Castration Induces Down-Regulation of A-Type K+ Channel in Rat Vas Deferens Smooth Muscle
Abstract
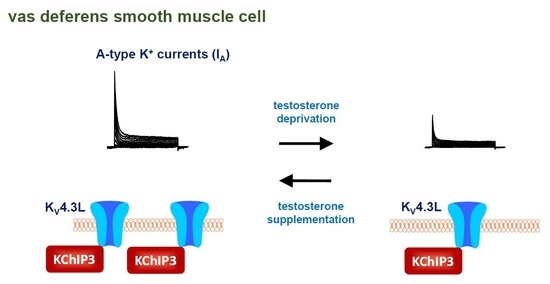
:1. Introduction
2. Results
2.1. Molecular Identification of Kv4 Channel α and β Subunits Expressed in the Rat VD
2.2. Changes in A-Type K+ Current Properties in Rat VDSMCs by Castration
2.3. Changes in the mRNA Expression of Kv4 Channel α and β Subunits Expressed in the Rat VD by Castration
2.4. Changes in the Protein Expression of Kv4 Channel α and β Subunits in Rat VD by Castration
2.5. Identification of DPP6 Isoforms Expressed in the Rat VD
3. Discussion
4. Materials and Methods
4.1. Surgery and Hormonal Manipulations
4.2. Total RNA Extraction, Reverse Transcription, and Real-Time PCR
4.3. Western Blotting
4.4. Cell Isolation and Electrophysiology
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wilson, J.D.; Griffin, J.E.; Leshin, M.; George, F.W. Role of gonadal hormones in development of the sexual phenotypes. Hum. Genet. 1981, 58, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Turner, K.J.; McIntyre, B.S.; Phillips, S.L.; Barlow, N.J.; Bowman, C.J.; Foster, P.M. Altered gene expression during rat Wolffian duct development in response to in utero exposure to the antiandrogen linuron. Toxicol. Sci. 2003, 74, 114–128. [Google Scholar] [CrossRef] [PubMed]
- Longhurst, P.A.; Brotcke, T.P.; Burrell, C.L.; Belis, J.A. Comparison of the effects of castration and streptozotocin-induced diabetes mellitus on contractile responses of the rat vas deferens. Pharmacology 1989, 38, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Tep-areenan, P.; Kendall, D.A.; Randall, M.D. Mechanisms of vasorelaxation to testosterone in the rat aorta. Eur. J. Pharmacol. 2003, 465, 125–132. [Google Scholar] [CrossRef]
- Thompson, J.; Khalil, R.A. Gender differences in the regulation of vascular tone. Clin. Exp. Pharmacol. Physiol. 2003, 30, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Boselli, C.; Barbieri, A.; Grana, E. Effect of cromakalim on spontaneous activity of castrated rat vas deferens. Pharmacol. Res. 1994, 29, 163–167. [Google Scholar] [CrossRef]
- Nagano, H.; Imaizumi, Y.; Watanabe, M. Effects of arachidonic acid on A-type potassium currents in smooth muscle cells of the guinea-pig. Am. J. Physiol. 1997, 272, C860–C869. [Google Scholar] [CrossRef] [PubMed]
- Amberg, G.C.; Koh, S.D.; Imaizumi, Y.; Ohya, S.; Sanders, K.M. A-type potassium currents in smooth muscle. Am. J. Physiol. 2003, 284, C583–C595. [Google Scholar] [CrossRef] [Green Version]
- Carrasquillo, Y.; Nerbonne, JM. IA channels: Diverse regulatory mechanisms. Neuroscientist 2014, 20, 104–111. [Google Scholar] [CrossRef]
- An, W.F.; Bowlby, M.R.; Betty, M.; Cao, J.; Ling, H.P.; Mendoza, G.; Hinson, J.W.; Mattsson, K.I.; Strassle, B.W.; Trimmer, J.S.; et al. Modulation of A-type potassium channels by a family of calcium sensors. Nature 2000, 403, 553–556. [Google Scholar] [CrossRef]
- Nakamura, T.Y.; Nakao, S.; Wakabayashi, S. Emerging roles of neuronal Ca2+ sensor-1 in cardiac and neuronal tissues: A mini review. Front. Mol. Neurosci. 2019, 12, 56. [Google Scholar] [CrossRef] [PubMed]
- Maffie, J.; Rudy, B. Weighting the evidence for a ternary protein complex mediating A-type K+ currents in neurons. J. Physiol. 2008, 586, 5609–5623. [Google Scholar] [CrossRef] [PubMed]
