming and M3 Macrophage “Switch” Phenotype. Biomed. Res. Int. 2015, 2015, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Mills, C.D. Anatomy of a Discovery: M1 and M2 Macrophages. Front. Immunol. 2015, 6, 212. [Google Scholar] [CrossRef]
- Yao, Y.; Xu, X.H.; Jin, L. Macrophage Polarization in Physiological and Pathological Pregnancy. Front. Immunol. 2019, 10, 792. [Google Scholar] [CrossRef]
- Melgert, B.N.; Spaans, F.; Borghuis, T.; Klok, P.A.; Groen, B.; Bolt, A.; de Vos, P.; van Pampus, M.G.; Wong, T.Y.; van Goor, H.; et al. Pregnancy and Preeclampsia Affect Monocyte Subsets in Humans and Rats. PLoS ONE 2012, 7, e45229. [Google Scholar] [CrossRef]
- Al-ofi, E.; Coffelt, S.B.; Anumba, D.O. Monocyte subpopulations from pre-eclamptic patients are abnormally skewed and exhibit exaggerated responses to toll-like receptor ligands. PLoS ONE 2012, 7, e42217. [Google Scholar] [CrossRef] [PubMed]
- Lampé, R.; Kövér, Á.; Szűcs, S.; Pál, L.; Árnyas, E.; Ádány, R.; Póka, R. Phagocytic index of neutrophil granulocytes and monocytes in healthy and preeclamptic pregnancy. J. Reprod. Immunol. 2015, 107, 26–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faas, M.M.; de Vos, P. Maternal monocytes in pregnancy and preeclampsia in humans and in rats. J. Reprod. Immunol. 2017, 119, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Faas, M.M.; Spaans, F.; De Vos, P. Monocytes and macrophages in pregnancy and pre-eclampsia. Front. Immunol. 2014, 5, 298. [Google Scholar] [CrossRef] [PubMed]
- Nonn, O.; Güttler, J.; Forstner, D.; Maninger, S.; Zadora, J.; Balogh, A.; Frolova, A.; Glasner, A.; Herse, F.; Gauster, M. Placental CX3CL1 is Deregulated by Angiotensin II and Contributes to a Pro-Inflammatory Trophoblast-Monocyte Interaction. Int. J. Mol. Sci. 2019, 20, 641. [Google Scholar] [CrossRef] [PubMed]
- Siwetz, M.; Sundl, M.; Kolb, D.; Hiden, U.; Herse, F.; Huppertz, B.; Gauster, M. Placental fractalkine mediates adhesion of THP-1 monocytes to villous trophoblast. Histochem. Cell Biol. 2015, 143, 565–574. [Google Scholar] [CrossRef] [Green Version]
- Hung, T.-H.; Charnock-Jones, D.S.; Skepper, J.N.; Burton, G.J. Secretion of tumor necrosis factor-alpha from human placental tissues induced by hypoxia-reoxygenation causes endothelial cell activation in vitro: A potential mediator of the inflammatory response in preeclampsia. Am. J. Pathol. 2004, 164, 1049–1061. [Google Scholar] [CrossRef]
- Sacks, G.P.; Clover, L.M.; Bainbridge, D.R.J.; Redman, C.W.G.; Sargent, I.L. Flow Cytometric Measurement of Intracellular Th1 and Th2 Cytokine Production by Human Villous and Extravillous Cytotrophoblast. Placenta 2001, 22, 550–559. [Google Scholar] [CrossRef]
- Dragovic, R.A.; Collett, G.P.; Hole, P.; Ferguson, D.J.P.; Redman, C.W.; Sargent, I.L.; Tannetta, D.S. Isolation of syncytiotrophoblast microvesicles and exosomes and their characterisation by multicolour flow cytometry and fluorescence Nanoparticle Tracking Analysis. Methods 2015, 87, 64–74. [Google Scholar] [CrossRef]
- Göhner, C.; Fledderus, J.; Fitzgerald, J.S.; Schleußner, E.; Markert, U.R.; Scherjon, S.A.; Plösch, T.; Faas, M.M. Syncytiotrophoblast exosomes guide monocyte maturation and activation of monocytes and granulocytes. Placenta 2015, 36, A47–A48. [Google Scholar] [CrossRef]
- Sokolov, D.I.; Ovchinnikova, O.M.; Korenkov, D.A.; Viknyanschuk, A.N.; Benken, K.A.; Onokhin, K.V.; Selkov, S.A. Influence of peripheral blood microparticles of pregnant women with preeclampsia on the phenotype of monocytes. Transl. Res. 2016, 170, 112–123. [Google Scholar] [CrossRef] [PubMed]
