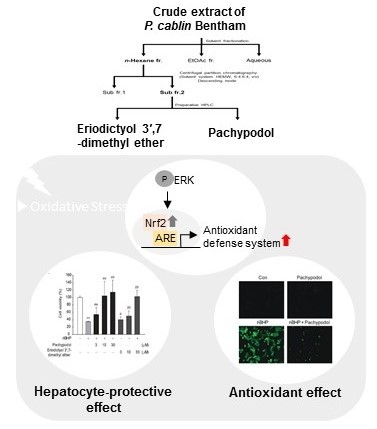
Pachypodol, a Methoxyflavonoid Isolated from Pogostemon cablin Bentham Exerts Antioxidant and Cytoprotective Effects in HepG2 Cells: Possible Role of ERK-Dependent Nrf2 Activation
Abstract
:1. Introduction
2. Results
2.1. Bioactivity-Guided Isolation of Pachypodol and Eriodictyol 3′,7-Dimethyl Ether from P. cablin
2.2. Effects of Pachypodol and Eriodictyol 3′,7-Dimethyl Ether on the Nrf2-ARE Pathway and t-BHP-Induced Cell Death
2.3. Effects of Pachypodol on the t-BHP-Induced ROS Production and the Intracellular Antioxidant System
2.4. Role of ERK Activation in the Nrf2 Activation and Cytoprotection by Pachypodol
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Plant Material and Extraction, and Isolation of Pachypodol
4.3. Cell Culture
4.4. Cell Viability Assay
4.5. Luciferase Assay
4.6. Preparation of Nuclear Extracts and Western Blot Analysis
4.7. Measurement of Intracellular ROS Generation
4.8. Measurement of Intracellular Glutathione (GSH) Content
4.9. Real-Time PCR Assay
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AMPK | AMP-activated protein kinase |
| ARE | Antioxidant response element |
| CM-H2DCFDA | 5-(and-6)-Chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate acetyl ester |
| CPC | Centrifugal partition chromatography |
| DMSO | Dimethyl sulfoxide |
| ERK | Extracellular signal-regulated kinase |
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase |
| GCL | Glutamate-cysteine ligase |
| GCLC | Catalytic subunit of glutamate-cysteine ligase |
| GCLM | Modifier subunit of glutamate-cysteine ligase |
| GSH | Glutathione |
| Keap1 | Kelch-like ECH-associated protein 1 |
| LPS | Lipopolysaccharide |
| MEK | Mitogen-activated protein kinase kinase |
| MTT | 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| Nrf2 | NF-E2-related factor-2 |
| NQO1 | NAD(P)H:quinone oxidoreductase 1 |
| PI3K | Phosphoinositide 3-kinase |
| ROS | Reactive oxygen species |
| t-BHP | tert-Butylhydroperoxide |
References
- Dröge, W. Free radicals in the physiological control of cell function. Physiol. Rev. 2002, 82, 47–95. [Google Scholar] [CrossRef]
- Ma, Q. Transcriptional responses to oxidative stress: pathological and toxicological implications. Pharmacol. Ther. 2010, 125, 376–393. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q. Role of nrf2 in oxidative stress and toxicity. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 401–426. [Google Scholar] [CrossRef] [PubMed]
- Niture, S.K.; Khatri, R.; Jaiswal, A.K. Regulation of Nrf2-an update. Free Radic. Biol. Med. 2014, 66, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Bryan, H.K.; Olayanju, A.; Goldring, C.E.; Park, B.K. The Nrf2 cell defense pathway: Keap1-dependent and -independent mechanisms of regulation. Biochem. Pharmacol. 2013, 85, 705–717. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Tan, H.Y.; Wang, N.; Zhang, Z.J.; Lao, L.; Wong, C.W.; Feng, Y. The role of oxidative stress and antioxidants in liver diseases. Int. J. Mol. Sci. 2015, 16, 26087–26124. [Google Scholar] [CrossRef] [PubMed]
- Bataille, A.M.; Manautou, J.E. Nrf2: a potential target for new therapeutics in liver disease. Clin. Pharmacol. Ther. 2012, 92, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.C.; Ji, J.A.; Jiang, Z.Y.; You, Q.D. The Keap1-Nrf2-ARE pathway as a potential preventive and therapeutic target: an update. Med. Res. Rev. 2016, 36, 924–963. [Google Scholar] [PubMed]
- Swamy, M.K.; Sinniah, U.R. A comprehensive review on the phytochemical constituents and pharmacological activities of Pogostemon cablin Benth.: an aromatic medicinal plant of industrial importance. Molecules 2015, 20, 8521–8547. [Google Scholar] [CrossRef]