- Morohashi, Y.; Hatano, N.; Ohya, S.; Takikawa, R.; Watabiki, T.; Takasugi, N.; Imaizumi, Y.; Tomita, T.; Iwatsubo, T. Molecular cloning and characterization of CALP/KChIP4, a novel EF-hand protein interacting with presenilin 2 and voltage-gated potassium channel subunit Kv4. J. Biol. Chem. 2002, 277, 14965–14975. [Google Scholar] [CrossRef]
- Zicha, S.; Xiao, L.; Stafford, S.; Cha, T.J.; Han, W.; Varro, A.; Nattel, S. Transmural expression of transient outward potassium current subunits in normal and failing canine and human hearts. J. Physiol. 2004, 561, 735–748. [Google Scholar] [CrossRef]
- Nishiyama, A.; Ishii, D.N.; Backx, P.H.; Pulford, B.E.; Birks, B.R.; Tamkun, M.M. Altered K+ channel gene expression in diabetic rat ventricle: Isoform switching between Kv4.2 and Kv1.4. Am. J. Physiol. 2001, 281, H1800–H1807. [Google Scholar] [CrossRef]
- Kaab, S.; Dixon, J.; Duc, J.; Ashen, D.; Nabauer, M.; Beuckelmann, D.J.; Steinbeck, G.; McKinnon, D.; Tomaselli, G.F. Molecular basis of transient outward potassium current downregulation in human heart failure: A decrease in Kv4.3 mRNA correlates with a reduction in current density. Circulation 1998, 98, 1383–1393. [Google Scholar] [CrossRef]
- Suzuki, T.; Takimoto, K. Differential expression of Kv4 pore-forming and KChIP auxiliary subunits in rat uterus during pregnancy. Am. J. Physiol. 2005, 288, E335–E341. [Google Scholar] [CrossRef] [Green Version]
- Song, M.; Helguera, G.; Eghbali, M.; Zhu, N.; Zarei, M.M.; Olcese, R.; Toro, L.; Stefani, E. Remodeling of Kv4.3 potassium channel gene expression under the control of sex hormones. J. Biol. Chem. 2001, 276, 31883–31890. [Google Scholar] [CrossRef] [PubMed]
- Ohya, S.; Tanaka, M.; Oku, T.; Asai, Y.; Watanabe, M.; Giles, W.R.; Imaizumi, Y. Molecular cloning and tissue distribution of an alternatively spliced variant of an A-type K+ channel alpha-subunit, Kv4.3 in the rat. FEBS Lett. 1997, 420, 47–53. [Google Scholar] [CrossRef]
- Wakade, A.R.; Garcia, A.G.; Kirpekar, S.M. Effect of castration on the smooth muscle cells of the internal sex organs of the rat: Influence of the smooth muscle on the sympathetic neurons innervating the vas deferens, seminal vesicle and coagulating gland. J. Pharmacol. Exp. Ther. 1975, 193, 424–434. [Google Scholar]
- Niwa, N.; Nerbonne, J.M. Molecular determinants of cardiac transient outward potassium current (Ito) expression and regulation. J. Mol. Cell. Cardiol. 2010, 48, 12–25. [Google Scholar] [CrossRef] [PubMed]
- Deschênes, I.; Tomasekki, G.F. Modulation of Kv4.3 current by accessory subunits. FEBS Lett. 2002, 528, 183–188. [Google Scholar] [CrossRef]
- Kin, Y.; Misumi, Y.; Ikehara, Y. Biosynthesis and characterization of the brain-specific membrane protein DPPX, a dipeptidyl peptidase IV-related protein. J. Biochem. 2001, 129, 289–295. [Google Scholar] [CrossRef]
- Nadal, M.S.; Amarillo, Y.; Vega-Saenz de Meira, E.; Rudy, B. Differential characterization of three alternative spliced isoforms of DPPX. Brain Res. 2006, 1094, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Lafayette, S.S.; Vladimirova, I.; Garcez-do-Carmo, L.; Monteforte, P.T.; Caricati Neto, A.; Jurkiewicz, A. Evidence for the participation of calcium in non-genomic relazations induced by androgenic steroids in rat vas deferens. Br. J. Pharmacol. 2008, 153, 1242–1250. [Google Scholar] [CrossRef] [PubMed]