- Tannetta, D.; Collett, G.; Vatish, M.; Redman, C.; Sargent, I. Syncytiotrophoblast extracellular vesicles–Circulating biopsies reflecting placental health. Placenta 2017, 52, 134–138. [Google Scholar] [CrossRef] [PubMed]
- Lang, T.J. Estrogen as an immunomodulator. Clin. Immunol. 2004, 113, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Janis, K.; Hoeltke, J.; Nazareth, M.; Fanti, P.; Poppenberg, K.; Aronica, S.M. Estrogen decreases expression of chemokine receptors, and suppresses chemokine bioactivity in murine monocytes. Am. J. Reprod. Immunol. 2004, 51, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Kramer, P.R.; Kramer, S.F.; Guan, G. 17?-estradiol regulates cytokine release through modulation of CD16 expression in monocytes and monocyte-derived macrophages. Arthritis Rheum. 2004, 50, 1967–1975. [Google Scholar] [CrossRef] [PubMed]
- Margaryan, S.; Hyusyan, A.; Martirosyan, A.; Sargsian, S.; Manukyan, G. Differential modulation of innate immune response by epinephrine and estradiol. Horm. Mol. Biol. Clin. Investig. 2017, 30. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.-H.; Kim, S.-C.; Joo, J.-K.; Kim, H.-G.; Na, Y.-J.; Kwak, J.-Y.; Lee, K.-S. Effects of 17β-estradiol on the release of monocyte chemotactic protein-1 and MAPK activity in monocytes stimulated with peritoneal fluid from endometriosis patients. J. Obstet. Gynaecol. Res. 2012, 38, 516–525. [Google Scholar] [CrossRef] [PubMed]
- Morishita, M.; Miyagi, M.; Iwamoto, Y. Effects of Sex Hormones on Production of Interleukin-1 by Human Peripheral Monocytes. J. Periodontol. 1999, 70, 757–760. [Google Scholar] [CrossRef]
- Habib, P.; Dreymueller, D.; Rösing, B.; Botung, H.; Slowik, A.; Zendedel, A.; Habib, S.; Hoffmann, S.; Beyer, C. Estrogen serum concentration affects blood immune cell composition and polarization in human females under controlled ovarian stimulation. J. Steroid Biochem. Mol. Biol. 2018, 178, 340–347. [Google Scholar] [CrossRef]
- Pelekanou, V.; Kampa, M.; Kiagiadaki, F.; Deli, A.; Theodoropoulos, P.; Agrogiannis, G.; Patsouris, E.; Tsapis, A.; Castanas, E.; Notas, G. Estrogen anti-inflammatory activity on human monocytes is mediated through cross-talk between estrogen receptor ERα36 and GPR30/GPER1. J. Leukoc. Biol. 2016, 99, 333–347. [Google Scholar] [CrossRef]
- Mor, G.; Sapi, E.; Abrahams, V.M.; Rutherford, T.; Song, J.; Hao, X.-Y.; Muzaffar, S.; Kohen, F. Interaction of the estrogen receptors with the Fas ligand promoter in human monocytes. J. Immunol. 2003, 170, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Pepe, G.; Locati, M.; Della Torre, S.; Mornata, F.; Cignarella, A.; Maggi, A.; Vegeto, E. The estrogen–macrophage interplay in the homeostasis of the female reproductive tract. Hum. Reprod. Update 2018, 24, 652–672. [Google Scholar] [CrossRef] [PubMed]
- Thiruchelvam, U.; Dransfield, I.; Saunders, P.T.K.; Critchley, H.O.D. The importance of the macrophage within the human endometrium. J. Leukoc. Biol. 2013, 93, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Cousins, F.L.; Kirkwood, P.M.; Saunders, P.T.K.; Gibson, D.A. Evidence for a dynamic role for mononuclear phagocytes during endometrial repair and remodelling. Sci. Rep. 2016, 6, 36748. [Google Scholar] [CrossRef] [Green Version]