- China Pharmacopoeia Commission. Pharmacopoeia of the People’s Republic of China; Chemical Industry Press: Beijing, China, 2010; Volume 1, pp. 42–46. [Google Scholar]
- Yang, Y.; Kinoshita, K.; Koyama, K.; Takahashi, K.; Tai, T.; Nunoura, Y.; Watanabe, K. Anti-emetic principles of Pogostemon cablin (Blanco) Benth. Phytomedicine 1999, 6, 89–93. [Google Scholar] [CrossRef]
- Liu, X.R.; Fan, R.; Zhang, Y.Y.; Zhu, M.J. Study on antimicrobial activities of extracts from Pogestemon cablin (Blanco) Benth. Food Sci. Technol. 2009, 24, 220–227. [Google Scholar]
- Miyazawa, M.; Okuno, Y.; Nakamura, S.I.; Kosaka, H. Antimutagenic activity of flavonoids from Pogostemon cablin. J. Agric. Food Chem. 2000, 48, 642–647. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.W.; Cho, S.J.; Kim, B.Y.; Cho, S.I.; Kim, Y.K. Pogostemon cablin as ROS scavenger in oxidant-induced cell death of human neuroglioma cells. Evid. Based Complement. Alternat. Med. 2010, 7, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Neupane, G.P.; Lee, S.O.; Lee, J.S.; Kim, M.Y.; Kim, S.Y.; Park, B.C.; Park, Y.J.; Kim, J.A. Protective effects of Pogostemon cablin Bentham water extract on inflammatory cytokine expression in TNBS-induced colitis in rats. Arch. Pharmacal. Res. 2014, 37, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.C.; Liao, J.C.; Huang, T.H.; Lin, Y.C.; Liu, C.Y.; Chiu, Y.J.; Peng, W.H. Analgesic and anti-inflammatory activities of the methanol extract from Pogostemon cablin. Evid. Based Complement. Alternat. Med. 2011, 2011, 671741. [Google Scholar] [CrossRef]
- Li, K.; Zhang, H.; Xie, H.; Liang, Y.; Wang, X.; Ito, Y. Preparative isolation and purification of five flavonoids from Pogostemon cablin Benth by high-speed countercurrent chromatography and preparative high-performance liquid chromatography. J. Liq. Chromatogr. Relat. Technol. 2011, 34, 1617–1629. [Google Scholar] [CrossRef]
- Hussin, N.; Mondello, L.; Costa, R.; Dugo, P.; Yusoff, N.I.; Yarmo, M.A.; AbWahab, A.; Said, M. Quantitative and physical evaluation of patchouli essential oils obtained from different sources of Pogostemon cablin. Nat. Prod. Commun. 2012, 7, 927–930. [Google Scholar] [CrossRef]
- Kocevski, D.; Du, M.; Kan, J.; Jing, C.; Lačanin, I.; Pavlović, H. Antifungal effect of Allium tuberosum, Cinnamomum cassia, and Pogostemon cablin essential oils and their components against population of Aspergillus species. J. Food Sci. 2013, 78, M731–M737. [Google Scholar] [CrossRef]
- Lin, R.F.; Feng, X.X.; Li, C.W.; Zhang, X.J.; Yu, X.T.; Zhou, J.Y.; Zhang, X.; Xie, Y.L.; Su, Z.R.; Zhan, J.Y. Prevention of UV radiation-induced cutaneous photoaging in mice by topical administration of patchouli oil. J. Ethnopharmacol. 2014, 154, 408–418. [Google Scholar] [CrossRef]
- Wu, H.; Li, B.; Wang, X.; Jin, M.; Wang, G. Inhibitory effect and possible mechanism of action of patchouli alcohol against Influenza A (H2N2) virus. Molecules 2011, 16, 6489–6501. [Google Scholar] [CrossRef]
- Wei, A.; Shibamoto, T. Antioxidant activities and volatile constituents of various essential oils. J. Agric. Food Chem. 2007, 55, 1737–1742. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.B.; Shin, Y.K.; Lee, S.H. Anti-inflammatory activity of patchouli alcohol in RAW264.7 and HT-29 cells. Food Chem. Toxicol. 2013, 55, 229–233. [Google Scholar] [CrossRef] [PubMed]
- Yi, Y.Y.; He, J.J.; Su, J.Q.; Kong, S.Z.; Su, J.Y.; Li, Y.C.; Huang, S.H.; Li, C.W.; Lai, X.P.; Su, Z.R. Synthesis and antimicrobial evaluation of pogostone and its analogues. Fitotherapia 2013, 84, 135–139. [Google Scholar] [CrossRef]