- Harhun, M.I.; Jurkiewicz, A.; Jurkiewicz, N.H.; Kryshtal, D.O.; Shuba, M.F.; Vladimirova, I.A. Voltage-gated potassium currents in rat vas deferens smooth muscle cells. Pflugers Arch. 2003, 446, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Imaizumi, Y.; Muraki, K.; Watanabe, M. Characteristics of transient outward currents in single smooth muscle cells from the ureter of the guinea-pig. J. Physiol. 1990, 427, 301–324. [Google Scholar] [CrossRef]
- Agus, Z.S.; Dukes, I.D.; Morad, M. Divalent cations modulate the transient outward current in rat ventricular myocytes. Am. J. Physiol. 1991, 261, C310–C318. [Google Scholar] [CrossRef] [PubMed]
- Faivre, J.F.; Calmels, T.P.; Rouanet, S.; Javre, J.L.; Cheval, B.; Bril, A. Characterisation of Kv4.3 in HEK293 cells: Comparison with the rat ventricular transient outward potassium current. Cardiovasc. Res. 1999, 41, 188–199. [Google Scholar] [CrossRef]
- Guo, W.; Malin, S.A.; Johns, D.C.; Jeromin, A.; Nerbonne, J.M. Modulation of Kv4-encoded K+ currents in the mammalian myocardium by neuronal calcium sensor-1. J. Biol. Chem. 2002, 277, 26436–26443. [Google Scholar] [CrossRef]
- Beckett, E.A.; McCloskey, C.; O’Kane, N.; Sanders, K.; Koh, S.D. Effects of female steroid hormones on A-type K+ currents in murine colon. J. Physiol. 2006, 573, 453–468. [Google Scholar] [CrossRef] [PubMed]
- Nerbonne, J.M.; Gerber, B.R.; Norris, A.; Burkhalter, A. Electrical remodeling maintains firing properties in cortical pyramidal neurons lacking KCND2-encoded A-type K+ currents. J. Physiol. 2008, 586, 1565–1579. [Google Scholar] [CrossRef] [PubMed]
- Fix, C.; Jordan, C.; Cano, P.; Walker, W.H. Testosterone activates mitogen-activated protein kinase and the cAMP response element binding protein transcription factor in Sertoli cells. Proc. Natl. Acad. Sci. USA 2004, 101, 10919–10924. [Google Scholar] [CrossRef] [Green Version]
- Ledo, F.; Kremer, L.; Mellstrom, B.; Naranjo, J.R. Ca2+-dependent block of CREB-CBP transcription by repressor DREAM. EMBO J. 2002, 21, 4583–4592. [Google Scholar] [CrossRef] [PubMed]
- Iusem, N.D.; De Larminat, M.A.; Tezon, J.G.; Blaquier, J.A.; Belocopitow, E. Androgen dependence of protein N-glycosylation in rat epididymis. Endocrinology 1984, 114, 1448–1453. [Google Scholar] [CrossRef]
- Ufret-Vincenty, C.A.; Baro, D.J.; Santana, L.F. Differential contribution of sialic acid to the function of repolarizing K+ currents in ventricular myocytes. Am. J. Physiol. 2001, 281, C464–C474. [Google Scholar] [CrossRef]
- Ohya, S.; Morohashi, Y.; Muraki, K.; Tomita, T.; Watanabe, M.; Iwatsubo, T.; Imaizumi, Y. Molecular cloning and expression of the novel splice variants of K+ channel-interacting protein 2. Biochem. Biophys. Res. Commun. 2001, 282, 96–102. [Google Scholar] [CrossRef]
- Yeung, S.Y.M.; Ohya, S.; Sergeant, G.P.; Pucovsky, V.; Greenwood, I.A. Pharmacological and molecular evidence for a role of Kv4.3 in ultra-fast activating K+ currents in murine portal vein myocytes. Br. J. Pharmacol. 2006, 149, 676–686. [Google Scholar] [CrossRef]