- Hunt, J.S.; Robertson, S.A. Uterine macrophages and environmental programming for pregnancy success. J. Reprod. Immunol. 1996, 32, 1–25. [Google Scholar] [CrossRef]
- Wira, C.R.; Fahey, J.V.; Rodriguez-Garcia, M.; Shen, Z.; Patel, M.V. Regulation of Mucosal Immunity in the Female Reproductive Tract: The Role of Sex Hormones in Immune Protection Against Sexually Transmitted Pathogens. Am. J. Reprod. Immunol. 2014, 72, 236–258. [Google Scholar] [CrossRef] [Green Version]
- PrabhuDas, M.; Bonney, E.; Caron, K.; Dey, S.; Erlebacher, A.; Fazleabas, A.; Fisher, S.; Golos, T.; Matzuk, M.; McCune, J.M.; et al. Immune mechanisms at the maternal-fetal interface: Perspectives and challenges. Nat. Immunol. 2015, 16, 328–334. [Google Scholar] [CrossRef]
- Schonkeren, D.; Van Der Hoorn, M.L.; Khedoe, P.; Swings, G.; Van Beelen, E.; Claas, F.; Van Kooten, C.; De Heer, E.; Scherjon, S. Differential distribution and phenotype of decidual macrophages in preeclamptic versus control pregnancies. Am. J. Pathol. 2011, 178, 709–717. [Google Scholar] [CrossRef]
- Smith, S.D.; Dunk, C.E.; Aplin, J.D.; Harris, L.K.; Jones, R.L. Evidence for Immune Cell Involvement in Decidual Spiral Arteriole Remodeling in Early Human Pregnancy. Am. J. Pathol. 2009, 174, 1959–1971. [Google Scholar] [CrossRef] [Green Version]
- Lash, G.E.; Pitman, H.; Morgan, H.L.; Innes, B.A.; Agwu, C.N.; Bulmer, J.N. Decidual macrophages: Key regulators of vascular remodeling in human pregnancy. J. Leukoc. Biol. 2016, 100, 315–325. [Google Scholar] [CrossRef]
- Hazan, A.D.; Smith, S.D.; Jones, R.L.; Whittle, W.; Lye, S.J.; Dunk, C.E. Vascular-Leukocyte Interactions. Am. J. Pathol. 2010, 177, 1017–1030. [Google Scholar] [CrossRef]
- Chen, Q.; Guo, F.; Jin, H.Y.; Lau, S.; Stone, P.; Chamley, L. Phagocytosis of apoptotic trophoblastic debris protects endothelial cells against activation. Placenta 2012, 33, 548–553. [Google Scholar] [CrossRef]
- Mor, G.; Cardenas, I.; Abrahams, V.; Guller, S. Inflammation and pregnancy: The role of the immune system at the implantation site. Ann. N. Y. Acad. Sci. 2011, 1221, 80–87. [Google Scholar] [CrossRef]
- Vargas-Rojas, M.I.; Solleiro-Villavicencio, H.; Soto-Vega, E. Th1, Th2, Th17 and Treg levels in umbilical cord blood in preeclampsia. J. Matern. Neonatal Med. 2016, 29, 1642–1645. [Google Scholar] [CrossRef]
- Reinhard, G.; Noll, A.; Schlebusch, H.; Mallmann, P.; Ruecker, A.V. Shifts in the TH1/TH2 Balance during Human Pregnancy Correlate with Apoptotic Changes. Biochem. Biophys. Res. Commun. 1998, 245, 933–938. [Google Scholar] [CrossRef]
- Piccinni, M.P.; Lombardelli, L.; Logiodice, F.; Kullolli, O.; Romagnani, S.; Bouteiller, P. Le T helper cell mediated-tolerance towards fetal allograft in successful pregnancy. Clin. Mol. Allergy 2015, 13, 9. [Google Scholar] [CrossRef]
- Bogdanova, I.M.; Boltovskaya, M.N. Immune control of reproduction, the role of regulatory t-cells in the induction of immunological tolerance during pregnancy. Clin. Exp. Morphol. 2015, 3, 59–67. [Google Scholar]
- La Rocca, C.; Carbone, F.; Longobardi, S.; Matarese, G. The immunology of pregnancy: Regulatory T cells control maternal immune tolerance toward the fetus. Immunol. Lett. 2014, 162, 41–48. [Google Scholar] [CrossRef]
- Sykes, L.; MacIntyre, D.A.; Yap, X.J.; Teoh, T.G.; Bennett, P.R. The Th1:Th2 Dichotomy of Pregnancy and Preterm Labour. Mediat. Inflamm. 2012, 2012, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Berkane, N.; Liere, P.; Oudinet, J.-P.; Hertig, A.; Lefèvre, G.; Pluchino, N.; Schumacher, M.; Chabbert-Buffet, N. From Pregnancy to Preeclampsia: A Key Role for Estrogens. Endocr. Rev. 2017, 38, 123–144. [Google Scholar] [CrossRef]