- Sun, C.Y.; Xu, L.Q.; Zhang, Z.B.; Chen, C.H.; Huang, Y.Z.; Su, Z.Q.; Guo, H.Z.; Chen, X.Y.; Zhang, X.; Liu, Y.H.; et al. Protective effects of pogostone against LPS-induced acute lung injury in mice via regulation of Keap1-Nrf2/NF-κB signaling pathways. Int. Immunopharmacol. 2016, 32, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.Y.; Dou, Y.X.; Luo, D.D.; Zhang, Z.B.; Li, C.L.; Zeng, H.F.; Su, Z.R.; Xie, J.H.; Lai, X.P.; Li, Y.C. β-Patchoulene from patchouli oil protects against LPS-induced acute lung injury via suppressing NF-κB and activating Nrf2 pathways. Int. Immunopharmacol. 2017, 50, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Huong, D.T.; Luong, D.V.; Thao, T.T.; Sung, T.V. A new flavone and cytotoxic activity of flavonoid constituents isolated from Miliusa balansae (Annonaceae). Pharmazie 2005, 60, 627–629. [Google Scholar] [CrossRef] [PubMed]
- Rauter, A.P.; Branco, I.; Tosrão, Z.; Pais, M.S.; Gonzalez, A.G.; Bermejo, J.B. Flavonoids from Artemisia campestris subsp. Marítima. Phytochemistry 1989, 28, 2173–2175. [Google Scholar] [CrossRef]
- Liang, C.; Xue, Z.; Cang, J.; Wang, H.; Li, P. Dimethyl sulfoxide induces heme oxygenase-1 expression via JNKs and Nrf2 pathways in human umbilical vein endothelial cells. Mol. Cell. Biochem. 2011, 355, 109–115. [Google Scholar] [CrossRef]
- Hix, S.; Kadiiska, M.B.; Mason, R.P.; Augusto, O. In vivo metabolism of tert-butyl hydroperoxide to methyl radicals. EPR spin-trapping and DNA methylation studies. Chem. Res. Toxicol. 2000, 13, 1056–1064. [Google Scholar] [CrossRef]
- Oh, J.M.; Jung, Y.S.; Jeon, B.S.; Yoon, B.I.; Lee, K.S.; Kim, B.H.; Oh, S.J.; Kim, S.K. Evaluation of hepatotoxicity and oxidative stress in rats treated with tert-butyl hydroperoxide. Food Chem. Toxicol. 2012, 50, 1215–1221. [Google Scholar] [CrossRef]
- Song, J.S.; Kim, E.K.; Choi, Y.W.; Oh, W.K.; Kim, Y.M. Hepatocyte-protective effect of nectandrin B, a nutmeg lignan, against oxidative stress: Role of Nrf2 activation through ERK phosphorylation and AMPK-dependent inhibition of GSK-3β. Toxicol. Appl. Pharmacol. 2016, 307, 138–149. [Google Scholar] [CrossRef] [PubMed]
- Na, H.K.; Kim, E.H.; Jung, J.H.; Lee, H.H.; Hyun, J.W.; Surh, Y.J. (−)-Epigallocatechin gallate induces Nrf2-mediated antioxidant enzyme expression via activation of PI3K and ERK in human mammary epithelial cells. Arch. Biochem. Biophys. 2008, 476, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Mo, C.; Wang, L.; Zhang, J.; Numazawa, S.; Tang, H.; Tang, X.; Han, X.; Li, J.; Yang, M.; Wang, Z.; et al. The crosstalk between Nrf2 and AMPK signal pathways is important for the anti-inflammatory effect of berberine in LPS-stimulated macrophages and endotoxin-shocked mice. Antioxid. Redox Signal. 2014, 20, 574–588. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.; Hou, D.X. Multiple regulations of Keap1/Nrf2 system by dietary phytochemicals. Mol. Nutr. Food Res. 2016, 60, 1731–1755. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.A.; Chowdhury, A.K.; Rahman, A.K.; Borkowski, T.; Nahar, L.; Sarker, S.D. Pachypodol, a flavonol from the leaves of Calycopteris floribunda, inhibits the growth of CaCo 2 colon cancer cell line in vitro. Phytother. Res. 2008, 22, 1684–1687. [Google Scholar] [CrossRef] [PubMed]
- Ishitsuka, H.; Ohsawa, C.; Ohiwa, T.; Umeda, I.; Suhara, Y. Antipicornavirus flavone Ro 09-0179. Antimicrob. Agents Chemother. 1982, 22, 611–616. [Google Scholar] [CrossRef] [Green Version]