- Ohya, S.; Yamamura, H.; Muraki, K.; Watanabe, M.; Imaizumi, Y. Comparative study of the molecular and functional expression of L-type Ca2+ channels and large-conductance, Ca2+-activated K+ channels in rabbit aorta and vas deferens smooth muscle. Pflugers Arch. 2001, 441, 611–620. [Google Scholar] [CrossRef]
- Ohno, A.; Ohya, S.; Yamamura, H.; Imaizumi, Y. Regulation of ryanodine receptor-mediated Ca2+ release in vas deferens smooth muscle cells. J. Pharmacol. Sci. 2009, 110, 78–86. [Google Scholar] [CrossRef]
- Masuda, K.; Takanari, H.; Morishima, M.; Ma, F.; Wang, Y.; Takahashi, N.; Ono, K. Testosterone-mediated up-regulation of delayed rectifier potassium channel in cardiomyocytes caused abbreviation of QT intervals in rats. J. Physiol. Sci. 2018, 68, 759–767. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Jiang, J.; Xia, J.; Jiang, R. Effect of low androgen levels on IKCa and SKCa3 channels in rat penile corpus cavernosum. Andrologia 2018, 50, e13075. [Google Scholar] [CrossRef] [PubMed]







| Sham | Cast. | MET | Veh. | |
|---|---|---|---|---|
| current amplitude at +40 mV | ||||
| current (nA) | 1.22 ± 0.06 | 0.63 ± 0.04 **,## | 1.11 ± 0.07 | 0.56 ± 0.05 **,## |
| capacitance (pF) | 53.2 ± 2.5 | 39.8 ± 0.9 **,## | 53.2 ± 2.3 | 34.8 ± 1.4 **,## |
| current density (pA/pF) | 23.2 ± 0.9 | 15.8 ± 0.9 **,## | 20.7 ± 0.8 | 16.4 ± 1.3 **,# |
| n = 15 | n = 20 | n = 17 | n = 16 | |
| activation/inactivation rate at +20 mV | ||||
| time to peak (ms) | 4.35 ± 0.25 | 4.13 ± 0.23 | 4.65 ± 0.25 | 4.44 ± 0.26 |
| inactivation rate, t1/2 (ms) | 18.0 ± 1.8 | 14.6 ± 0.6 | 19.7 ± 1.4 | 19.3 ± 1.8 |
| n = 15 | n = 20 | n = 17 | n = 16 | |
| voltage-dependence of activation | ||||
| V1/2 (mV) | −13.9 ± 2.4 | −9.2 ± 3.5 | −10.4 ± 6.1 | −11.3 ± 4.0 |
| slope factor | 12.7 ± 0.7 | 9.0 ± 1.0 | 11.7 ± 1.0 | 13.5 ± 1.8 |
| n = 5 | n = 6 | n = 5 | n = 5 | |
| voltage-dependence of inactivation | ||||
| V1/2 (mV) | −58.6 ± 1.4 | −57.0 ± 1.5 | −60.3 ± 1.1 | −57.7 ± 0.9 |
| slope factor | 4.47 ± 0.21 | 4.97 ± 0.15 | 4.58 ± 0.10 | 5.36 ± 0.23 *,# |
| n = 14 | n = 16 | n = 14 | n = 14 | |
| recovery from inactivation | ||||
| τ (ms) | 47.9 ± 8.9 | 37.5 ± 3.1 | 51.6 ± 5.2 | 50.2 ± 5.2 |
| n = 10 | n = 15 | n = 15 | n = 14 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ohya, S.; Ito, K.; Hatano, N.; Ohno, A.; Muraki, K.; Imaizumi, Y. Castration Induces Down-Regulation of A-Type K+ Channel in Rat Vas Deferens Smooth Muscle. Int. J. Mol. Sci. 2019, 20, 4073. https://doi.org/10.3390/ijms20174073
Ohya S, Ito K, Hatano N, Ohno A, Muraki K, Imaizumi Y. Castration Induces Down-Regulation of A-Type K+ Channel in Rat Vas Deferens Smooth Muscle. International Journal of Molecular Sciences. 2019; 20(17):4073. https://doi.org/10.3390/ijms20174073
Chicago/Turabian StyleOhya, Susumu, Katsunori Ito, Noriyuki Hatano, Akitoshi Ohno, Katsuhiko Muraki, and Yuji Imaizumi. 2019. "Castration Induces Down-Regulation of A-Type K+ Channel in Rat Vas Deferens Smooth Muscle" International Journal of Molecular Sciences 20, no. 17: 4073. https://doi.org/10.3390/ijms20174073
APA StyleOhya, S., Ito, K., Hatano, N., Ohno, A., Muraki, K., & Imaizumi, Y. (2019). Castration Induces Down-Regulation of A-Type K+ Channel in Rat Vas Deferens Smooth Muscle. International Journal of Molecular Sciences, 20(17), 4073. https://doi.org/10.3390/ijms20174073