- Napso, T.; Yong, H.E.J.; Lopez-Tello, J.; Sferruzzi-Perri, A.N. The Role of Placental Hormones in Mediating Maternal Adaptations to Support Pregnancy and Lactation. Front. Physiol. 2018, 9, 1091. [Google Scholar] [CrossRef]
- Schmidt, M.; Kreutz, M.; Löffler, G.; Schölmerich, J.; Straub, R.H. Conversion of dehydroepiandrosterone to downstream steroid hormones in macrophages. J. Endocrinol. 2000, 164, 161–169. [Google Scholar] [CrossRef]
- Tang, Z.; Tadesse, S.; Norwitz, E.; Mor, G.; Abrahams, V.M.; Guller, S. Isolation of hofbauer cells from human term placentas with high yield and purity. Am. J. Reprod. Immunol. 2011, 66, 336–348. [Google Scholar] [CrossRef]
- Trenti, A.; Tedesco, S.; Boscaro, C.; Trevisi, L.; Bolego, C.; Cignarella, A. Estrogen, Angiogenesis, Immunity and Cell Metabolism: Solving the Puzzle. Int. J. Mol. Sci. 2018, 19, 859. [Google Scholar] [CrossRef]
- Villa, A.; Rizzi, N.; Vegeto, E.; Ciana, P.; Maggi, A. Estrogen accelerates the resolution of inflammation in macrophagic cells. Sci. Rep. 2015, 5, 15224. [Google Scholar] [CrossRef]
- Salas, S.P.; Marshall, G.; Gutiérrez, B.L.; Rosso, P. Time Course of Maternal Plasma Volume and Hormonal Changes in Women With Preeclampsia or Fetal Growth Restriction. Hypertension 2006, 47, 203–208. [Google Scholar] [CrossRef]
- Hertig, A.; Liere, P.; Chabbert-Buffet, N.; Fort, J.; Pianos, A.; Eychenne, B.; Cambourg, A.; Schumacher, M.; Berkane, N.; Lefevre, G.; et al. Steroid profiling in preeclamptic women: Evidence for aromatase deficiency. Am. J. Obstet. Gynecol. 2010, 203, e1–e477. [Google Scholar] [CrossRef]
- Bussen, S.; Bussen, D. Influence of the vascular endothelial growth factor on the development of severe pre-eclampsia or HELLP syndrome. Arch. Gynecol. Obstet. 2011, 284, 551–557. [Google Scholar] [CrossRef]
- Jobe, S.O.; Tyler, C.T.; Magness, R.R. Aberrant synthesis, metabolism, and plasma accumulation of circulating estrogens and estrogen metabolites in preeclampsia implications for vascular dysfunction. Hypertens. (Dallas, Tex. 1979) 2013, 61, 480–487. [Google Scholar] [CrossRef]
- Yin, G.; Zhu, X.; Guo, C.; Yang, Y.; Han, T.; Chen, L.; Yin, W.; Gao, P.; Zhang, H.; Geng, J.; et al. Differential expression of estradiol and estrogen receptor α in severe preeclamptic pregnancies compared with normal pregnancies. Mol. Med. Rep. 2013, 7, 981–985. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, T.; Shen, Y.; Wang, X.; Baker, P.N.; Zhao, A. 2-Methoxyestradiol deficiency is strongly related to hypertension in early onset severe pre-eclampsia. Pregnancy Hypertens. 2014, 4, 215–219. [Google Scholar] [CrossRef]
- Açıkgöz, Ş.; Özmen Bayar, Ü.; Can, M.; Güven, B.; Mungan, G.; Doğan, S.; Sümbüloğlu, V. Levels of Oxidized LDL, Estrogens, and Progesterone in Placenta Tissues and Serum Paraoxonase Activity in Preeclampsia. Mediat. Inflamm. 2013, 2013, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Makieva, S.; Saunders, P.T.K.; Norman, J.E. Androgens in pregnancy: Roles in parturition. Hum. Reprod. Update 2014, 20, 542–559. [Google Scholar] [CrossRef]
- Kallak, T.K.; Hellgren, C.; Skalkidou, A.; Sandelin-Francke, L.; Ubhayasekhera, K.; Bergquist, J.; Axelsson, O.; Comasco, E.; Campbell, R.E.; Poromaa, I.S. Maternal and female fetal testosterone levels are associated with maternal age and gestational weight gain. Eur. J. Endocrinol. 2017, 177, 379–388. [Google Scholar] [CrossRef]