- Sandoval, I.V.; Carrasco, L. Poliovirus infection and expression of the poliovirus protein 2B provoke the disassembly of the Golgi complex, the organelle target for the antipoliovirus drug Ro-090179. J. Virol. 1997, 71, 4679–4693. [Google Scholar]
- Arita, M.; Philipov, S.; Galabov, A.S. Phosphatidylinositol 4-kinase III beta is the target of oxoglaucine and pachypodol (Ro 09-0179) for their anti-poliovirus activities, and is located at upstream of the target step of brefeldin A. Microbiol. Immunol. 2015, 59, 338–347. [Google Scholar] [CrossRef] [PubMed]
- Shan, X.Q.; Aw, T.Y.; Jones, D.P. Glutathione-dependent protection against oxidative injury. Pharmacol. Ther. 1990, 47, 61–71. [Google Scholar] [CrossRef]
- Joo, M.S.; Kim, W.D.; Lee, K.Y.; Kim, J.H.; Koo, J.H.; Kim, S.G. AMPK facilitates nuclear accumulation of Nrf2 by phosphorylating at Serine 550. Mol. Cell. Biol. 2016, 36, 1931–1942. [Google Scholar] [CrossRef]
- Zipper, L.M.; Mulcahy, R.T. Erk activation is required for Nrf2 nuclear localization during pyrrolidine dithiocarbamate induction of glutamate cysteine ligase modulatory gene expression in HepG2 cells. Toxicol. Sci. 2003, 73, 124–134. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Xu, C.; Pan, Z.; Keum, Y.S.; Kim, J.H.; Shen, G.; Yu, S.; Oo, K.T.; Ma, J.; Kong, A.N. Butylated hydroxyanisole regulates ARE-mediated gene expression via Nrf2 coupled with ERK and JNK signaling pathway in HepG2 cells. Mol. Carcinog. 2006, 45, 841–850. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.Y.; Lee, J.H.; Jegal, K.H.; Cho, I.J.; Kim, Y.W.; Kim, S.C. Oxyresveratrol abrogates oxidative stress by activating ERK-Nrf2 pathway in the liver. Chem. Biol. Interact. 2016, 245, 110–121. [Google Scholar] [CrossRef] [PubMed]
- Noh, K.; Kim, Y.M.; Kim, Y.W.; Kim, S.G. Farnesoid X receptor activation by chenodeoxycholic acid induces detoxifying enzymes through AMP-activated protein kinase and extracellular signal-regulated kinase 1/2-mediated phosphorylation of CCAAT/enhancer binding protein β. Drug Metab. Dispos. 2011, 39, 1451–1459. [Google Scholar] [CrossRef] [PubMed]





© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, E.K.; Kim, J.H.; Jeong, S.; Choi, Y.W.; Choi, H.J.; Kim, C.Y.; Kim, Y.-M. Pachypodol, a Methoxyflavonoid Isolated from Pogostemon cablin Bentham Exerts Antioxidant and Cytoprotective Effects in HepG2 Cells: Possible Role of ERK-Dependent Nrf2 Activation. Int. J. Mol. Sci. 2019, 20, 4082. https://doi.org/10.3390/ijms20174082
Kim EK, Kim JH, Jeong S, Choi YW, Choi HJ, Kim CY, Kim Y-M. Pachypodol, a Methoxyflavonoid Isolated from Pogostemon cablin Bentham Exerts Antioxidant and Cytoprotective Effects in HepG2 Cells: Possible Role of ERK-Dependent Nrf2 Activation. International Journal of Molecular Sciences. 2019; 20(17):4082. https://doi.org/10.3390/ijms20174082
Chicago/Turabian StyleKim, Eun Kyung, Ji Hoon Kim, Soyeon Jeong, Yong Won Choi, Hyun Jung Choi, Chul Young Kim, and Young-Mi Kim. 2019. "Pachypodol, a Methoxyflavonoid Isolated from Pogostemon cablin Bentham Exerts Antioxidant and Cytoprotective Effects in HepG2 Cells: Possible Role of ERK-Dependent Nrf2 Activation" International Journal of Molecular Sciences 20, no. 17: 4082. https://doi.org/10.3390/ijms20174082
APA StyleKim, E. K., Kim, J. H., Jeong, S., Choi, Y. W., Choi, H. J., Kim, C. Y., & Kim, Y. -M. (2019). Pachypodol, a Methoxyflavonoid Isolated from Pogostemon cablin Bentham Exerts Antioxidant and Cytoprotective Effects in HepG2 Cells: Possible Role of ERK-Dependent Nrf2 Activation. International Journal of Molecular Sciences, 20(17), 4082. https://doi.org/10.3390/ijms20174082