- Benten, W.P.M.; Guo, Z.; Krücken, J.; Wunderlich, F. Rapid effects of androgens in macrophages. Steroids 2004, 69, 585–590. [Google Scholar] [CrossRef]
- Wunderlich, F.; Benten, W.P.M.; Lieberherr, M.; Guo, Z.; Stamm, O.; Wrehlke, C.; Sekeris, C.E.; Mossmann, H. Testosterone signaling in T cells and macrophages. Steroids 2002, 67, 535–538. [Google Scholar] [CrossRef]
- Becerra-Díaz, M.; Strickland, A.B.; Keselman, A.; Heller, N.M. Androgen and Androgen Receptor as Enhancers of M2 Macrophage Polarization in Allergic Lung Inflammation. J. Immunol. 2018, 201, 2923–2933. [Google Scholar] [CrossRef] [Green Version]
- Biró, O.; Fóthi, Á.; Alasztics, B.; Nagy, B.; Orbán, T.I.; Rigó, J. Circulating exosomal and Argonaute-bound microRNAs in preeclampsia. Gene 2019, 692, 138–144. [Google Scholar] [CrossRef] [Green Version]
- Shen, L.; Li, Y.; Li, R.; Diao, Z.; Yany, M.; Wu, M.; Sun, H.; Yan, G.; Hu, Y. Placenta-associated serum exosomal miR-155 derived from patients with preeclampsia inhibits eNOS expression in human umbilical vein endothelial cells. Int. J. Mol. Med. 2018, 41, 1731–1739. [Google Scholar] [CrossRef]
- Sammar, M.; Dragovic, R.; Meiri, H.; Vatish, M.; Sharabi-Nov, A.; Sargent, I.; Redman, C.; Tannetta, D. Reduced placental protein 13 (PP13) in placental derived syncytiotrophoblast extracellular vesicles in preeclampsia–A novel tool to study the impaired cargo transmission of the placenta to the maternal organs. Placenta 2018, 66, 17–25. [Google Scholar] [CrossRef]
- Aouache, R.; Biquard, L.; Vaiman, D.; Miralles, F.; Aouache, R.; Biquard, L.; Vaiman, D.; Miralles, F. Oxidative Stress in Preeclampsia and Placental Diseases. Int. J. Mol. Sci. 2018, 19, 1496. [Google Scholar] [CrossRef]
- Brownfoot, F.C.; Hannan, N.J.; Cannon, P.; Nguyen, V.; Hastie, R.; Parry, L.J.; Senadheera, S.; Tuohey, L.; Tong, S.; Kaitu’u-Lino, T.J. Sulfasalazine reduces placental secretion of antiangiogenic factors, up-regulates the secretion of placental growth factor and rescues endothelial dysfunction. EBioMedicine 2019, 41, 636–648. [Google Scholar] [CrossRef] [Green Version]
- Valencia-Ortega, J.; Zárate, A.; Saucedo, R.; Hernández-Valencia, M.; Cruz, J.G.; Puello, E. Placental Proinflammatory State and Maternal Endothelial Dysfunction in Preeclampsia. Gynecol. Obstet. Invest. 2019, 84, 12–19. [Google Scholar] [CrossRef]
- Harmon, A.C.; Ibrahim, T.; Cornelius, D.C.; Amaral, L.M.; Cunningham, M.W.; Wallace, K.; LaMarca, B. Placental CD4 + T cells isolated from preeclamptic women cause preeclampsia-like symptoms in pregnant nude-athymic rats. Pregnancy Hypertens. 2019, 15, 7–11. [Google Scholar] [CrossRef]
- Geldenhuys, J.; Rossouw, T.M.; Lombaard, H.A.; Ehlers, M.M.; Kock, M.M. Disruption in the Regulation of Immune Responses in the Placental Subtype of Preeclampsia. Front. Immunol. 2018, 9, 1659. [Google Scholar] [CrossRef]
- Wang, J.; Zhu, Q.W.; Cheng, X.Y.; Liu, J.Y.; Zhang, L.L.; Tao, Y.M.; Cui, Y.B.; Wei, Y. Assessment efficacy of neutrophil-lymphocyte ratio and monocyte-lymphocyte ratio in preeclampsia. J. Reprod. Immunol. 2019, 132, 29–34. [Google Scholar] [CrossRef]
- Brien, M.E.; Boufaied, I.; Soglio, D.D.; Rey, E.; Leduc, L.; Girard, S. Distinct inflammatory profile in preeclampsia and postpartum preeclampsia reveal unique mechanisms. Biol. Reprod. 2019, 100, 187–194. [Google Scholar] [CrossRef]
- Jabalie, G.; Ahmadi, M.; Koushaeian, L.; Eghbal-Fard, S.; Mehdizadeh, A.; Kamrani, A.; Abdollahi-Fard, S.; Farzadi, L.; Hojjat- Farsangi, M.; Nouri, M.; et al. Metabolic syndrome mediates proinflammatory responses of inflammatory cells in preeclampsia. Am. J. Reprod. Immunol. 2019, 81, e13086. [Google Scholar] [CrossRef]
- Alahakoon, T.I.; Medbury, H.; Williams, H.; Fewings, N.; Wang, X.M.; Lee, V.W. Characterization of fetal monocytes in preeclampsia and fetal growth restriction. J. Perinat. Med. 2019. [Google Scholar] [CrossRef]
- Alahakoon, T.I.; Medbury, H.; Williams, H.; Fewings, N.; Wang, X.M.; Lee, V.W. Distribution of monocyte subsets and polarization in preeclampsia and intrauterine fetal growth restriction. J. Obstet. Gynaecol. Res. 2018, 44, 2135–2148. [Google Scholar] [CrossRef]
- Ma, Y.; Ye, Y.; Zhang, J.; Ruan, C.-C.; Gao, P.-J. Immune imbalance is associated with the development of preeclampsia. Medicine (Baltimore) 2019, 98, e15080. [Google Scholar] [CrossRef]
- Yang, S.W.; Cho, E.H.; Choi, S.Y.; Lee, Y.K.; Park, J.H.; Kim, M.K.; Park, J.Y.; Choi, H.J.; Lee, J.I.; Ko, H.M.; et al. DC-SIGN expression in Hofbauer cells may play an important role in immune tolerance in fetal chorionic villi during the development of preeclampsia. J. Reprod. Immunol. 2017, 124, 30–37. [Google Scholar] [CrossRef]
- Tang, Z.; Buhimschi, I.A.; Buhimschi, C.S.; Tadesse, S.; Norwitz, E.; Niven-Fairchild, T.; Huang, S.-T.J.; Guller, S. Decreased Levels of Folate Receptor-β and Reduced Numbers of Fetal Macrophages (Hofbauer Cells) in Placentas from Pregnancies with Severe Pre-Eclampsia. Am. J. Reprod. Immunol. 2013, 70, 104–115. [Google Scholar] [CrossRef]
- Evsen, M.S.; Kalkanli, S.; Deveci, E.; Sak, M.E.; Ozler, A.; Baran, O.; Erdem, E.; Seker, U. Human placental macrophages (Hofbauer cells) in severe preeclampsia complicated by HELLP syndrome: Immunohistochemistry of chorionic villi. Anal. Quant. Cytopathol. Histopathol. 2013, 35, 283–288. [Google Scholar]
- Al-Khafaji, L.A.; Al-Yawer, M.A. Localization and counting of CD68-labelled macrophages in placentas of normal and preeclamptic women. In AIP Conference Proceedings; AIP Publishing: Melville, NY, USA, 2017; Volume 1888, p. 20011. [Google Scholar]
- Saeed, I.; Yousaf, A.; Ali, S. Number of Hofbauer Cells in Placentae from Normal and Pre Eclamptic Gestation. J. Rawalpindi Med. Coll. 2018, 22, 76–78. [Google Scholar]
- Przybyl, L.; Haase, N.; Golic, M.; Rugor, J.; Solano, M.E.; Arck, P.C.; Gauster, M.; Huppertz, B.; Emontzpohl, C.; Stoppe, C.; et al. CD74-downregulation of placental macrophage-trophoblastic interactions in preeclampsia. Circ. Res. 2016, 119, 55–68. [Google Scholar] [CrossRef]
- Milosevic-Stevanovic, J.; Krstic, M.; Radovic-Janosevic, D.; Popovic, J.; Tasic, M.; Stojnev, S. Number of decidual natural killer cells & macrophages in pre-eclampsia. Indian J. Med. Res. 2016, 144, 823–830. [Google Scholar]
- Williams, P.J.; Bulmer, J.N.; Searle, R.F.; Innes, B.A.; Robson, S.C. Altered decidual leucocyte populations in the placental bed in pre-eclampsia and foetal growth restriction: A comparison with late normal pregnancy. Reproduction 2009, 138, 177–184. [Google Scholar] [CrossRef]
- Bürk, M.R.; Troeger, C.; Brinkhaus, R.; Holzgreve, W.; Hahn, S. Severely reduced presence of tissue macrophages in the basal plate of pre-eclamptic placentae. Placenta 2001, 22, 309–316. [Google Scholar] [CrossRef]
- Brien, M.-E.; Baker, B.; Duval, C.; Gaudreault, V.; Jones, R.L.; Girard, S. Alarmins at the maternal–fetal interface: Involvement of inflammation in placental dysfunction and pregnancy complications. Can. J. Physiol. Pharmacol. 2018, 97, 206–212. [Google Scholar] [CrossRef]
- Conrad, K.P.; Rabaglino, M.B.; Post Uiterweer, E.D. Emerging role for dysregulated decidualization in the genesis of preeclampsia. Placenta 2017, 60, 119–129. [Google Scholar] [CrossRef] [Green Version]
- Sargent, I.L.; Borzychowski, A.M.; Redman, C.W.G. Immunoregulation in normal pregnancy and pre-eclampsia: An overview. Reprod. Biomed. Online 2006, 13, 680–686. [Google Scholar] [CrossRef]
- Pinheiro, M.B.; Gomes, K.B.; Dusse, L.M.S. Fibrinolytic system in preeclampsia. Clin. Chim. Acta 2013, 416, 67–71. [Google Scholar] [CrossRef]
- Xu, J.; Gu, Y.; Sun, J.; Zhu, H.; Lewis, D.F.; Wang, Y. Reduced CD200 expression is associated with altered Th1/Th2 cytokine production in placental trophoblasts from preeclampsia. Am. J. Reprod. Immunol. 2018, 79, 1–8. [Google Scholar] [CrossRef]
- Aggarwal, R.; Jain, A.K.; Mittal, P.; Kohli, M.; Jawanjal, P.; Rath, G. Association of pro- and anti-inflammatory cytokines in preeclampsia. J. Clin. Lab. Anal. 2019, 1–10. [Google Scholar] [CrossRef]
- Abumaree, M.H.; Al Harthy, S.; Al Subayyil, A.M.; Alshabibi, M.A.; Abomaray, F.M.; Khatlani, T.; Kalionis, B.; El-Muzaini, M.F.; Al Jumah, M.A.; Jawdat, D.; et al. Decidua Basalis Mesenchymal Stem Cells Favor Inflammatory M1 Macrophage Differentiation In Vitro. Cells 2019, 8, 173. [Google Scholar] [CrossRef]
- Abumaree, M.H.; Al Jumah, M.A.; Kalionis, B.; Jawdat, D.; Al Khaldi, A.; Abomaray, F.M.; Fatani, A.S.; Chamley, L.W.; Knawy, B.A. Human Placental Mesenchymal Stem Cells (pMSCs) Play a Role as Immune Suppressive Cells by Shifting Macrophage Differentiation from Inflammatory M1 to Anti-inflammatory M2 Macrophages. Stem Cell Rev. Rep. 2013, 9, 620–641. [Google Scholar] [CrossRef]
- Wang, S.; Sun, F.; Han, M.; Liu, Y.; Zou, Q.; Wang, F.; Tao, Y.; Li, D.; Du, M.; Li, H.; et al. Trophoblast-derived hyaluronan promotes the regulatory phenotype of decidual macrophages. Reproduction 2019, 157, 189–198. [Google Scholar] [CrossRef] [Green Version]
- FDA Approved Cellular and Gene Therapy Products. Available online: https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products/approved-cellular-and-gene-therapy-products (accessed on 5 June 2019).
- Fidler, I.J. Inhibition of pulmonary metastasis by intravenous injection of specifically activated macrophages. Cancer Res. 1974, 34, 1074–1078. [Google Scholar]
- Liu, M.; Connor, R.S.O.; Trefely, S.; Graham, K.; Snyder, N.W.; Beatty, G.L. Metabolic rewiring of macrophages by CpG potentiates clearance of cancer cells and overcomes tumor-expressed CD47-mediated ‘don’t-eat-me’ signal. Nat. Immunol. 2019, 20, 265–275. [Google Scholar] [CrossRef]
- Van den Bosch, T.P.P.; Kannegieter, N.M.; Hesselink, D.A.; Baan, C.C.; Rowshani, A.T. Targeting the Monocyte–macrophage Lineage in Solid Organ Transplantation. Front. Immunol. 2017, 8, 153. [Google Scholar] [CrossRef]
- Wynn, T.A.; Vannella, K.M. Macrophages in Tissue Repair, Regeneration, and Fibrosis. Immunity 2016, 44, 450–462. [Google Scholar] [CrossRef] [Green Version]
- Weagel, E.; Smith, C.; Liu, P.G.; Robison, R.; O’Neill, K. Macrophage Polarization and Its Role in Cancer. J. Clin. Cell. Immunol. 2015, 6, 4–11. [Google Scholar]
- Herold, J.; Pipp, F.; Fernandez, B.; Xing, Z.; Heil, M.; Tillmanns, H.; Braun-dullaeus, R.C. Transplantation of Monocytes: A Novel Strategy for In Vivo Augmentation of Collateral Vessel Growth. Hum. Gene Ther. 2004, 12, 1–12. [Google Scholar] [CrossRef]
- Tsuboki, J.; Komohara, Y.; Nishimura, Y.; Imamura, Y.; Senju, S.; Haruta, M.; Tashiro, H.; Ohba, T.; Takaishi, K.; Dashdemberel, N.; et al. Novel therapeutic strategies for advanced ovarian cancer by using induced pluripotent stem cell-derived myelomonocytic cells producing interferon beta. Cancer Sci. 2018, 109, 3403–3410. [Google Scholar]
- Chernykh, E.; Shevela, E.; Kafanova, M.; Sakhno, L.; Polovnikov, E.; Ostanin, A. Monocyte-derived macrophages for treatment of cerebral palsy: A study of 57 cases. J. Neurorestoratology 2018, 6, 41–47. [Google Scholar] [CrossRef]
- Li, J.; Li, C.; Zhuang, Q.; Peng, B.; Zhu, Y.; Ye, Q.; Ming, Y. The Evolving Roles of Macrophages in Organ Transplantation. J. Immunol. Res. 2019, 2019, 5763430. [Google Scholar] [CrossRef]
- Jeanty, C.; Derderian, S.C.; Mackenzie, T.C. Maternal-fetal cellular trafficking: Clinical implications and consequences. Curr. Opin. Pediatr. 2014, 26, 377–382. [Google Scholar] [CrossRef]
- Fleck, S.; Bautista, G.; Keating, S.M.; Lee, T.H.; Keller, R.L.; Moon-Grady, A.J.; Gonzales, K.; Norris, P.J.; Busch, M.P.; Kim, C.J.; et al. Fetal production of growth factors and inflammatory mediators predicts pulmonary hypertension in congenital diaphragmatic hernia. Pediatr. Res. 2013, 74, 290–298. [Google Scholar] [CrossRef] [Green Version]

| Subject | Observation in PE | Quantity: Control vs. PE | Reference |
|---|---|---|---|
| Monocyte | ↑ Monocyte count ↑ Monocyte-lymphocyte ratio | 161/302 | [88] |
| ↑ Monocyte count | 20/20 | [89] | |
| ↓ CD14++CD16−, ↑ CD14+CD16++ | 11/17 | [23] | |
| 40/35 | [90] | ||
| 8/4 (umbilical cord blood) | [91] | ||
| ↓ CD14++CD16−; ↑ (CD14+CD16++ and CD14++CD16+) | 24/9 | [92] | |
| 23/26 | [22] | ||
| ↑ CD14+CD11c+CD163- | 30/22 | [93] | |
| Macrophages in placenta | |||
| Hofbauer cells | ↓ CD14+ | 30/10 | [94] |
| ↓ CD68+ | 11/10 | [95] | |
| ↑ CD68+ | 20/20 | [96] | |
| 6/6 | [97] | ||
| ↑ Hofbauer cells number | 50/50 | [98] | |
| ↓ CD163+ | 30/10 | [94] | |
| ↓ CD163+ | 11/10 | [95] | |
| ↓ CD74+ | 28/24 | [99] | |
| ↓ CD11b+Arg1+ ↑ CD11b+ iNOS+ | 22/30 | [93] | |
| Decidual macrophages | ↑ CD14+ | 5/6 | [50] |
| ↑ CD68+ | 20/30 | [100] | |
| 6/6 | [97] | ||
| ↓ CD14+ | 12/12 | [101] | |
| 6/6 | [102] | ||
| ↓ CD163/CD14 | 5/6 | [50] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vishnyakova, P.; Elchaninov, A.; Fatkhudinov, T.; Sukhikh, G. Role of the Monocyte–Macrophage System in Normal Pregnancy and Preeclampsia. Int. J. Mol. Sci. 2019, 20, 3695. https://doi.org/10.3390/ijms20153695
Vishnyakova P, Elchaninov A, Fatkhudinov T, Sukhikh G. Role of the Monocyte–Macrophage System in Normal Pregnancy and Preeclampsia. International Journal of Molecular Sciences. 2019; 20(15):3695. https://doi.org/10.3390/ijms20153695
Chicago/Turabian StyleVishnyakova, Polina, Andrey Elchaninov, Timur Fatkhudinov, and Gennady Sukhikh. 2019. "Role of the Monocyte–Macrophage System in Normal Pregnancy and Preeclampsia" International Journal of Molecular Sciences 20, no. 15: 3695. https://doi.org/10.3390/ijms20153695
APA StyleVishnyakova, P., Elchaninov, A., Fatkhudinov, T., & Sukhikh, G. (2019). Role of the Monocyte–Macrophage System in Normal Pregnancy and Preeclampsia. International Journal of Molecular Sciences, 20(15), 3695. https://doi.org/10.3390/ijms20153695